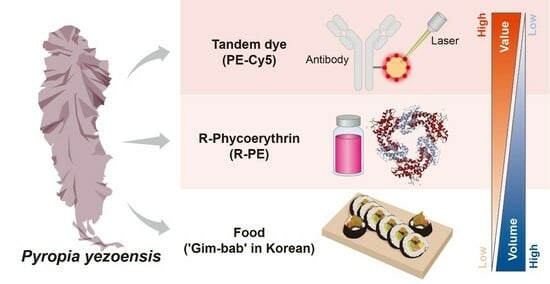
Purified Pyropia yezoensis Pigment Extract-Based Tandem Dye Synthesis
Abstract
:1. Introduction
2. Results
2.1. Purification of Phycoerythrin
2.2. Development of a Tandem Dye with Purified Phycoerythrin and Cyanine5
2.3. Economic Viability
3. Discussion
| Species | R-PE Extraction and Purification Steps | PE Extraction (mg·g−1) | Purity Index (A565/A280) | Reference |
|---|---|---|---|---|
| Amphiroa anceps | Extract PBPs from fresh algae using 50 mM PBS (pH 7.4) with 150 mM NaCl, NaN3, 2-mercaptoethanol, and EDTA. Extract biliproteins using (NH4)2SO4 and perform ion-exchange chromatography on R-PE samples using a Q Sepharose™ Fast Flow column. Elute red-colored fractions using linear gradient elution with NaCl and monitor at wavelength 280 nm. Purify R-PE using gel filtration chromatography with Sepharose™ CL-6B and fast protein liquid chromatography. Collect bright-red fractions for characterization. | 0.71 | NA | Kawsar et al. [45] |
| Gracilaria gracilis | Homogenize algae in liquid nitrogen, suspended in phosphate buffer (20 mM, pH 7.1), extract at a 1:20 ratio, centrifuge, and collect supernatant as the crude extract. Apply the crude extract on a DEAE Sepharose™ Fast Flow column, perform elution using 200 mM NaCl, adjust the flow rate to 4 mL·min–1, and collect the fractions containing R-PE. | 0.24 | 3.25 | Nguyen et al. [46] |
| Gracilaria lemaneiformis | Cut G. lemaneiformis and lyse cells with hypotonicity and break further with a refiner, filter, and centrifuge. Precipitate with 65% saturated ammonium sulfate to obtain a crude sample for centrifugal partition chromatography separation. Fill the upper and lower centrifugal partition chromatography channels with 50% saturated ammonium sulfate solution. Load the sample into the inlet terminal of the water channel and elute with a linear gradient of ammonium sulfate solution and water at designated flow rates. Collect eluents and analyze absorbance values. Record the absorption spectrum of purified R-PE using a UV-visible spectrophotometer with a scanned wavelength from 250 to 700 nm. For native PAGE analysis, mix the sample with native PAGE sample buffer and deionized water. Analyze on 3–12% Bis-Tris protein gel and stain with CBB R250. For SDS-PAGE analysis, mix concentrated samples with SDS protein gel loading solution, heat, analyze on NuPAGE™ 4–12% Bis-Tris protein gel, and stain with CBB R250. | 5 | 6.5 | Gu et al. [47] |
| Gracilariatenuistipitata | Homogenize 1 kg of G. tenuistipitata in distilled water and freeze–thaw twice. Filter the mixture to remove the cell residue. Add 0.2 M ammonium sulfate to the crude extract and incubate at 4 °C overnight. Filter the crude extract to remove the precipitate. Determine the R-PE quantity using empirical formulae. Isolate R-PE using a STREAMLINE™ column filled with Phenyl Sepharose™ 6 Fast Flow. Wash the column with 0.5 M ammonium sulfate and elute with three different concentrations (0.5, 0.2, and 0.05 M) of ammonium sulfate. Collect the fractions containing PBPs and record the absorption spectrum. Dialyze the eluate against different concentrations of ammonium sulfate. Purify the dialyzed eluate using ion-exchange chromatography on a DEAE Sepharose™ column. Load the fractions separately onto the column and elute with 10 mM sodium acetate at a pH gradient from 4.5 to 4.0, to collect R-PE. | 0.03 | >4 | Zhao et al. [48] |
| Heterosiphonia japonica | Extract PBPs with 50 mM phosphate buffer, NaN3, 2-mercaptoethanol, and EDTA. Salt out biliproteins with (NH4)2SO4 at 85% saturation and collect the R-PE extract. Purify R-PE via gel filtration with Sepharose™ CL-4B and Sephadex™ G-200, and perform ion-exchange chromatography on a DEAE Sepharose™ Fast Flow column. Evaluate R-PE purity via native PAGE in neutral and alkaline buffer systems. | NA | 4.89 | Sun et al. [49] |
