Broad-Spectrum Anti-Adhesive Coating Based on an Extracellular Polymer from a Marine Cyanobacterium
Abstract
:1. Introduction
2. Results
2.1. Biopolymer Production
2.2. CyanoCoating Production and Characterization
2.2.1. Thickness
2.2.2. Wettability
2.2.3. Surface Functional Groups
2.2.4. Topography
2.2.5. Surface Charge
2.3. CyanoCoating Biological Performance
2.3.1. Protein Adsorption
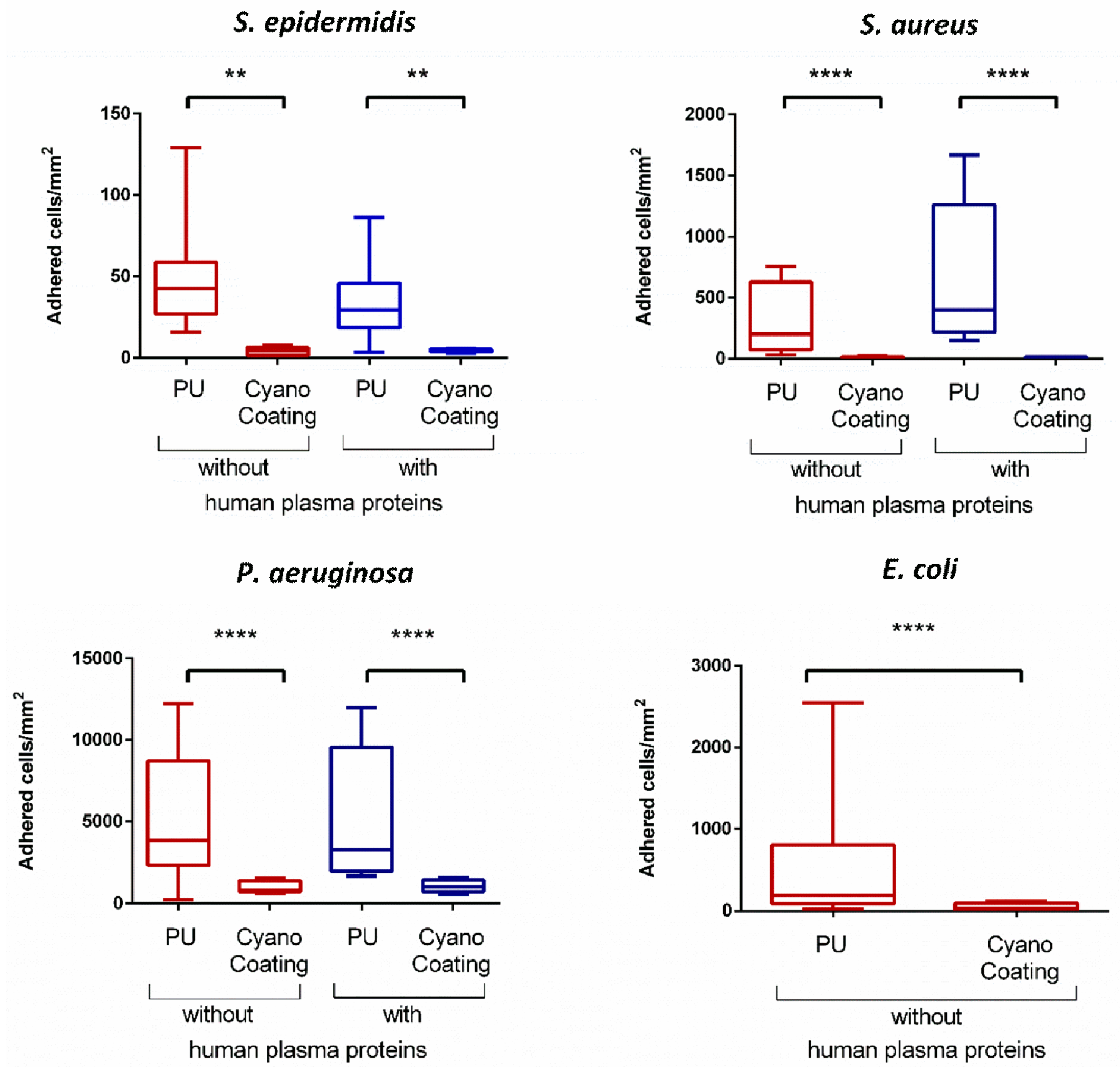
2.3.2. Bacterial Adhesion
2.3.3. Platelet Adhesion and Activation
2.3.4. Biocompatibility
3. Discussion
4. Materials and Methods
4.1. Cyanobacterium Growth Conditions and Biopolymer Isolation
4.2. CyanoCoating Development
4.2.1. Substrate Preparation
4.2.2. Substrate Activation
4.2.3. Polyurethane (PU) Coating Production
4.2.4. CyanoCoating Production
4.3. Surface Characterization
4.3.1. Ellipsometry
4.3.2. Water Contact Angle (WCA)
4.3.3. Fourier Transform–Infrared Reflection Absorption Spectroscopy (FT–IRRAS)
4.3.4. Scanning Electron Microscopy with Energy-Dispersive Spectroscopy (SEM/EDS)
4.3.5. Electrokinetic Analyzer
4.4. CyanoCoating Biological Performance Evaluation
4.4.1. Protein Adsorption Studies: Quartz Crystal Microbalance with Dissipation (QCM–D)
4.4.2. Bacterial Assays
4.4.2.1. Bacterial Strains, Media and Growth Conditions
4.4.2.2. Bacterial Adhesion Assays
4.4.2.3. Bacterial Viability Assay
4.4.2.4. Image Acquisition and Analysis
4.4.2.5. Scanning Electron Microscopy (SEM) analysis of Adhered Bacteria
4.4.3. Platelet Adhesion and Activation Assay
4.4.4. Biocompatibility Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yu, Q.; Sun, H. Novel strategies for the prevention and treatment of biofilm related infections. Int. J. Mol. Sci. 2013, 14, 18488–18501. [Google Scholar] [CrossRef]
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Ascencio, F.; Gama, N.L.; De Philippis, R.; Ho, B. Effectiveness of Cyanothece spp. and Cyanospira capsulata exocellular polysaccharides as antiadhesive agents for blocking attachment of Helicobacter pylori to human gastric cells. Folia Microbiol. (Praha) 2004, 49, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.P.; Mota, R.; Durao, J.; Neves, S.C.; Barrias, C.C.; Tamagnini, P.; Gales, L. Cyanobacterium-Derived Extracellular Carbohydrate Polymer for the Controlled Delivery of Functional Proteins. Macromol. Biosci. 2017, 17, 1600206. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Mota, R.; Leite, J.P.; Tamagnini, P.; Gales, L.; Rocha, F. Application of a cyanobacterial extracellular polymeric substance in the microencapsulation of vitamin B12. Powder. Technol. 2019, 343, 644–651. [Google Scholar] [CrossRef]
- Loke, M.F.; Lui, S.Y.; Ng, B.L.; Gong, M.; Ho, B. Antiadhesive property of microalgal polysaccharide extract on the binding of Helicobacter pylori to gastric mucin. FEMS Immunol. Med. Microbiol. 2007, 50, 231–238. [Google Scholar] [CrossRef]
