The Influence of Various Crosslinking Conditions of EDC/NHS on the Properties of Fish Collagen Film
Abstract
:1. Introduction
2. Results
2.1. Fourier Transform Infrared Spectroscopy
2.2. Mechanical Testing
2.3. Swelling and Degradation Properties
2.4. Atomic Force Microscopy
2.5. Contact Angle and Surface Energy
3. Discussion
4. Materials and Methods
4.1. Preparation of Collagen Films
4.2. Fourier Transform Infrared Spectroscopy
4.3. Mechanical Testing
4.4. Swelling and Degradation Properties
4.5. Atomic Force Microscopy
4.6. Contact Angle and Surface Energy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coppola, D.; Olivero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-S.; Ok, Y.-J.; Hwang, S.-Y.; Kwak, J.-Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.L.; Marques, A.L.P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic Potential of Marine Fish Skin Collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef]
- Rico-Llanos, G.A.; Borrego-Gonzalez, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Davison-Kotler, E.; Marshall, W.S.; Garcia-Gareta, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef]
- Rigogliuso, S.; Campora, S.; Notarbartolo, M.; Ghersi, G. Recovery of Bioactive Compounds from Marine Organism: Focus on the Future Perspectives for Pharmacological Biomedical and Regenerative Medicine Applications of Marine Collagen. Molecules 2023, 28, 1152. [Google Scholar] [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- Cavallo, A.; Al Kayal, T.; Mero, A.; Mezzetta, A.; Pisani, A.; Foffa, I.; Vecoli, C.; Buscemi, M.; Guazzelli, L.; Soldani, G.; et al. Marine Collagen—Based Bioink for SD Bioprinting of a Bilayered Skin Model. Pharmaceutics 2023, 15, 1331. [Google Scholar] [CrossRef]
- Diogo, G.S.; López-Senra, E.L.; Pirraco, R.P.; Canadas, R.F.; Fernandes, E.M.; Serra, J.; Pérez-Martín, R.I.; Sotelo, C.G.; Marques, A.P.; González, P.; et al. Marine Collagen/Apatite Composite Scaffolds Envisaging Hard Tissue Applications. Mar. Drugs 2018, 16, 269. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.-C.; Chiu, C.-S.; Chan, Y.-J.; Mulio, A.T.; Li, P.-H. Characterization and Biological Properties of Marine By-Product Collagen Through Ultrasound-Assisted Extraction. Aquac. Rep. 2023, 29, 101514. [Google Scholar] [CrossRef]
- Rajabimashhadi, Z.; Gallo, N.; Salvatore, L.; Lionetto, F. Collagen Derived from Fish Industry Waste: Progresses and Challenges. Polymers 2023, 15, 544. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A Review on Recent Advances and Applications of Fish Collagen. Crit. Rev. Food Sci Nutr. 2020, 61, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Lewandowska, K.; Adamiak, K. The Influence of UV Light on Rheological Properties of Collagen Extracted from Silver Carp Skin. Materials 2020, 13, 4453. [Google Scholar] [CrossRef] [PubMed]
- Oslan, S.N.H.; Li, C.X.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.; Huda, N. Extraction and Characterization of Bioactive Fish By-Product Collagen as Promising for Potential Wound Healing Agent in Pharmaceutical Applications: Current Trend and Future perspective. Int. J. Food Sci. 2022, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, L.; Xu, H.; Yamamoto, M.; Shinoda, M.; Kishimoto, M.; Tanaka, T.; Yamane, H. Effect of the Application of a Dehydrothermal Treatment on the Structure and the Mechanical Properties of Collagen Film. Materials 2020, 13, 377. [Google Scholar] [CrossRef]
