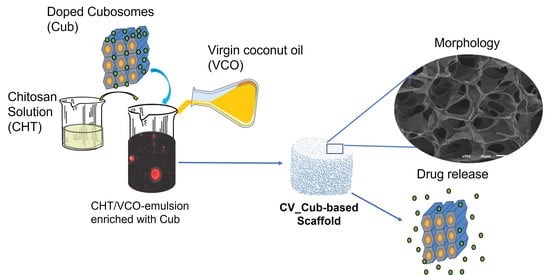
Chitosan/Virgin-Coconut-Oil-Based System Enriched with Cubosomes: A 3D Drug-Delivery Approach
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Morphological Features
2.2. Mechanical Properties
2.3. Stability and Degree of Swelling
2.4. Release Study
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Cubosome Preparation
3.2.2. Preparation of CHT/VCO-Based Emulsion Scaffolds
3.3. Characterization
3.3.1. Dynamic Light-Scattering (DLS) Measurements
3.3.2. Small-Angle X-ray-Scattering (SAXS) Measurements
3.3.3. Scanning Electron Microscope
3.3.4. Fourier Transform Infrared Spectroscopy
3.3.5. Mechanical Properties
3.3.6. Swelling
3.3.7. Diclofenac-Release Quantification
3.3.8. Confocal Analysis
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, M.-C.; Qin, W.; Lei, C.; Li, Q.-H.; Meng, M.; Fang, M.; Song, W.; Chen, J.-H.; Tay, F.; Niu, L.-N. Biomaterials from the sea: Future building blocks for biomedical applications. Bioact. Mater. 2021, 6, 4255–4285. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Almeida, M.; Varela Coelho, A.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.S.; et al. Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Mar. Drugs 2022, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Reys, L.L.; Silva, S.S.; Oliveira, C.; Lopez-Cebral, R.; Neves, N.M.; Martins, A.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Marine-origin Polysaccharides for Tissue Engineering and Regenerative Medicine. In Encyclopedia of Marine Biotechnology; Wiley: Hoboken, NJ, USA, 2020; pp. 2619–2650. [Google Scholar]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceuticals 2023, 15, 1313. [Google Scholar] [CrossRef]
- Thananukul, K.; Kaewsaneha, C.; Opaprakasit, P.; Lebaz, N.; Errachid, A.; Elaissari, A. Smart gating porous particles as new carriers for drug delivery. Adv. Drug Deliv. Rev. 2021, 174, 425–446. [Google Scholar] [CrossRef]
- Silva, S.S.; Rodrigues, L.C.; Fernandes, E.M.; Reis, R.L. Chapter 1—Fundamentals on biopolymers and global demand. In Biopolymer Membranes and Films; de Moraes, M.A., da Silva, C.F., Vieira, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–34. [Google Scholar]
- Silva, S.S.; Rodrigues, L.C.; Fernandes, E.M.; Reis, R.L. Chapter 6—Biopolymer membranes in tissue engineering. In Biopolymer Membranes and Films; de Moraes, M.A., da Silva, C.F., Vieira, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 141–163. [Google Scholar]
- Piaia, L.; Silva, S.S.; Gomes, J.M.; Franco, A.R.; Fernandes, E.M.; Lobo, F.C.M.; Rodrigues, L.C.; Leonor, I.B.; Fredel, M.C.; Salmoria, G.V.; et al. Chitosan/β-TCP composites scaffolds coated with silk fibroin: A bone tissue engineering approach. Biomed. Mater. 2022, 17, 015003. [Google Scholar] [CrossRef]
- Silva, S.S.; Popa, E.G.; Gomes, M.E.; Cerqueira, M.; Marques, A.P.; Caridade, S.G.; Teixeira, P.; Sousa, C.; Mano, J.F.; Reis, R.L. An investigation of the potential application of chitosan/aloe-based membranes for regenerative medicine. Acta Biomater. 2013, 9, 6790–6797. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.S.; Rodrigues, L.C.; Fernandes, E.M.; Gomes, J.M.; Vilas-Boas, Â.; Pirraco, R.P.; Reis, R.L. Approach on chitosan/virgin coconut oil-based emulsion matrices as a platform to design superabsorbent materials. Carbohydr. Polym. 2020, 249, 116839. [Google Scholar] [CrossRef]
