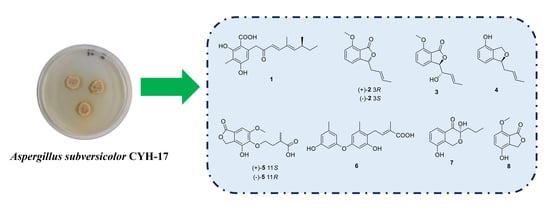
New Phenol Derivatives from the Haima Cold Seep-Derived Fungus Aspergillus subversicolor CYH-17
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Elucidation
| Position | 1 a | 5 b | 6 b | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1 | 173.23, C | 173.84, C | 159.53, C | |||
| 2 | 104.89, C | 103.91, CH | 6.17 (t, 2.1) | |||
| 3 | 162.93, C | 69.19, CH2 | 5.22 (s) | 160, C | ||
| 3a | 129.65, C | |||||
| 4 | 108.61, C | 146.91, C | 111.46, CH | 6.26 (s) | ||
| 5 | 159.10, C | 142.04, C | 141.57, C | |||
| 6 | 110.94, CH | 6.15 (s) | 156.63, C | 111.71, CH | 6.33 (s) | |
| 7 | 136.70, C | 99.61, CH | 6.94 (s) | 157.12, C | ||
| 7a | 121.58, C | |||||
| 8 | 47.47, CH2 | 4.09–4.16 (m) | 104.77, CH | 6.30 (s) | ||
| 9 | 197.23, C | 72.84, CH2 | 4.12 (m) 4.03–4.09 (m) | 157.35, C | ||
| 10 | 124.45, CH | 6.12 (d, 15.9) | 35.75, CH2 | 2.00–2.08 (m) 1.81–1.89 (m) | 120.99, C | |
| 11 | 146.61, CH | 7.22 (d, 15.9) | 39.92, CH | 2.68 (m) | 139.9, C | |
| 12 | 131.76, C | 184.64, C | 113.02, CH | 6.30 (s) | ||
| 13 | 148.35, CH | 5.81 (d, 9.7) | 18.46, CH3 | 1.19 (d, 6.8) | 26.46, CH2 | 3.48 (d, 7.0) |
| 14 | 34.37, CH | 2.44–2.48 (m) | 56.66, CH3 | 3.88 (s) | 140.17, CH | 6.60 (t, 6.8) |
| 15 | 29.48, CH2 | 1.36–1.42 (m) 1.24–1.30 (m) | 130.37, C | |||
| 16 | 11.84, CH3 | 0.81 (t, 7.4) | 175.62, C | |||
| 17 | 20.09, CH3 | 0.96 (d, 6.6) | 13.09, CH3 | 1.96 (s) | ||
| 18 | 12.29, CH3 | 1.75 (s) | 19.99, CH3 | 2.20 (s) | ||
| 19 | 8.14, CH3 | 1.94 (s) | 21.55, CH3 | 2.21 (s) | ||
| Position | 2 a | 3 a | 4 b | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1 | 171.03, C | 171.06, C | 58.97, CH2 | 4.95 (s) | ||
| 3 | 81.58, CH | 5.46–5.53 (m) | 84.49, CH | 5.42 (d, 4.0) | 70.89, CH | 4.82 (dd, 7.9, 5.3) |
| 3a | 154.00, C | 151.26, C | 142.03, C | |||
| 4 | 115.06, CH | 7.10 (d, 7.3) | 116.16, CH | 7.15 (d, 7.6) | 118.13, CH | 6.98 (d, 7.7) |
| 5 | 137.92, CH | 7.69 (dd, 8.1, 7.7) | 137.57, CH | 7.67 (t, 8.0) | 129.32, CH | 7.20 (t, 7.9) |
| 6 | 112.03, CH | 7.08 (d, 8.0) | 112.25, CH | 7.09 (d, 8.3) | 116.16, CH | 6.82 (d, 8.0) |
| 7 | 159.92, C | 159.90, C | 156.63, C | |||
| 7a | 114.35, C | 114.97, C | 122.95, C | |||
| 8 | 38.31, CH2 | 2.68–2.75 (m) 2.46–2.59 (m) | 74.23, CH | 4.42–4.45 (m) | 41.85, CH2 | 2.38–2.45 (m) |
| 9 | 124.92, CH | 5.25–5.35 (m) | 129.15, CH | 5.51 (m) | 126.79, CH | 5.37–5.52 (m) |
| 10 | 131.17, CH | 5.58 (m) | 130.49, CH | 5.73 (m) | 130.04, CH | 5.59–5.64 (m) |
| 11 | 18.12, CH3 | 1.61 (dd, 6.5, 1.4) | 17.94, CH3 | 1.68 (m) | 18.23, CH3 | 1.70 (d, 6.3) |
| 12 | 56.33, CH3 | 3.96 (s, 3H) | 56.30, CH3 | 3.96 (s) | ||
| Position | 7 a | 8 a | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| 1 | 58.42, CH2 | 5.03 (d, 16.2) 4.84 (d, 16.2) | 172.00, C | |
| 3 | 97.69, C | 68.88, CH2 | 5.22 (s) | |
| 3a | 136.44, C | |||
| 4 | 193.27, C | 147.22, C | ||
| 4a | 130.64, C | |||
| 5 | 118.59, CH | 7.46 (d, 7.6) | 123.37, CH | 7.05 (d, 8.7) |
| 6 | 128.87, CH | 7.23 (t, 7.9) | 113.43, CH | 6.92 (d, 8.7) |
| 7 | 120.56, CH | 7.00 (dd, 8.0, 0.7) | 152.87, C | |
| 7a | 114.58, C | |||
| 8 | 154.19, C | 56.53, CH3 | 3.87 (s) | |