- Elias, J.; Matheson, B.-A.; Gowe, L. Influence of Crosslinking Methods on Biomimetically Mineralized Collagen Matrices for Bone-like Biomaterials. Polymers 2023, 15, 1981. [Google Scholar] [CrossRef] [PubMed]
- Sapuła, P.; Bialik-Wąs, K.; Malarz, K. Are Natural Compounds a Promising Alternative to Synthetic Cross-Linking Agents in the Preparation of Hydrogels? Pharmaceutics 2023, 15, 253. [Google Scholar] [CrossRef]
- Velmurugan, P.; Jonnalagadda, R.R.; Nair, B.U. Biochemical and Biophysical Characterization of EDC Treated Rattus Type I Collagen. Process Biochem. 2013, 48, 1059–1064. [Google Scholar] [CrossRef]
- Bax, D.V.; Davidenko, N.; Gullber, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental Insight into the Effect of Carbodiimide Crosllinking on Cellular Recognition of Collagen-Based Scaffolds. Acta Biomater. 2017, 49, 218–234. [Google Scholar] [CrossRef]
- Yang, C. Enhanced Physicochemical Properties of Collagen by Using EDC/NHS-Crosslinking. Bull. Mater. Sci. 2012, 35, 913–918. [Google Scholar] [CrossRef]
- Nam, K.; Kimura, T.; Kishida, A. Reaction of EDC and NHS for Preparation of Collagen Gels Using Ethanol/Water Co-Solvents. Macromol. Biosci. 2008, 8, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Usha, R.; Sreeram, K.J.; Rajam, A. Stabilization of Collagen with EDC/NHS in the Presence of L-Lysine: A Comprehensive Study. Colloids Surf. B Biointerfaces 2012, 90, 83–90. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Lou, Y.Y.; Li, T.H.; Liu, B.Z.; Chen, K.; Zhang, D.; Li, T. Cross-Linking Methods of Type I Collagen-Based Scaffolds for Cartilage Tissue Engineering. Am. J. Transl. Res. 2022, 14, 1146–1159. [Google Scholar] [PubMed]
- Nair, M.; Johal, R.K.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Tunable Bioactivity and Mechanics of Collagen-Based Tissue Engineering Constructs: A Comparison of EDC-NHS, Genipin and TG2 Crosslinkers. Biomaterials 2020, 254, 120109. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Zielińska, S.; Sionkowska, A.; Carvalho, Â.; Monteiro, F.J. Biomaterials with Potential Use in Bone Tissue Regeneration —Collagen/Chitosan/Silk Fibroin Scaffolds Cross-Linked by EDC/NHS. Materials 2021, 14, 1105. [Google Scholar] [CrossRef] [PubMed]
- Everaerts, F.; Torrianni, M.; Hendriks, M.; Feijen, J. Biomechanical Properties of Carbodiimide Crosslinked Collagen: Influence of the Formation of Ester Crosslinks. J. Biomed. Mater. Res. A 2008, 85A, 547–555. [Google Scholar] [CrossRef]
- Wang, Y.; Green, A.; Yao, X.; Liu, H.; Nisar, S.; Gorski, J.P.; Hass, V. Cranberry Juice Extract Rapidly Protects Demineralized Dentin against Digestion and Inhibits Its Gelatinolytic Activity. Materials 2021, 14, 3637. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, C.; Shen, Q.; Kong, Q.; Wu, J.; Yang, J.; Wang, Y.; Wu, H.; Peng, Z.; Yan, Y. Study on the Preparation and Physicochemical Properties of Fish Swim Bladder Membrane. Chin. J. Reparative Reconstr. Surg. 2019, 33, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.N.; Gwynne, J.H.; Pugh, N.; Hamaia, S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Crosslinking and Composition Influence the Surface Properties, Mechanical Stiffness and Cell Reactivity of Collagen-Based Films. Acta Biomater. 2012, 8, 3080–3090. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Bao, B.; Bao, C.; Wang, S.; Rahman, S.U.; Hou, C.; Elango, J.; Wu, W. Resveratrol and Celastrol Loaded Collagen Dental Implants Regulate Periodontal Ligament Fibroblast Growth and Osteoclastogenesis of Bone Marrow Macrophages. Chem. Biodivers. 2020, 17, e2000295. [Google Scholar] [CrossRef] [PubMed]