| Mastocarpus stellatus | Prepare seaweed extract by homogenizing dry algal powder in phosphate buffer (20 mM, pH 7.1); centrifuge the extract and repeat the process four times to maximize yield. Fractionate the crude extract with ammonium sulfate, recover the precipitate via centrifugation, and dissolve it in phosphate buffer, followed by dialysis overnight. Apply the extract on an anion-exchange column and develop elution with a three-step increase in buffer ionic strength. Collect fractions containing R-PE at 200 mM NaCl using a high-performance liquid chromatography system with two buffers and a diode array detector. | 0.11 | 1.91 | Nguyen et al. [50] |
| Michrochaete | Harvest cyanobacterial biomass via centrifugation and wash with distilled water. Dry overnight at 50 °C. Extract PE in 0.1 M potassium phosphate buffer (pH 7.1) by repeated freezing and thawing. Record absorbance. Optimise PE extraction using 0.1 M acetate buffer (pH 6.0). Add solid ammonium sulfate to the crude extract to achieve 65% saturation. Allow the solution to stand for 12 h and then centrifuge. Resuspend pellets in a small volume of 50 mM acetic acid-sodium acetate buffer (pH 7.1) and dialyze overnight against the same buffer. Apply the dialyzed solution to a pre-equilibrated column of DEAE-cellulose in 50 mM acetate buffer (pH 7.1) at a flow rate of 0.5 mL·min–1. Collect pink-colored elutes and analyze each fraction using absorption spectroscopy to characterize PE. Calculate the purity of PE at each step in terms of the A555/A280 ratio. | 0.198 (mg·mL–1) | 4.1 (A555/A280) | Afreen and Fatma [51] |
| Polysiphonia urceolata | Mix frozen P. urceolata with distilled water and allow cell lysis. Filter and centrifuge the supernatant. Add solid ammonium sulfate to achieve 0.50 M concentration. Load the extract onto a STREAMLINE™ column with Phenyl Sepharose™ and wash with 0.50 M ammonium sulfate. Elute with 0.20, 0.10, and 0.05 M ammonium sulfate solutions and distilled water successively. Apply eluates onto an ion-exchange column with Q Sepharose™ and wash with 0.05 M NaAc and 0.05 M phosphate buffer (pH 7.0). Elute with NaCl gradient from 0 to 0.30 M in 0.05 M phosphate buffer (pH 7.0). Measure the volumes and optical densities of all eluates to determine the quantity and purity of R-PE. | 0.40 | 3.26 | Niu et al. [31] |
| Polysiphonia urceolata | Thaw frozen algae in 0.02 M Na-phosphate buffer (pH 7.0), filter, and centrifuge to obtain the PE-containing supernatant. Fractionate supernatant with ammonium sulfate at 25% and 45% (w/v), collect red supernatant and precipitate, dissolve precipitate in 20 mM phosphate buffer (pH 7.0), and dialyze overnight. Apply dialyzed R-PE samples on DEAE Sepharose™ Fast Flow column pre-equilibrated with 20 mM phosphate buffer (pH 5.6) containing 0.05 M NaCl. Wash the column with the same buffer, elute with 20 mM phosphate buffer containing 0.05 M NaCl with a pH gradient (pH 5.6–4.0, 2 × 50 mL) at 1 mL·min−1, monitor eluate at 280 nm, and collect 2-mL fractions. Perform native PAGE and SDS-PAGE using the Bio-Rad vertical slab discontinuous gel electrophoresis apparatus with 5% stacking gel as well as 7.5% and 15% separating gel, respectively. Stain gels with CBB R250. | 1.79 (ca.) | 5.6 | Liu et al. [52] |