| 8a | 130.29, C | |||
| 9 | 39.75, CH2 | 2.02 (ddd, 13.5, 11.7, 4.8) 1.77 (ddd, 13.6, 11.7, 4.8) | ||
| 10 | 17.61, CH2 | 1.51 (m) 1.31–1.39 (m) | ||
| 11 | 14.76, CH3 | 0.93 (t, 7.4) | ||
2.2. Biological Test
3. Materials and Methods
3.1. Fungal Materials
3.2. General
3.3. Fermentation, Extraction and Purification
3.3.1. Subversin A (1)
3.3.2. Subversin B (2)
3.3.3. Subversin C (3)
3.3.4. Subversin D (4)
3.3.5. Subversin E (5)
3.3.6. Subversic Acid A (6)
3.3.7. epi-Wortmannine G (7)
3.3.8. 4-Hydroxy-7-methoxyphthalide (8)
3.4. X-ray Crystal Structure Analysis
3.5. Bioassays
3.5.1. Antibacterial Assay
3.5.2. AChE Inhibitory Assay
3.5.3. α-Glucosidase Inhibitory Assay
3.5.4. DPPH Radical Scavenging Assay
3.6. Chiral HPLC Separation of Compounds 2 and 5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paull, C.K.; Hecker, B.; Commeau, R.; Freeman-Lynde, R.P.; Neumann, C.; Corso, W.P.; Golubic, S.; Hook, J.E.; Sikes, E.; Curray, J. Biological communities at the Florida escarpment resemble hydrothermal vent taxa. Science 1984, 226, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Feng, J.; Kong, J.; Huang, Y.; Chen, X.; Zhang, S. Differences in bacterial co-occurrence networks and ecological niches at the surface sediments and bottom seawater in the Haima cold seep. Microorganisms 2023, 11, 3001. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Guan, H.; Liu, L.; Tao, J.; Li, J.; Dong, J.; Zhang, S. The diversity, composition, and putative functions of gill-associated bacteria of bathymodiolin mussel and vesicomyid clam from haima cold seep, South China Sea. Microorganisms 2020, 8, 1699. [Google Scholar] [CrossRef] [PubMed]
- Nethupul, H.; Stöhr, S.; Zhang, H. Review of Ophioplinthaca Verrill, 1899 (Echinodermata, Ophiuroidea, Ophiacanthidae), description of new species in Ophioplinthaca and Ophiophthalmus, and new records from the Northwest Pacific and the South China Sea. Zookeys 2022, 1099, 155–202. [Google Scholar] [CrossRef]
- Leduc, D. Six new species of free-living nematodes (Nematoda: Enoplida) from deep-sea cold seeps on Hikurangi Margin, New Zealand. PeerJ 2023, 11, e14867. [Google Scholar] [CrossRef]
- Shekarriz, E.; Chen, J.; Xu, Z.; Liu, H. Disentangling the functional role of fungi in cold seep sediment. Microbiol. Spectr. 2023, 11, e0197822. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhang, C.; Peng, Y.; Zhang, H.X.; Shi, L.D.; Wei, G.; Hubert, C.R.J.; Wang, Y.; Greening, C. Phylogenetically and catabolically diverse diazotrophs reside in deep-sea cold seep sediments. Nat. Commun. 2022, 13, 4885. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Zhou, L.; Wang, W.; Anjum, K.; Zhang, J.; Zhang, G.; Zhu, T.; Li, D.; Che, Q. Glycosylated 24-membered lactones and unsaturated fatty acids from cold-seep-derived Bacillus sp. HDN 20-1259. Tetrahedron Lett. 2023, 121, 154477. [Google Scholar] [CrossRef]
- Cong, M.; Pang, X.; Zhao, K.; Song, Y.; Liu, Y.; Wang, J. Deep-sea natural products from extreme environments: Cold seeps and hydrothermal vents. Mar. Drugs 2022, 20, 404. [Google Scholar] [CrossRef]