- Gadenne, V.; Lebrun, L.; Jouenne, T.; Thebault, P. Antiadhesive activity of ulvan polysaccharides covalently immobilized onto titanium surface. Colloids Surf. B Biointerfaces 2013, 112, 229–236. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Maia, S.; Gomes, J.; Gomes, P.; Martins, M.C. Characterization of hLF1-11 immobilization onto chitosan ultrathin films, and its effects on antimicrobial activity. Acta Biomater. 2014, 10, 3513–3521. [Google Scholar] [CrossRef]
- Costa, F.M.; Maia, S.R.; Gomes, P.A.; Martins, M.C. Dhvar5 antimicrobial peptide (AMP) chemoselective covalent immobilization results on higher antiadherence effect than simple physical adsorption. Biomaterials 2015, 52, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou Zeid, A.H.; Aboutabl, E.A.; Sleem, A.A.; El-Rafie, H.M. Water soluble polysaccharides extracted from Pterocladia capillacea and Dictyopteris membranacea and their biological activities. Carbohydr. Polym. 2014, 113, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Pierre, G.; Sopena, V.; Juin, C.; Mastouri, A.; Graber, M.; Maugard, T. Antibacterial Activity of a Sulfated Galactan Extracted from the Marine Alga Chaetomorpha aerea Against Staphylococcus aureus. Biotechnol. Bioprocess. Eng. 2011, 16, 937–945. [Google Scholar] [CrossRef]
- Saeki, Y.; Kato, T.; Okuda, K. Inhibitory effects of funoran on the adherence and colonization of oral bacteria. Bull. Tokyo Dent. Coll. 1996, 37, 77–92. [Google Scholar] [PubMed]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; De Philippis, R. Exocellular Polysaccharides in Microalgae and Cyanobacteria: Chemical Features, Role and Enzymes and Genes Involved in Their Biosynthesis. In The Physiology of Microalgae; Springer International Publishing: Basel, Switzerland, 2016; Volume 6, pp. 565–590. [Google Scholar]
- Mota, R.; Guimaraes, R.; Buttel, Z.; Rossi, F.; Colica, G.; Silva, C.J.; Santos, C.; Gales, L.; Zille, A.; De Philippis, R.; et al. Production and characterization of extracellular carbohydrate polymer from Cyanothece sp. CCY 0110. Carbohydr. Polym. 2013, 92, 1408–1415. [Google Scholar] [CrossRef]
- Mota, R. Cyanobacterial Extracellular Polymeric Substances (EPS): Biosynthesis, Characteristics and Applications. Ph.D. Thesis, University of Porto, Porto, Portugal, July 2017. [Google Scholar]
- Goncalves, D.; Irene, E.A. Fundamentals and applications of spectroscopic ellipsometry. Quim. Nova 2002, 25, 794–800. [Google Scholar] [CrossRef] [Green Version]
- Brubaker, C.E.; Messersmith, P.B. The present and future of biologically inspired adhesive interfaces and materials. Langmuir 2012, 28, 2200–2205. [Google Scholar] [CrossRef]
- Sileika, T.S.; Kim, H.D.; Maniak, P.; Messersmith, P.B. Antibacterial performance of polydopamine-modified polymer surfaces containing passive and active components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. [Google Scholar] [CrossRef]