- Morello, G.; De Iaco, G.; Gigli, G.; Polini, A.; Gervaso, F. Chitosan and Pectin Hydrogels for Tissue Engineering and In Vitro Modeling. Gels 2023, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Marangon, C.A.; Martins, V.C.A.; Leite, P.M.F.; Santos, D.A.; Nitschke, M.; Plepis, A.M.G. Chitosan/gelatin/copaiba oil emulsion formulation and its potential on controlling the growth of pathogenic bacteria. Ind. Crops Prod. 2017, 99, 163–171. [Google Scholar] [CrossRef]
- Hasan, M.; Rusman, R.; Khaldun, I.; Ardana, L.; Mudatsir, M.; Fansuri, H. Active edible sugar palm starch-chitosan films carrying extra virgin olive oil: Barrier, thermo-mechanical, antioxidant, and antimicrobial properties. Int. J. Biol. Macromol. 2020, 163, 766–775. [Google Scholar] [CrossRef]
- Binsi, P.K.; Ravishankar, C.N.; Srinivasa Gopal, T.K. Development and characterization of an edible composite film based on chitosan and virgin coconut oil with improved moisture sorption properties. J. Food Sci. 2013, 78, E526–E534. [Google Scholar] [CrossRef] [PubMed]
- Vieira, T.M.; Moldão-Martins, M.; Alves, V.D. Design of Chitosan and Alginate Emulsion-Based Formulations for the Production of Monolayer Crosslinked Edible Films and Coatings. Foods 2021, 10, 1654. [Google Scholar] [CrossRef]
- Ghani, N.A.A.; Channip, A.A.; Chok Hwee Hwa, P.; Ja’afar, F.; Yasin, H.M.; Usman, A. Physicochemical properties, antioxidant capacities, and metal contents of virgin coconut oil produced by wet and dry processes. Food Sci. Nutr. 2018, 6, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The Next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [Green Version]
- Sivadasan, D.; Sultan, M.H.; Alqahtani, S.S.; Javed, S. Cubosomes in Drug Delivery- A Comprehensive Review on Its Structural Components, Preparation Techniques and Therapeutic Applications. Biomedicines 2023, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Villalva, D.G.; França, C.G.; Loh, W. Characterization of cubosomes immobilized in hydrogels of hyaluronic acid and their use for diclofenac controlled delivery. Colloids Surf. B Biointerfaces 2022, 212, 112352. [Google Scholar] [CrossRef] [PubMed]
- Poletto, F.S.; Lima, F.S.; Lundberg, D.; Nylander, T.; Loh, W. Tailoring the internal structure of liquid crystalline nanoparticles responsive to fungal lipases: A potential platform for sustained drug release. Colloids Surf. B Biointerfaces 2016, 147, 210–216. [Google Scholar] [CrossRef]
- Zakaria, F.; Ashari, S.E.; Mat Azmi, I.D.; Abdul Rahman, M.B. Recent advances in encapsulation of drug delivery (active substance) in cubosomes for skin diseases. J. Drug Deliv. Sci. Technol. 2022, 68, 103097. [Google Scholar] [CrossRef]
- Nasr, M.; Younes, H.; Abdel-Rashid, R.S. Formulation and evaluation of cubosomes containing colchicine for transdermal delivery. Drug Deliv. Transl. Res. 2020, 10, 1302–1313. [Google Scholar] [CrossRef]
- Bessone, C.D.V.; Akhlaghi, S.P.; Tártara, L.I.; Quinteros, D.A.; Loh, W.; Allemandi, D.A. Latanoprost-loaded phytantriol cubosomes for the treatment of glaucoma. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2021, 160, 105748. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Young, K.; Wilson, R.; Rizwan, S.; Kemp, R.; Rades, T.; Hook, S. Chitosan hydrogels containing liposomes and cubosomes as particulate sustained release vaccine delivery systems. J. Liposome Res. 2012, 22, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, S.; Park, J.; Lee, S.E.; Kim, C.; Kang, D. Diclofenac: A Nonsteroidal Anti-Inflammatory Drug Inducing Cancer Cell Death by Inhibiting Microtubule Polymerization and Autophagy Flux. Antioxidants 2022, 11, 1009. [Google Scholar] [CrossRef]