- Yan, L.H.; Du, F.Y.; Li, X.M.; Yang, S.Q.; Wang, B.G.; Li, X. Antibacterial indole diketopiperazine alkaloids from the deep-sea cold seep-derived fungus Aspergillus chevalieri. Mar. Drugs 2023, 21, 195. [Google Scholar] [CrossRef]
- Song, Q.; Yang, S.Q.; Li, X.M.; Hu, X.Y.; Li, X.; Wang, B.G. Aromatic polyketides from the deep-sea cold-seep mussel associated endozoic fungus Talaromyces minioluteus CS-138. Mar. Drugs 2022, 20, 529. [Google Scholar] [CrossRef]
- Chi, L.P.; Li, X.M.; Wan, Y.P.; Li, X.; Wang, B.G. Ophiobolin sesterterpenoids and farnesylated phthalide derivatives from the deep sea cold-seep-derived fungus Aspergillus insuetus SD-512. J. Nat. Prod. 2020, 83, 3652–3660. [Google Scholar] [CrossRef]
- Yang, S.Q.; Song, Q.; Li, X.M.; Li, X.; Li, H.L.; Meng, L.H.; Wang, B.G. Antimicrobial polyketides and sesquiterpene lactones from the deep-sea cold-seep-derived fungus Talaromyces minioluteus CS-113 triggered by the histone deacetylase inhibitor SAHA. Org. Biomol. Chem. 2023, 21, 2575–2585. [Google Scholar] [CrossRef]
- Li, C.P.; Song, Y.P.; Wang, B.G.; Ji, N.Y. Sulfurated and iodinated metabolites from the cold-seep fungus Cladosporium cladosporioides 8-1. Tetrahedron Lett. 2022, 93, 153689. [Google Scholar] [CrossRef]
- Che, Y.H.; Wang, J.F.; Shi, X.F.; Ding, W.P.; Xiao, Z.H.; Wu, J.M.; Wang, F.Z.; Zhang, S. 8R-methoxy-9R-hydroxyl-fumitremorgin C, a new diketopiperazine alkaloid from Haima cold seep-derived fungus Aspergillus fumigatus CYH-5. Nat. Prod. Res. 2023. [Google Scholar] [CrossRef]
- Che, Y.H.; Wang, J.F.; Ding, W.P.; Xiao, Z.H.; Shi, X.F.; Wu, J.M.; Wang, F.Z.; Zhang, S. New diketopiperazine alkaloids from the Haima cold seep-derived fungus Toxicocladosporium sp. CYH-18. Phytochem. Lett. 2024, 60, 96–100. [Google Scholar] [CrossRef]
- Weng, H.Z.; Zhu, J.Y.; Yuan, F.Y.; Tang, Z.Y.; Tian, X.Q.; Chen, Y.; Fan, C.Q.; Tang, G.H.; Yin, S. Homo/hetero-dimers of aromatic bisabolane sesquiterpenoids with neuroprotective activity from the fungus Aspergillus versicolor A18 from south China sea. Mar. Drugs 2022, 20, 322. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, T.; Takenaka, Y.; Nagakura, N.; Hamada, N. Dibenzofurans from the cultured lichen mycobionts of Lecanora cinereocarnea. Phytochemistry 2001, 58, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wang, J.; Zhang, X.; Nong, X.; Xu, X.; Qi, S. Cytotoxic polyketides from the deep-sea-derived fungus Engyodontium album DFFSCS021. Mar. Drugs 2014, 12, 5902–5915. [Google Scholar] [CrossRef] [PubMed]
- Fredimoses, M.; Zhou, X.; Ai, W.; Tian, X.; Yang, B.; Lin, X.; Liu, J.; Liu, Y. Emerixanthone E, a new xanthone derivative from deep sea fungus Emericella sp. SCSIO 05240. Nat. Prod. Res. 2019, 33, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Tahara, H.; Inokoshi, J.; Tanaka, H.; Masuma, R.; Omura, S. New brominated and halogen-less derivatives and structure-activity relationship of azaphilones inhibiting gp120-CD4 binding. J. Antibiot. 1998, 51, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, J.M.; Tauber, J.; Krische, M.J. Enantioselective iridium-catalyzed phthalide formation through internal redox allylation of phthalaldehydes. Angew. Chem. Int. Ed. Engl. 2018, 57, 1390–1393. [Google Scholar] [CrossRef]