- Nashchekina, Y.A.; Sirotkina, M.Y.; Darvish, D.M.; Barsuk, I.A.; Moskalyuk, O.A.; Mikhailova, N.A. The Effect of Carbodiimide on the Structural, Mechanical and Biological Properties of Collagen Films. Cell Tissue Biol. 2021, 15, 272–280. [Google Scholar] [CrossRef]
- Safandowska, M.; Pietrucha, K. Effect of Fish Collagen Modification on its Thermal and Rheological Properties. Int. J. Biol. Macromol. 2013, 53, 32–37. [Google Scholar] [CrossRef]



| Sample | Band Position [cm−1] | |||||||
|---|---|---|---|---|---|---|---|---|
| Amide A | Amide B | CH2 Asymmetric Stretch | Amide I | Amide II | CH2 Bend | COO− Symmetric Stretch | Amide III | |
| Coll | 3293 | 3071 | 2933 | 1629 | 1541 | 1450 | 1386 | 1235 |
| Coll_EDC | 3308 | 3068 | 2935 | 1632 | 1548 | 1448 | 1378 | 1237 |
| Coll_EDC/NHS | 3302 | 3070 | 2935 | 1629 | 1544 | 1451 | 1379 | 1233 |
| Coll_EDC_d | 3291 | 3072 | 2935 | 1629 | 1543 | 1450 | 1386 | 1236 |
| Coll_EDC/NHS_d | 3291 | 3074 | 2935 | 1629 | 1541 | 1451 | 1387 | 1235 |
| Sample | Time [h] | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 8 | 24 | 48 | 72 | |
| Coll | 498 ± 18 | 663 ± 17 | 889 ± 38 | 800 ± 15 | - | - | - | - |
| Coll_EDC | 426 ± 24 | 498 ± 13 | 685 ± 38 | - | - | - | - | - |
| Coll_EDC/NHS | 782 ± 113 | 1019 ± 136 | 1045 ± 92 | 945 ± 151 | - | - | - | - |
| Coll_EDC_d | 146 ± 29 | 135 ± 32 | 132 ± 33 | 146 ± 24 | 119 ± 39 | 133 ± 31 | 148 ± 26 | 131 ± 31 |
| Coll_EDC/NHS_d | 181 ± 46 | 195 ± 31 | 190 ± 19 | 191 ± 40 | 195 ± 32 | 198 ± 32 | 173 ± 51 | 128 ± 92 |
| Rq [nm] | Ra [nm] | |
|---|---|---|
| Coll | 16.79 ± 0.50 | 13.57 ± 0.75 |
| Coll_EDC | 58.32 ± 4.97 | 46.79 ± 3.71 |
| Coll_EDC/NHS | 13.31 ± 0.42 | 10.70 ± 0.16 |
| Coll_EDC_d | 19.80 ± 2.04 | 15.75 ± 1.89 |
| Coll_EDC/NHS_d | 40.72 ± 6.29 | 33.01 ± 5.02 |
Coll | |
Coll_EDC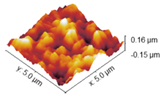 | Coll_EDC/NHS |
Coll_EDC_d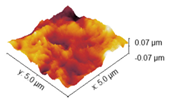 | Coll_EDC/NHS_d |
| Sample | Θ Glycerine [°] | Θ Diodomethane [°] | IFT (s) [mJ/m2] | IFT (s, D) [mJ/m2] | IFT (s, P) [mJ/m2] |
|---|---|---|---|---|---|
| Coll | 70.8 | 58.9 | 62.85 | 15.12 | 47.73 |
| Coll_EDC | 87.7 | 52.9 | 32.18 | 31.00 | 1.17 |
| Coll_EDC/NHS | 73.7 | 60.7 | 30.7 | 22.49 | 8.08 |
| Coll_EDC_d | 78.3 | 58.2 | 30.28 | 25.13 | 5.15 |
| Coll_EDC/NHS_d | 75.9 | - | - | - | - |
| Coll | Coll_EDC | Coll_EDC/NHS | Coll_EDC_d | Coll_EDC/NHS_d |
|---|---|---|---|---|
 |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sionkowska, A.; Kulka-Kamińska, K.; Brudzyńska, P.; Lewandowska, K.; Piwowarski, Ł. The Influence of Various Crosslinking Conditions of EDC/NHS on the Properties of Fish Collagen Film. Mar. Drugs 2024, 22, 194. https://doi.org/10.3390/md22050194
Sionkowska A, Kulka-Kamińska K, Brudzyńska P, Lewandowska K, Piwowarski Ł. The Influence of Various Crosslinking Conditions of EDC/NHS on the Properties of Fish Collagen Film. Marine Drugs. 2024; 22(5):194. https://doi.org/10.3390/md22050194
Chicago/Turabian StyleSionkowska, Alina, Karolina Kulka-Kamińska, Patrycja Brudzyńska, Katarzyna Lewandowska, and Łukasz Piwowarski. 2024. "The Influence of Various Crosslinking Conditions of EDC/NHS on the Properties of Fish Collagen Film" Marine Drugs 22, no. 5: 194. https://doi.org/10.3390/md22050194