| Pyropia yezoensis | Homogenize 50 g dried P. yezoensis in 250 mL PBSE (pH 6.8) with EDTA, centrifuge at 8500× g and 4 °C for 15 min. Precipitate supernatant with 20% ammonium sulfate and discard the pellet. Precipitate the supernatant again with 50% ammonium sulfate and dissolve the pellet in PBSE. Precipitate the solution with 10% ammonium sulfate, collect the supernatant, precipitate again with 40% ammonium sulfate, and discard the supernatant. Dissolve the final pellet in 50 mM PBSE and centrifuge at 19,000× g and 4 °C for 30 min. Desalt with Sephadex™ G-25 and purify with hydroxyapatite. Perform gradient elution with PBSE at different concentrations to collect the PE-containing eluent and purify it with regenerated hydroxyapatite. Analyze PE purity and content via protein absorption, SDS-PAGE (14% separating and 5% stacking gel), and native PAGE (5% separating and 5% stacking gel). Determine purity based on the A565/A280 ratio. | NA | 5.5 | Cai et al. [53] |
| Pyropia yezoensis | Chop and immerse 200 g Py. yezoensis in PBSE buffer (50 mmol·L−1 sodium phosphate and 1 mmol·L−1 EDTA) at a ratio of 1:5 g·mL−1. Homogenise and centrifuge the mixture at 10,000× g and 4 °C for 15 min. Collect 980 mL supernatant and precipitate 420 mL with ammonium sulfate using seven gradients of three parts/concentration × 20 mL/part. Collect the supernatant and dissolve the pellet in PBSE after overnight precipitation and centrifugation. Measure solution spectra at each step with an Ultrospec 2000 spectrophotometer to calculate PBP purity and content. | 0.94 | 1.94 | Cai et al. [54] |
| Porphyra yezoensis | Fragment 28 g of the leafy gametophyte of P. yezoensis in 300 mL 10 mM phosphate buffer (pH 6.8) using a triturator for 30 min, followed by freeze–thawing. Filter the slurry with gauze, repeat the procedure three times, and pool all supernatants. Add powdered (NH4)2SO4 to a final concentration of 0.50 M and maintain at 4 °C overnight. Centrifuge at 3000× g for 10 min and determine the quantity of R-PE. Fill STREAMLINE™ column with Phenyl Sepharose™ and equilibrate with 0.50 M ammonium sulfate solution. Apply the crude R-PE extract at room temperature and conduct the expanded bed run. Determine the quantity and purity of isolated R-PE in each eluate. Combine eluates and dialyze against MilliQ water. Pump into an anion-exchange column (20 × 1 cm) loaded with 15 mL DEAE Sepharose™ in a downward direction. Wash with 10 mM NaAc (pH 4.2) and develop with 50 mM phosphate buffer (pH 6.8) containing an increasing step gradient of NaCl (0–0.20 M) at a flow rate of 2.5 mL·min−1. Collect the red-colored eluate and record the UV-visible and fluorescence spectra at room temperature. | 0.82 | 4.5 | Niu et al. [55] |
| Porphyra yezoensis | Store freshly harvested P. yezoensis at −80 °C until use. Isolate PE from lyophilized P. yezoensis and measure the visible absorption spectrum of the fraction containing PE using a spectrophotometer. Use an extinction coefficient of 80.2 at 565 nm to quantify PE. Obtain phycoerythrobilin from the methanolysis of PE by heating at 90 °C for 3 h, followed by centrifugation and extraction from the chloroform layer. Use an extinction coefficient of 25,200 at 591 nm to quantify phycoerythrobilin. Analyze PE using an HPLC system with a photodiode array detector, using an ODS-80Ts column and a mobile phase of methanol:water:acetic acid (50:50:1, v:v:v) at a flow rate of 1.0 mL·min–1, with the column temperature maintained at 40 °C. | NA | NA | Sakai et al. [56] |
| Portieria hornemannii | Prepare P. hornemannii by adding 50 g of fresh thallus to 125 mL of 0.02 mM phosphate buffer at pH 7.2, then pulverize, filter, and repeatedly freeze–thaw. Centrifuge at 12,500× g for 20 min, precipitate with 35% and 55% saturated ammonium sulfate and dialyze against 0.05 M phosphate buffer at pH 7.2. Record levels of R-PE, phycocyanin, and allophycocyanin. Load the dialyzed sample onto a Q Sepharose™ anionic-exchange column (30 × 2.5 cm) and elute with increasing concentrations of NaCl. Collect R-PE-rich fractions, dialyze, concentrate, and store. Conduct native PAGE of R-PE using a Bio-Rad Electrophoresis Apparatus. Perform two-dimensional electrophoresis of R-PE and stain with silver nitrate. | 0.86 | 5.21 | Senthilkumar et al. [32] |