- Zanardi, M.M.; Sarotti, A.M. Sensitivity analysis of DP4+ with the probability distribution terms: Development of a universal and customizable method. J. Org. Chem. 2021, 86, 8544–8548. [Google Scholar] [CrossRef] [PubMed]
- Rebollar-Ramos, D.; Macías-Ruvalcaba, M.L.; Figueroa, M.; Raja, H.A.; González-Andrade, M.; Mata, R. Additional α-glucosidase inhibitors from Malbranchea flavorosea (Leotiomycetes, Ascomycota). J. Antibiot. 2018, 71, 862–871. [Google Scholar] [CrossRef]
- Höller, U.; Gloer, J.B.; Wicklow, D.T. Biologically active polyketide metabolites from an undetermined fungicolous hyphomycete resembling Cladosporium. J. Nat. Prod. 2002, 65, 876–882. [Google Scholar] [CrossRef]
- Katoh, N.; Nakahata, T.; Kuwahara, S. Synthesis of novel antifungal phthalides produced by a wheat rhizosphere fungus. Tetrahedron 2008, 64, 9073–9077. [Google Scholar] [CrossRef]
- Takahashi, K.; Koshino, H.; Narita, Y.; Yoshihara, T. Novel antifungal compounds produced by Sterile Dark, an unidentified wheat rhizosphere fungus. Biosci. Biotechnol. Biochem. 2005, 69, 1018–1020. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.Y.; Li, L.; Yang, C.G.; Luo, D.Q.; Zheng, Z.H.; Lu, X.H.; Shi, B.Z. A novel oxybis cresol verticilatin with highly varying degrees of biological activities from the insect pathogenic fungus Paecilomyces verticillatus. J. Asian Nat. Prod. Res. 2014, 16, 1153–1157. [Google Scholar] [CrossRef]
- Zhao, J.W.; Yang, Z.D.; Zhou, S.Y.; Yang, L.J.; Sun, J.H.; Yao, X.J.; Shu, Z.M.; Li, S. Wortmannine F and G, two new pyranones from Talaromyces wortmannii LGT-4, the endophytic fungus of Tripterygium wilfordii. Phytochem. Lett. 2019, 29, 115–118. [Google Scholar] [CrossRef]
- Keay, B.A.; Rodrigo, R. A convergent synthesis of (±)daunomycinoni. Tetrahedron 1984, 40, 4597–4607. [Google Scholar] [CrossRef]
- Shou, Q.; Banbury, L.K.; Renshaw, D.E.; Lambley, E.H.; Mon, H.; Macfarlane, G.A.; Griesser, H.J.; Heinrich, M.M.; Wohlmuth, H. Biologically active dibenzofurans from Pilidiostigma glabrum, an endemic Australian Myrtaceae. J. Nat. Prod. 2012, 75, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Oramas-Royo, S.; Pantoja, K.D.; Amesty, Á.; Romero, C.; Lorenzo-Castrillejo, I.; Machín, F.; Estévez-Braun, A. Synthesis and antibacterial activity of new symmetric polyoxygenated dibenzofurans. Eur. J. Med. Chem. 2017, 141, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.J.; Li, X.A.; Jin, M.Y.; Guo, W.X.; Lei, L.R.; Liu, R.; Zhang, M.Z.; Guo, D.L.; Wang, D.; Zhou, Y.; et al. Two previously undescribed phthalides from Talaromyces amestolkiae, a symbiotic fungus of Syngnathus acus. J. Asian Nat. Prod. Res. 2023, 25, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.J.; Ren, H.G.; Jiang, J.M.; Xu, G.; Xu, Y.; Chen, S.M.; Chen, G.; Zheng, D.; Yuan, M.; Zhang, H.; et al. Two novel phenylpropanoid trimers from Ligusticum chuanxiong Hort with inhibitory activities on alpha-hemolysin secreted by Staphylococcus aureus. Front. Chem. 2022, 10, 877469. [Google Scholar] [CrossRef]
- Huang, L.; Peng, C.; Guo, L.; Feng, R.; Shu, H.Z.; Tian, Y.C.; Zhou, Q.M.; Xiong, L. Six pairs of enantiomeric phthalide dimers from the rhizomes of Ligusticum chuanxiong and their absolute configurations and anti-inflammatory activities. Bioorg. Chem. 2022, 127, 105970. [Google Scholar] [CrossRef]
