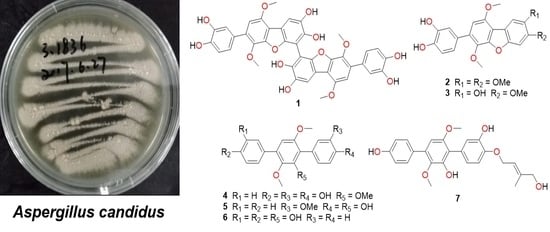
Polyhydroxy p-Terphenyls from a Mangrove Endophytic Fungus Aspergillus candidus LDJ-5
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation and Extraction
3.4. Isolation
3.5. Cytotoxicity Assay
3.6. Anti-Influenza A viral (H1N1) Assay
3.7. Antimicrobial Activities
3.8. PTP1B Inhibitory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Li, X.B.; Lou, H.X. Structural and biological diversity of natural p-terphenyls. J. Asian Nat. Prod. Res. 2018, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K. Natural terphenyls: Developments since 1877. Chem. Rev. 2006, 106, 2209–2223. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J.; Lu, F.M.; Bao, L.; Wang, H.Y.; Chen, B.S.; Li, E.W.; Wang, Z.D.; Xie, L.P.; Guo, C.B.; Xue, Y.F.; et al. Terphenyl derivatives and terpenoids from a wheat-born mold Aspergillus candidus. J. Antibiot. 2020, 73, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, X.L.; Wang, Y.; Zheng, J.Y.; Wang, C.Y.; Shao, C.L. Aspergivones A and B, two new flavones isolated from a gorgonian-derived Aspergillus candidus fungus. Nat. Prod. Res. 2017, 31, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Buttachon, S.; Ramos, A.A.; Inácio, Â.; Dethoup, T.; Gales, L.; Lee, M.; Costa, P.M.; Silva, A.M.S.; Sekeroglu, N.; Rocha, E.; et al. Bis-indolyl benzenoids, hydroxypyrrolidine derivatives and other constituents from cultures of the marine sponge-associated fungus Aspergillus candidus KUFA0062. Mar. Drugs 2018, 16, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.L.; Chen, X.H.; Zhang, X.M.; Che, Q.; Zhang, G.J.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Prenylated p-terphenyls from a mangrove endophytic fungus, Aspergillus candidus LDJ-5. J. Nat. Prod. 2020, 83, 8–13. [Google Scholar] [CrossRef]
- Stead, P.; Affleck, K.; Sidebottom, P.J.; Taylor, N.L.; Drake, C.S.; Todd, M.; Jowett, A.; Webb, G. Isolation and characterisation of a prenylated p-terphenyl metabolite of Aspergillus candidus possessing potent and selective cytotoxic activity; studies on mechanism of action. J. Antibiot. 1999, 52, 89–95. [Google Scholar]
- Cai, S.; Sun, S.; Zhou, H.; Kong, X.; Zhu, T.; Li, D.; Gu, Q. Prenylated polyhydroxy-p-terphenyls from Aspergillus taichungensis ZHN-7-07. J. Nat. Prod. 2011, 74, 1106–1110. [Google Scholar] [CrossRef]
- Yan, W.; Li, S.J.; Guo, Z.K.; Zhang, W.J.; Wei, W.; Tan, R.X.; Jiao, R.H. New p-terphenyls from the endophytic fungus Aspergillus sp. YXf3. Bioorg. Med. Chem. Lett. 2017, 27, 51–54. [Google Scholar] [CrossRef]
- Quang, D.N.; Hashimoto, T.; Hitaka, Y.; Tanaka, M.; Nukada, M.; Yamamoto, I.; Asakawa, Y. Thelephantins I-N: P-terphenyl derivatives from the inedible mushroom Hydnellum caeruleum. Phytochemistry 2004, 65, 1179–1184. [Google Scholar] [CrossRef]