| Red algae | Homogenise algal powder in extraction buffer (1 g/20 mL ratio). Agitate system (150 rpm) for 20 min to 12 h. Centrifuge at 25,000× g for 30 min and recover supernatant. Perform multiple extractions if necessary. Pool all supernatants. Pre-equilibrate DEAE Sepharose™ Fast Flow column with buffer A (10-times column void volume). Load supernatant on top of the column without overloading. Rinse the column with buffer A at a flow rate of 4 mL·min–1. Elute R-PE with a three-step increase in buffer ionic strength: 150 mM, 200 mM, and 1 M NaCl, for 10 min each, at a flow rate of 4 mL·min–1. Monitor absorption at 280 and 565 nm. Collect fraction eluted with 200 mM NaCl. Desalt overnight via dialysis against phosphate buffer. Estimate the purity index of the fraction based on the A565/A280 ratio. | NA | NA | Dumay et al. [57] |
4. Materials and Methods
4.1. Sample Material
4.2. Purification of Phycoerythrin
4.2.1. Fractionation and Ultrafiltration
4.2.2. Ion Exchange
4.2.3. Gel Filtration
4.2.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
4.2.5. Analyzes of Purified Proteins
4.3. Development of a Tandem Dye with Purified R-PE and Cy5
4.3.1. Conjugation of Cy5 to R-PE
4.3.2. Conjugation of Succinimidyl-4-(N-maleimidomethyl) Cyclohexane-1-Carboxylate (SMCC) to PE-Cy5
4.3.3. Reduction in Immunoglobulin G (IgG)
4.3.4. Covalent Conjugation of Antibodies to SMCC-PE-Cy5
4.3.5. Techno-Economic Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Fishery and Aquaculture Statistics. Global Production Statistics 1950–2019. In: FAO Fisheries Division [Online]. FishStaJ–Software for Fishery and Aquaculture Statistical Time Series. 2021. Available online: www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 14 March 2023).
- Pereira, L. A review of the nutrient composition of selected edible seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V., Ed.; Nova Science Publishers: New York, NY, USA, 2011; pp. 15–47. [Google Scholar]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.J.; Rhee, M.S. Health functionality and quality control of laver (Porphyra, Pyropia): Current issues and future perspectives as an edible seaweed. Mar. Drugs 2020, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, J.; Wang, S.; Xu, X. Porphyra species: A mini-review of its pharmacological and nutritional properties. J. Med. Food 2016, 19, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Bito, T.; Teng, F.; Watanabe, F. Bioactive compounds of edible purple laver Porphyra sp. (Nori). J. Agric. Food Chem. 2017, 65, 10685–10692. [Google Scholar] [CrossRef] [PubMed]
- Dagnino-Leone, J.; Pinto Figueroa, C.; Latorre Castañeda, M.; Donoso Youlton, A.; Vallejos-Almirall, A.; Agurto-Muñoz, A.; Pavón Pérez, J.; Agurto-Muñoz, C. Phycobiliproteins: Structural aspects, functional characteristics, and biotechnological perspectives. Comput. Struct. Biotechnol. J. 2022, 20, 1506–1527. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Kumar, D.; Singh, V.; Sinha, R.P. Advances in phycobiliproteins research: Innovations and commercialization. In Natural Bioactive Compounds; Sinha, R.P., Häder, D.-P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 57–81. [Google Scholar] [CrossRef]
- Glazer, A.N. Phycobiliproteins—A family of valuable, widely used fluorophores. J. Appl. Phycol. 1994, 6, 105–112. [Google Scholar] [CrossRef]
- Lee, H.-A.; Kim, I.-H.; Nam, T.-J. Bioactive peptide from Pyropia yezoensis and its anti-inflammatory activities. Int. J. Mol. Med. 2015, 36, 1701–1706. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Choi, S.; Pandey, L.K.; Depuydt, S.; De Saeger, J.; Park, J.-T.; Han, T. Extracts of red seaweed, Pyropia yezoensis, inhibit melanogenesis but stimulate collagen synthesis. J. Appl. Phycol. 2021, 33, 653–662. [Google Scholar] [CrossRef]