- Amanullah, A.; Upadhyay, A.; Dhiman, R.; Singh, S.; Kumar, A.; Ahirwar, D.K.; Gutti, R.K.; Mishra, A. Development and Challenges of Diclofenac-Based Novel Therapeutics: Targeting Cancer and Complex Diseases. Cancers 2022, 14, 4385. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [Green Version]
- Wickramasinghe Mudiyanselage, D.R.; Wickramasinghe, I. Comparison of physicochemical characteristics of virgin coconut oils from traditional and hybrid coconut varieties. J. Agric. Food Res. 2023, 12, 100554. [Google Scholar] [CrossRef]
- Dumancas, G.G.; Kasi Viswanath, L.C.; de Leon, A.R.; Ramasahayam, S.; Maples, R.; Koralege, R.H.; Perera, U.D.N.; Langford, J.; Shakir, A.; Castles, S. Health benefits of virgin coconut oil. In Vegetable Oil: Properties, Uses and Benefits; Holt, B., Ed.; Nova Science Publisher: Hauppauge, NY, USA, 2016; pp. 161–194. [Google Scholar]
- Mahmoud, T.M.; Nafady, M.M.; Farouk, H.O.; Mahmoud, D.M.; Ahmed, Y.M.; Zaki, R.M.; Hamad, D.S. Novel Bile Salt Stabilized Vesicles-Mediated Effective Topical Delivery of Diclofenac Sodium: A New Therapeutic Approach for Pain and Inflammation. Pharmaceuticals 2022, 15, 1106. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B.; Ismail, A.; Hashim, P. Application of FTIR Spectroscopy for the Determination of Virgin Coconut Oil in Binary Mixtures with Olive Oil and Palm Oil. J. Am. Oil Chem. Soc. 2010, 87, 601–606. [Google Scholar] [CrossRef]
- Reys, L.L.; Silva, S.S.; Oliveira, J.M.; Caridade, S.G.; Mano, J.F.; Silva, T.H.; Reis, R.L. Revealing the potential of squid chitosan-based structures for biomedical applications. Biomed. Mater. 2013, 8, 045002. [Google Scholar] [CrossRef]
- Aranaz, I.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Preparation of chitosan nanocomposites with a macroporous structure by unidirectional freezing and subsequent freeze-drying. Mar. Drugs 2014, 12, 5619–5642. [Google Scholar] [CrossRef] [Green Version]
- Yonguep, E.; Kapiamba, K.F.; Kabamba, K.J.; Chowdhury, M. Formation, stabilization and chemical demulsification of crude oil-in-water emulsions: A review. Pet. Res. 2022, 7, 459–472. [Google Scholar] [CrossRef]
- Ribeiro, S.; Pugliese, E.; Korntner, S.H.; Fernandes, E.M.; Gomes, M.E.; Reis, R.L.; Bayon, Y.; Zeugolis, D.I. Modulation of stem cell response using biodegradable polyester films with different stiffness. Biomed. Eng. Adv. 2021, 2, 100007. [Google Scholar] [CrossRef]
- Park, J.S.; Chu, J.S.; Tsou, A.D.; Diop, R.; Tang, Z.; Wang, A.; Li, S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials 2011, 32, 3921–3930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levengood, S.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Kocak, F.Z.; Yar, M.; Rehman, I.U. Hydroxyapatite-Integrated, Heparin- and Glycerol-Functionalized Chitosan-Based Injectable Hydrogels with Improved Mechanical and Proangiogenic Performance. Int. J. Mol. Sci. 2022, 23, 5370. [Google Scholar] [CrossRef]