- Zhou, X.F.; Zhang, T.Z.; Jiang, X.L.; Gu, N. The Surface Modification of Medical Polyurethane to Improve the Hydrophilicity and Lubricity: The Effect of Pretreatment. J. Appl. Polym. Sci. 2010, 116, 1284–1290. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Butruk, B.A.; Zietek, P.A.; Ciach, T. Simple method of fabrication of hydrophobic coatings for polyurethanes. Cent. Eur. J. Chem. 2011, 9, 1039–1045. [Google Scholar] [CrossRef] [Green Version]
- Felgueiras, H.P.; Wang, L.M.; Ren, K.F.; Querido, M.M.; Jin, Q.; Barbosa, M.A.; Ji, J.; Martins, M.C. Octadecyl Chains Immobilized onto Hyaluronic Acid Coatings by Thiol-ene “Click Chemistry” Increase the Surface Antimicrobial Properties and Prevent Platelet Adhesion and Activation to Polyurethane. ACS Appl. Mater. Interfaces 2017, 9, 7979–7989. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17 (Suppl. 2), 68–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588. [Google Scholar] [CrossRef] [PubMed]
- McArthur, S.L.; McLean, K.M.; Kingshott, P.; St John, H.A.W.; Chatelier, R.C.; Griesser, H.J. Effect of polysaccharide structure on protein adsorption. Colloids Surf. B Biointerfaces 2000, 17, 37–48. [Google Scholar] [CrossRef]
- Vaudaux, P.; Yasuda, H.; Velazco, M.I.; Huggler, E.; Ratti, I.; Waldvogel, F.A.; Lew, D.P.; Proctor, R.A. Role of host and bacterial factors in modulating staphylococcal adhesion to implanted polymer surfaces. J. Biomater. Appl. 1990, 5, 134–153. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An Overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef]
- Goncalves, I.C.; Martins, M.C.; Barbosa, M.A.; Naeemi, E.; Ratner, B.D. Selective protein adsorption modulates platelet adhesion and activation to oligo(ethylene glycol)-terminated self-assembled monolayers with C18 ligands. J. Biomed. Mater. Res. A 2009, 89, 642–653. [Google Scholar] [CrossRef]
- De Philippis, R.; Vincenzini, M. Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Singh, S.; Das, S. Screening, production, optimization and characterization of cyanobacterial polysaccharide. World J. Microbiol. Biotechnol. 2011, 27, 1971–1980. [Google Scholar] [CrossRef]
- Cao, X.; Pettit, M.E.; Conlan, S.L.; Wagner, W.; Ho, A.D.; Clare, A.S.; Callow, J.A.; Callow, M.E.; Grunze, M.; Rosenhahn, A. Resistance of polysaccharide coatings to proteins, hematopoietic cells, and marine organisms. Biomacromolecules 2009, 10, 907–915. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Cangini, I.; Selan, L.; Vercellino, M.; Montanaro, L.; Visai, L.; Arciola, C.R. An overview of the methodological approach to the in vitro study of anti-infective biomaterials. Int. J. Artif. Organs. 2012, 35, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Poh, C.; Wang, W. Titanium with surface-grafted dextran and immobilized bone morphogenetic protein-2 for inhibition of bacterial adhesion and enhancement of osteoblast functions. Tissue Eng. Part A 2009, 15, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.N.; Goncalves, I.C.; Martins, M.C.; Barbosa, M.A.; Ratner, B.D. Fibrinogen adsorption, platelet adhesion and activation on mixed hydroxyl-/methyl-terminated self-assembled monolayers. Biomaterials 2006, 27, 5357–5367. [Google Scholar] [CrossRef] [PubMed]