- Cian, R.E.; Bacchetta, C.; Rossi, A.; Cazenave, J.; Drago, S.R. Red seaweed Pyropia columbina as antioxidant supplement in feed for cultured juvenile Pacú (Piaractus mesopotamicus). J. Appl. Phycol. 2019, 31, 1455–1465. [Google Scholar] [CrossRef]
- He, D.; Wu, S.; Yan, L.; Zuo, J.; Cheng, Y.; Wang, H.; Liu, J.; Zhang, X.; Wu, M.; Choi, J.; et al. Antitumor bioactivity of porphyran extracted from Pyropia yezoensis Chonsoo2 on human cancer cell lines. J. Sci. Food Agric. 2019, 99, 6722–6730. [Google Scholar] [CrossRef]
- Contreras-Martel, C.; Martinez-Oyanedel, J.; Bunster, M.; Legrand, P.; Piras, C.; Vernede, X.; Fontecilla-Camps, J.-C. Crystallization and 2.2 Å resolution structure of R-phycoerythrin from Gracilaria chilensis: A case of perfect hemihedral twinning. Acta. Crystallogr. D. Biol. Crystallogr. 2001, 57, 52–60. [Google Scholar] [CrossRef]
- Isailovic, D.; Li, H.-W.; Yeung, E.S. Isolation and characterization of R-phycoerythrin subunits and enzymatic digests. J. Chromatogr. A 2004, 1051, 119–130. [Google Scholar] [CrossRef]
- Glazer, A.N.; Hixson, C.S. Characterization of R-phycocyanin. Chromophore content of R-phycocyanin and C-phycoerythrin. J. Biol. Chem. 1975, 250, 5487–5495. [Google Scholar] [CrossRef]
- D’Agnolo, E.; Murano, E.; Rizzo, R.; Paoletti, S. A biliprotein from the red alga Gracilaria longa: Thermal stability of R-phycoerythrin. Ital. J. Biochem. 1993, 42, 316A–318A. [Google Scholar]
- Bharmoria, P.; Correia, S.F.H.; Martins, M.; Hernández-Rodríguez, M.A.; Ventura, S.P.M.; Ferreira, R.A.S.; Carlos, L.D.; Coutinho, J.A.P. Protein cohabitation: Improving the photochemical stability of R-phycoerythrin in the solid state. J. Phys. Chem. Lett. 2020, 11, 6249–6255. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Jagatheesan, A.; Perumal, K.; Arunkumar, K. Methods of phycobiliprotein extraction from Gracilaria crassa and its applications in food colourants. Algal Res. 2015, 8, 115–120. [Google Scholar] [CrossRef]
- Huang, B.; Wang, G.C.; Zeng, C.K.; Li, Z.G. The experimental research of R-phycoerythrin subunits on cancer treatment: A new photosensitizer in PDT. Cancer Biother. Radiopharm. 2002, 17, 35–42. [Google Scholar] [CrossRef]
- Yañuk, J.G.; Cabrerizo, F.M.; Dellatorre, F.G.; Cerdá, M.F. Photosensitizing role of R-phycoerythrin red protein and β-carboline alkaloids in Dye sensitized solar cell. Electrochemical and spectroscopic characterization. Energy Rep. 2020, 6, 25–36. [Google Scholar] [CrossRef]
- Ma, J.; Chen, H.; Qin, S.; Lin, H. Applications of natural and artificial phycobiliproteins in solar cells. Curr. Bioethanol. 2015, 4, 275–281. [Google Scholar] [CrossRef]
- Frias, A.R.; Correia, S.F.H.; Martins, M.; Ventura, S.P.M.; Pecoraro, E.; Ribeiro, S.J.L.; André, P.S.; Ferreira, R.A.S.; Coutinho, J.A.P.; Carlos, L.D. Sustainable liquid luminescent solar concentrators. Adv. Sustain. Syst. 2019, 3, 1800134. [Google Scholar] [CrossRef]
- Ferreira, R.A.S.; Correia, S.F.H.; Monguzzi, A.; Liu, X.; Meinardi, F. Spectral converters for photovoltaics–What’s ahead. Mater. Today 2016, 33, 105–121. [Google Scholar] [CrossRef]
- Saluri, M.; Kaldmäe, M.; Tuvikene, R. Reliable quantification of R-phycoerythrin from red algal crude extracts. J. Appl. Phycol. 2020, 32, 1421–1428. [Google Scholar] [CrossRef]
- Leney, A.C.; Tschanz, A.; Heck, A.J.R. Connecting color with assembly in the fluorescent B-phycoerythrin protein complex. FEBS J. 2018, 285, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Manirafasha, E.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Jing, K. Phycobiliprotein: Potential microalgae derived pharmaceutical and biological reagent. Biochem. Eng. J. 2016, 109, 282–296. [Google Scholar] [CrossRef]