- Smith, D.R.; Escobar, A.P.; Andris, M.N.; Boardman, B.M.; Peters, G.M. Understanding the Molecular-Level Interactions of Glucosamine-Glycerol Assemblies: A Model System for Chitosan Plasticization. ACS Omega 2021, 6, 25227–25234. [Google Scholar] [CrossRef]
- Yepuri, N.R.; Clulow, A.J.; Prentice, R.N.; Gilbert, E.P.; Hawley, A.; Rizwan, S.B.; Boyd, B.J.; Darwish, T.A. Deuterated phytantriol—A versatile compound for probing material distribution in liquid crystalline lipid phases using neutron scattering. J. Colloid Interface Sci. 2019, 534, 399–407. [Google Scholar] [CrossRef]
- Salonen, A.; Moitzi, C.; Salentinig, S.; Glatter, O. Material Transfer in Cubosome−Emulsion Mixtures: Effect of Alkane Chain Length. Langmuir 2010, 26, 10670–10676. [Google Scholar] [CrossRef]
- Tran, N.; Hawley, A.M.; Zhai, J.; Muir, B.W.; Fong, C.; Drummond, C.J.; Mulet, X. High-Throughput Screening of Saturated Fatty Acid Influence on Nanostructure of Lyotropic Liquid Crystalline Lipid Nanoparticles. Langmuir 2016, 32, 4509–4520. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Signini, R.; Campana Filho, S. Purificação e caracterização de quitosana comercial. Polymers 1998, 8, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Akhlaghi, S.P.; Ribeiro, I.R.; Boyd, B.J.; Loh, W. Impact of preparation method and variables on the internal structure, morphology, and presence of liposomes in phytantriol-Pluronic® F127 cubosomes. Colloids Surf. B Biointerfaces 2016, 145, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.C.; Silva, S.S.; Reis, R.L. Acemannan-based films: An improved approach envisioning biomedical applications. Mater. Res. Express 2019, 6, 095406. [Google Scholar] [CrossRef]
- Halim, R.; Webley, P.A. Nile Red Staining for Oil Determination in Microalgal Cells: A New Insight through Statistical Modelling. Int. J. Chem. Eng. 2015, 2015, 695061. [Google Scholar] [CrossRef]







| Cub | Mean Diameter (nm) |
|---|---|
| Cub | 215 ± 30 |
| Cub_D | 215 ± 12 |
| CV_Cub 1 | 550 ± 37 |
| CV_CubD 1 | 748 ± 90 |
| Sample | Mean Diameter | |
|---|---|---|
| Cubosomes (nm) | Oil Droplets (µm) | |
| Cub_NR | 307.8 ± 28.2 | - |
| CV_NR | - | 3.43 ± 1.01 |
| CV_Cub_NR | 728.3 ± 112.0 | 3.00 ± 0.97 |
| Identification | CHT (%) | VCO (%) | Gly (%) | Cubosomes (%) | Diclofenac (%) | Loaded Cubosomes (%) |
|---|---|---|---|---|---|---|
| CV | 98 | 1 | 1 | _ | _ | _ |
| CV_Cub | 97 | 1 | 1 | 1 | _ | _ |
| CV_CubD | 97 | 1 | 1 | _ | _ | 1 |
| CV_D | 97 | 1 | 1 | _ | 1 | _ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.S.; Rodrigues, L.C.; Fernandes, E.M.; Soares da Costa, D.; Villalva, D.G.; Loh, W.; Reis, R.L. Chitosan/Virgin-Coconut-Oil-Based System Enriched with Cubosomes: A 3D Drug-Delivery Approach. Mar. Drugs 2023, 21, 394. https://doi.org/10.3390/md21070394
Silva SS, Rodrigues LC, Fernandes EM, Soares da Costa D, Villalva DG, Loh W, Reis RL. Chitosan/Virgin-Coconut-Oil-Based System Enriched with Cubosomes: A 3D Drug-Delivery Approach. Marine Drugs. 2023; 21(7):394. https://doi.org/10.3390/md21070394
Chicago/Turabian StyleSilva, Simone S., Luísa C. Rodrigues, Emanuel M. Fernandes, Diana Soares da Costa, Denise G. Villalva, Watson Loh, and Rui L. Reis. 2023. "Chitosan/Virgin-Coconut-Oil-Based System Enriched with Cubosomes: A 3D Drug-Delivery Approach" Marine Drugs 21, no. 7: 394. https://doi.org/10.3390/md21070394