- Guo, Z.K.; Yan, T.; Guo, Y.; Song, Y.C.; Jiao, R.H.; Tan, R.X.; Ge, H.M. p-Terphenyl and diterpenoid metabolites from endophytic Aspergillus sp. YXf3. J. Nat. Prod. 2012, 75, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, N.; Quang, D.N.; Hashimoto, T.; Nukada, M.; Asakawa, Y. Terrestrins A-G: P-terphenyl derivatives from the inedible mushroom Thelephora terrestris. Phytochemistry 2005, 66, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, E.; Surup, F.; Herrmann, J.; Huch, V.; Müller, R.; Stadler, M. Rickenyls A-E, antioxidative terphenyls from the fungus Hypoxylon rickii (Xylariaceae, Ascomycota). Phytochemistry 2015, 118, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, W.; Zhang, M.; Li, Y.; Li, L.; Li, X.; Chang, W.; Zhao, Z.; Lou, H. p-Terphenyl derivatives from the endolichenic fungus Floricola striata. J. Nat. Prod. 2016, 79, 2188–2194. [Google Scholar] [CrossRef]
- Geraci, C.; Neri, P.; Paterno, C.; Rocco, C.; Tringali, C. An unusual nitrogenous terphenyl derivative from fruiting bodies of the basidiomycete Sarcodon leucopus. J. Nat. Prod. 2000, 63, 347–351. [Google Scholar] [CrossRef]
- Cali, V.; Spatafora, C.; Tringali, C. Sarcodonins and sarcoviolins, bioactive polyhydroxy-p-terphenyl pyrazinediol dioxide conjugates from fruiting bodies of the basidiomycete Sarcodon leucopus. Eur. J. Org. Chem. 2004, 592–599. [Google Scholar] [CrossRef]
- Ma, B.J.; Liu, J.K. An unusual nitrogenous terphenyl derivative from fruiting bodies of the basidiomycete Sarcodon scabrosus. Z. Naturforsch. B 2005, 60, 565–568. [Google Scholar] [CrossRef]
- Yamazoe, A.; Hayashi, K.; Kuboki, A.; Ohira, S.; Nozaki, H. The isolation, structural determination, and total synthesis of terfestatin A, a novel auxin signaling inhibitor from Streptomyces sp. Tetrahedron Lett. 2004, 45, 8359–8362. [Google Scholar] [CrossRef]
- Guo, H.; Hu, H.; Liu, S.; Liu, X.; Zhou, Y.; Che, Y. Bioactive p-terphenyl derivatives from a Cordyceps-colonizing isolate of Gliocladium sp. J. Nat. Prod. 2007, 70, 1519–1521. [Google Scholar] [CrossRef]
- Andernach, L.; Sandjo, L.P.; Liermann, J.C.; Schlamann, R.; Richter, C.; Ferner, J.; Schwalbe, H.; Schüffler, A.; Thines, E.; Opatz, T. Terphenyl derivatives from Allantophomopsis lycopodina. J. Nat. Prod. 2016, 79, 2718–2725. [Google Scholar] [CrossRef]
- Ren, F.; Chen, S.; Zhang, Y.; Zhu, S.; Xiao, J.; Liu, X.; Su, R.; Che, Y. Hawaiienols A-D, highly oxygenated p-terphenyls from an insect-associated fungus, Paraconiothyrium hawaiiense. J. Nat. Prod. 2018, 81, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Belofsky, G.N.; Gloer, K.B.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. New p-terphenyl and polyketide metabolites from the sclerotia of Penicillium raistrickii. J. Nat. Prod. 1998, 61, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Han, J.J.; Ma, K.; Jin, T.; Bao, L.; Pei, Y.F.; Liu, H.W. New α-glucosidase inhibitors with p-terphenyl skeleton from the mushroom Hydnellum concrescens. Fitoterapia 2014, 98, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, B.; Lu, C.; Huang, J.; Shen, Y. Two new p-terphenyl derivatives from the marine fungal strain Aspergillus sp. AF119. Nat. Prod. Commun. 2012, 7, 1057–1062. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Inada, H.; Hayashi, A.; Higashimoto, K.; Pruksakorn, P.; Kamada, S.; Arai, M.; Ishida, S.; Kobayashi, M. Prenylterphenyllin and its dehydroxyl analogs, new cytotoxic substances from a marine-derived fungus Aspergillus candidus IF10. J. Antibiot. 2007, 60, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, A.; Takemoto, A.; Koshimizu, K.; Kawazu, K. p-Terphenyls with cytotoxic activity toward sea Urchin Embryos. Agric. Biol. Chem. 1985, 49, 867–868. [Google Scholar] [CrossRef]