- Wei, X.; Zeng, Y.; Sun, C.; Meng, F.; Wang, Y. Recent advances in natural phthalides: Distribution, chemistry, and biological activities. Fitoterapia 2022, 160, 105223. [Google Scholar] [CrossRef]
- León, A.; Del-Ángel, M.; Ávila, J.L.; Delgado, G. Phthalides: Distribution in nature, chemical reactivity, synthesis, and biological activity. Prog. Chem. Org. Nat. Prod. 2017, 104, 127–246. [Google Scholar]
- Liao, H.X.; Li, X.B.; Shao, T.M.; Yu, Z.X. A new phthalide derivative from the mangrove-derived fungus Eupenicillium sp. HJ002. Chem. Nat. Compd. 2023, 59, 441–443. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Zhang, L.; Zhang, Q.; Zhang, W.; Chen, Y.; Zhang, W.; Zhang, H.; Zhang, C. Dassonmycins A and B, polycyclic thioalkaloids from a marine sponge-derived Nocardiopsis dassonvillei SCSIO 40065. Org. Lett. 2021, 23, 2858–2862. [Google Scholar] [CrossRef]
- Yang, B.; Qi, C.; Yao, Z.; Lin, S.; Li, F.; Sun, W.; Hu, Z.; Zhang, Y. Hybeanones A and B, two highly modified polycyclic polyprenylated acylphloroglucinols from Hypericum beanii. Chin. J. Chem. 2022, 40, 53–58. [Google Scholar] [CrossRef]
- Ding, W.; Li, Y.; Tian, X.; Xiao, Z.; Li, R.; Zhang, S.; Yin, H. Investigation on metabolites in structure and biosynthesis from the deep-sea sediment-derived Actinomycete Janibacter sp. SCSIO 52865. Molecules 2023, 28, 2133. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.M.; Wang, J.F.; Shi, X.F.; Wei, X.Y.; Chen, Y.C.; Zeng, Q.; Xiang, Y.; Chen, X.Y.; Tian, X.P.; Xiao, Z.H.; et al. Eurotiumins A⁻E, five new alkaloids from the marine-derived fungus Eurotium sp. SCSIO F452. Mar. Drugs 2018, 16, 136. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Exploration of chemical compound, conformer, and reaction space with meta-dynamics simulations based on tight-binding quantum chemical calculations. J. Chem. Theory Comput. 2019, 15, 2847–2862. [Google Scholar]
- Lu, T. Molclus Program, Version 1.9.9.9. Available online: http://www.keinsci.com/research/molclus.html (accessed on 6 March 2022).
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Pecitelli, G. SpecDis Version 1.71, Berlin, Germany. 2017. Available online: https://specdis-software.jimdo.co (accessed on 6 March 2022).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, Y.-H.; Ding, W.-P.; Xiao, Z.-H.; Wu, J.-M.; Yin, H.; Wang, F.-Z.; Zhang, S. New Phenol Derivatives from the Haima Cold Seep-Derived Fungus Aspergillus subversicolor CYH-17. Mar. Drugs 2024, 22, 117. https://doi.org/10.3390/md22030117
Che Y-H, Ding W-P, Xiao Z-H, Wu J-M, Yin H, Wang F-Z, Zhang S. New Phenol Derivatives from the Haima Cold Seep-Derived Fungus Aspergillus subversicolor CYH-17. Marine Drugs. 2024; 22(3):117. https://doi.org/10.3390/md22030117
Chicago/Turabian StyleChe, Yi-Hao, Wen-Ping Ding, Zhi-Hui Xiao, Jia-Min Wu, Hao Yin, Fa-Zuo Wang, and Si Zhang. 2024. "New Phenol Derivatives from the Haima Cold Seep-Derived Fungus Aspergillus subversicolor CYH-17" Marine Drugs 22, no. 3: 117. https://doi.org/10.3390/md22030117