- Mikhalovska, L.I.; Santin, M.; Denyer, S.P.; Lloyd, A.W.; Teer, D.G.; Field, S.; Mikhalovsky, S.V. Fibrinogen adsorption and platelet adhesion to metal and carbon coatings. Thromb. Haemost. 2004, 92, 1032–1039. [Google Scholar]
- Xu, L.C.; Bauer, J.W.; Siedlecki, C.A. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf. B Biointerfaces 2014, 124, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.C.; Ratner, B.D.; Barbosa, M.A. Protein adsorption on mixtures of hydroxyl- and methyl-terminated alkanethiols self-assembled monolayers. J. Biomed. Mater. Res. A 2003, 67, 158–171. [Google Scholar] [CrossRef]
- Antunes, J.C.; Pereira, C.L.; Molinos, M.; Ferreira-da-Silva, F.; Dessi, M.; Gloria, A.; Ambrosio, L.; Goncalves, R.M.; Barbosa, M.A. Layer-by-layer self-assembly of chitosan and poly(gamma-glutamic acid) into polyelectrolyte complexes. Biomacromolecules 2011, 12, 4183–4195. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Sousa, D.M.; Parreira, P.; Lamghari, M.; Gomes, P.; Martins, M.C.L. N-acetylcysteine-functionalized coating avoids bacterial adhesion and biofilm formation. Sci. Rep. 2017, 7, 17374. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Polikarpov, I.; Craievich, A.F. Average protein density is a molecular-weight-dependent function. Protein Sci. 2004, 13, 2825–2828. [Google Scholar] [CrossRef] [PubMed]
- Chick, H.; Lubrzynska, E. The Viscosity of Some Protein Solutions. Biochem. J. 1914, 8, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Sommer, C.; Straehle, C.; Kothe, U.; Hamprecht, F.A. Ilastik: Interactive Learning and Segmentation Toolkit. In Proceedings of the 2011 8th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Chicago, IL, USA, 30 March–2 April 2011; pp. 230–233. [Google Scholar]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, B.; Mota, R.; Parreira, P.; Tamagnini, P.; L. Martins, M.C.; Costa, F. Broad-Spectrum Anti-Adhesive Coating Based on an Extracellular Polymer from a Marine Cyanobacterium. Mar. Drugs 2019, 17, 243. https://doi.org/10.3390/md17040243
Costa B, Mota R, Parreira P, Tamagnini P, L. Martins MC, Costa F. Broad-Spectrum Anti-Adhesive Coating Based on an Extracellular Polymer from a Marine Cyanobacterium. Marine Drugs. 2019; 17(4):243. https://doi.org/10.3390/md17040243
Chicago/Turabian StyleCosta, Bruna, Rita Mota, Paula Parreira, Paula Tamagnini, M. Cristina L. Martins, and Fabíola Costa. 2019. "Broad-Spectrum Anti-Adhesive Coating Based on an Extracellular Polymer from a Marine Cyanobacterium" Marine Drugs 17, no. 4: 243. https://doi.org/10.3390/md17040243