- Kenji, K.; Mitsuaki, O.; Ryuji, S.; Akinori, A. Preparation and Formulation of p-Terphenyl Compounds as lgE Production inhibitors. WO Patent 9,804,508 A1, 5 February 1998. [Google Scholar]
- Oh, H.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. Arenarins A-C: New cytotoxic fungal metabolites from the sclerotia of Aspergillus arenarius. J. Nat. Prod. 1998, 61, 702–705. [Google Scholar] [CrossRef]
- He, R.J.; Yu, Z.H.; Zhang, R.U.; Zhang, Z.Y. Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol. Sin. 2014, 35, 1227–1246. [Google Scholar] [CrossRef] [Green Version]
- Lessard, L.; Stuible, M.; Tremblay, M.L. The two faces of PTP1B in cancer. Biochim. Biophys. Acta 2010, 1804, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhu, T.J.; Liu, H.B.; Fang, Y.C.; Zhu, W.M.; Gu, Q.Q. Cytotoxic polyketides from a marine-derived fungus Aspergillus glaucus. J. Nat. Prod. 2008, 71, 1837–1842. [Google Scholar] [CrossRef]
- Peng, J.X.; Jiao, J.Y.; Li, J.; Wang, W.; Gu, Q.Q.; Zhu, T.J.; Li, D.H. Pyronepolyene C-glucosides with NF-ϰB inhibitory and anti-influenza A viral (H1N1) activities from the sponge-associated fungus Epicoccum sp. JJY40. Bioorg. Med. Chem. Lett. 2012, 22, 3188–3190. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubben, T.; Clampit, J.; Stashko, M.; Trevillyan, J.; Jirousek, M.R. In vitro enzymatic assays of protein tyrosine phosphatase 1B. Curr. Protoc. Pharmacol. 2001, 13, 3.8.1–3.8.18. [Google Scholar] [CrossRef] [PubMed]


| Position | δC, Type | δH (J in Hz) |
|---|---|---|
| 1, 1′ | 113.2, C | |
| 2, 2′ | 148.9, C | |
| 3, 3′ | 105.1, C | |
| 4, 4′ | 144.7, C | |
| 5, 5′ | 142.8, C | |
| 6, 6′ | 106.6, CH | 7.51, s |
| 4, 4′-OH | 8.74, br s | |
| 5, 5′-OH | 9.56, br s | |
| 7, 7′ | 114.6, C | |
| 8, 8′ | 148.7, C | |
| 9, 9′ | 136.3, C | |
| 10, 10′ | 130.9, C | |
| 11, 11′ | 105.9, CH | 6.69, s |
| 12, 12′ | 149.7, C | |
| 9, 9′-OMe | 60.7, CH3 | 3.51, s |
| 12, 12′-OMe | 56.3, CH3 | 4.01, s |
| 13, 13′ | 129.6, C | |
| 14, 14′ | 117.2, CH | 6.97, d (1.8) |
| 15, 15′ | 145.1, C | |
| 16, 16′ | 145.2, C | |
| 17, 17′ | 115.9, CH | 6.77, d (8.2) |
| 18, 18′ | 120.7, CH | 6.82, dd (8.2, 1.8) |
| 15, 15′-OH | 8.94, s | |
| 16, 16′-OH | 8.94, s |
| Position | 2 | 3 | 4 | |||
|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 114.6, C | 115.0, C | 124.9, C | |||
| 2 | 150.4, C | 149.7, C | 118.5, CH | 6.66, d (2.1) | ||
| 3 | 96.8, CH | 7.45, s | 96.6, CH | 7.38, s | 145.1, C | |
| 4 | 149.6, C | 148.4, C | 144.9, C | |||
| 5 | 146.6, C | 144.0, C | 115.6, CH | 6.72, d (8.1) | ||
| 6 | 104.4, CH | 7.46, s | 107.3, CH | 7.39, s | 121.8, CH | 6.52, dd |
| (8.1, 2.1) | ||||||
| 4-OH/OMe | 56.5, CH3 | 3.87, s | 56.5, CH3 | 3.87, s | ||
| 5-OH/OMe | 56.6, CH3 | 3.86, s | 9.02, s | |||
| 1′ | 114.1, C | 114.1, C | 124.5, C | |||
| 2′ | 149.1, C | 149.1, C | 151.9, C | |||
| 3′ | 136.4, C | 136.4, C | 144.6, C | |||
| 4′ | 131.8, C | 131.7, C | 134.1, C | |||
| 5′ | 106.2, CH | 6.73, s | 106.1, CH | 6.70, s | 108.1, CH | 6.65, s |
| 6′ | 149.9, C | 149.9, C | 153.2, C | |||
| 2′-OMe | 60.7, CH3 | 3.50, s | ||||
| 3′-OMe | 61.1, CH3 | 3.78, s | 61.0, CH3 | 3.77, s | 60.7, CH3 | 3.49, s |
| 6′-OMe | 56.3, CH3 | 4.00, s | 56.3, CH3 | 3.98, s | 56.2, CH3 | 3.65, s |
| 1″ | 129.4, C | 129.5, C | 128.9, C | |||
| 2″ | 117.2, CH | 7.04, d (1.8) | 117.2, CH | 7.03, d (2.0) | 130.4, CH | 7.39, d (8.6) |
| 3″ | 145.3, C | 145.2, C | 115.5, CH | 6.83, d (8.6) | ||
| 4″ | 145.3, C | 145.3, C | 157.3, C | |||
| 5″ | 115.9, CH | 6.82, d (8.1) | 115.9, CH | 6.81, d (8.2) | 115.5, CH | 6.83, d (8.6) |
| 6″ | 120.8, CH | 6.89, dd | 120.8, CH | 6.88, dd | 130.4, CH | 7.39, d (8.6) |
| (8.1, 1.8) | (8.2, 2.0) | |||||
| 3″-OH | 9.00, s | |||||
| 4″-OH | 9.02, s | |||||
| Position | 5 | 6 | 7 | |||
|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 125.3, C | 134.8, C | 127.2, C | |||
| 2 | 115.7, CH | 6.85, d (1.8) | 131.3, CH | 7.29, d (7.1) | 118.9, CH | 6.73, s |
| 3 | 147.2, C | 127.8, CH | 7.36, dd | 146.3, C | ||
| (7.5, 7.5) | ||||||
| 4 | 145.7, C | 126.7, CH | 7.26, dd | 145.8, C | ||
| (7.3, 7.3) | ||||||
| 5 | 115.2, CH | 6.78, d (8.1) | 127.8, CH | 7.36, dd | 113.5, CH | 6.90, d (8.3) |
| (7.5, 7.5) | ||||||
| 6 | 123.9, CH | 6.71, dd | 131.3, CH | 7.29, d (7.1) | 122.1, CH | 6.64, d (8.3) |
| (8.1, 1.8) | ||||||
| 3-OH/OMe | 56.1, CH3 | 3.74, s | 8.76, s | |||
| 4-OH | 8.87, s | |||||
| 1′ | 118.4, C | 117.1, C | 117.3, C | |||
| 2′ | 148.7, C | 148.5, C | 148.6, C | |||
| 3′ | 139.9, C | 139.7, C | 139.7, C | |||
| 4′ | 132.9, C | 133.6, C | 132.9, C | |||
| 5′ | 103.7, CH | 6.45, s | 103.4, CH | 6.39, s | 103.4, CH | 6.38, s |
| 6′ | 153.6, C | 153.2, C | 153.5, C | |||
| 2′-OH | 8.58, s | 8.61, s | 8.49, s | |||
| 3′-OMe | 60.8, CH3 | 3.30, s | 60.5, CH3 | 3.32, s | 60.5, CH3 | 3.30, s |
| 6′-OMe | 56.1, CH3 | 3.66, s | 56.0, CH3 | 3.64, s | 56.0, CH3 | 3.64, s |
| 1″ | 138.7, C | 129.6, C | 129.1, C | |||
| 2″ | 129.1, CH | 7.60, d (7.2) | 116.5, CH | 7.06, d (1.9) | 130.1, CH | 7.43, d (8.5) |
| 3″ | 128.8, CH | 7.46, dd | 145.4, C | 115.6, CH | 6.84, d (8.5) | |
| (7.5, 7.5) | ||||||
| 4″ | 127.6, CH | 7.37, dd | 145.3, C | 157.2, C | ||
| (7.4, 7.4) | ||||||
| 5″ | 128.8, CH | 7.46, dd | 116.0, CH | 6.81, d (8.1) | 115.6, CH | 6.84, d (8.5) |
| (7.5, 7.5) | ||||||
| 6″ | 129.1, CH | 7.60, d (7.2) | 120.1, C | 6.90, dd | 130.1, CH | 7.43, d (8.5) |
| (8.1, 1.9) | ||||||
| 3″-OH | 8.98, s | |||||
| 4″-OH | 9.00, s | 9.51, s | ||||
| 1‴ | 65.3, CH2 | 4.62, d (6.2) | ||||
| 2‴ | 119.3, CH | 5.71, t (6.2) | ||||
| 3‴ | 140.2, C | |||||
| 4‴ | 14.3, CH3 | 1.67, s | ||||
| 5‴ | 66.0, CH3 | 3.86, d (4.4) | ||||
| 5‴-OH | 4.89, t (4.4) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, G.; Zhang, X.; Shah, M.; Che, Q.; Zhang, G.; Gu, Q.; Zhu, T.; Li, D. Polyhydroxy p-Terphenyls from a Mangrove Endophytic Fungus Aspergillus candidus LDJ-5. Mar. Drugs 2021, 19, 82. https://doi.org/10.3390/md19020082
Zhou G, Zhang X, Shah M, Che Q, Zhang G, Gu Q, Zhu T, Li D. Polyhydroxy p-Terphenyls from a Mangrove Endophytic Fungus Aspergillus candidus LDJ-5. Marine Drugs. 2021; 19(2):82. https://doi.org/10.3390/md19020082
Chicago/Turabian StyleZhou, Guoliang, Xiaomin Zhang, Mudassir Shah, Qian Che, Guojian Zhang, Qianqun Gu, Tianjiao Zhu, and Dehai Li. 2021. "Polyhydroxy p-Terphenyls from a Mangrove Endophytic Fungus Aspergillus candidus LDJ-5" Marine Drugs 19, no. 2: 82. https://doi.org/10.3390/md19020082