- Sathuvan, M.; Thangam, R.; Venkateshbabu, G.; Cheong, K.-L.; Kang, H.; Liu, Y. Single-step purified R-phycoerythrin transmits cellular imaging functionalities in vitro. Int. J. Biol. Macromol. 2022, 194, 563–570. [Google Scholar] [CrossRef]
- Munier, M.; Morançais, M.; Dumay, J.; Jaouen, P.; Fleurence, J. One-step purification of R-phycoerythrin from the red edible seaweed Grateloupia turuturu. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 992, 23–29. [Google Scholar] [CrossRef]
- Niu, J.-F.; Wangm, G.-C.; Tseng, C.-K. Method for large-scale isolation and purification of R-phycoerythrin from red alga Polysiphonia urceolata Grev. Protein Expr. Purif. 2006, 49, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, N.; Suresh, V.; Thangam, R.; Kurinjimalar, C.; Kavitha, G.; Murugan, P.; Kannan, S.; Rengasamy, R. Isolation and characterization of macromolecular protein R-Phycoerythrin from Portieria hornemannii. Int. J. Biol. Macromol. 2013, 55, 150–160. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, F.; Benavides, J.; Rito-Palomares, M. Scaling-up of a B-phycoerythrin production and purification bioprocess involving aqueous two-phase systems: Practical experiences. Process Biochem. 2013, 48, 738–745. [Google Scholar] [CrossRef]
- Chang, Y.K.; Show, P.L.; Lan, J.C.W.; Tsai, J.C.; Huang, C.R. Isolation of C-phycocyanin from Spirulina platensis microalga using Ionic liquid based aqueous two-phase system. Bioresour. Technol. 2018, 270, 320–327. [Google Scholar] [CrossRef]
- Acién, F.G.; Molina, E.; Fernández-Sevilla, J.M.; Barbosa, M.; Gouveia, L.; Sepúlveda, C.; Bazaes, J.; Arbib, Z. Economics of microalgae production. In Microalgae-Based Biofuels and Bioproducts; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 485–503. [Google Scholar] [CrossRef]
- Molina Grima, E.; Belarbi, E.-H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Morais, M.G.; Prates, D.F.; Moreira, J.B.; Duarte, J.H.; Costa, J.A.V. Phycocyanin from microalgae: Properties, extraction, and purification, with some recent applications. Ind. Biotechnol. 2018, 14, 30–37. [Google Scholar] [CrossRef]
- Heinemann, J.A.; Massaro, M.; Coray, D.S.; Agapito-Tenfen, S.Z.; Wen, J.D. Sustainability and innovation in staple crop production in the US midwest. Int. J. Agr. Sustain. 2014, 12, 71–88. [Google Scholar] [CrossRef]
- Moraes, C.C.; Kalil, S.J. Strategy for a protein purification design using C-phycocyanin extract. Bioresour. Technol. 2009, 100, 5312–5317. [Google Scholar] [CrossRef]
- Mittal, R.; Lamdande, A.G.; Sharma, R.; Raghavarao, K.S.M.S. Membrane processing for purification of R-Phycoerythrin from marine macro-alga, Gelidium pusillum and process integration. Sep. Purif. Technol. 2020, 252, 117470. [Google Scholar] [CrossRef]
- Moore, P.A.; Kery, V. High-Throughput protein concentration and buffer exchange: Comparison of ultrafiltration and ammonium sulfate precipitation. In Methods in Molecular Biology: High Throughput Protein Expression and Purification; Doyle, S.A., Ed.; Humana Press: New York, NY, USA, 2009; pp. 309–314. [Google Scholar] [CrossRef]
- Chen, H.; Deng, J.; Li, L.; Liu, Z.; Sun, S.; Xiong, P. Recent progress of natural and recombinant phycobiliproteins as fluorescent probes. Mar. Drugs 2023, 21, 572. [Google Scholar] [CrossRef]
- Korea Agriculture and Forestry Export and Import Statistics. 2023. Available online: https://www.kati.net/index.do (accessed on 7 January 2024).
- Phycoerythrin Market Outlook (2022 to 2032). Available online: https://www.futuremarketinsights.com/reports/phycoerythrin-market (accessed on 7 January 2024).
- Kawsar, S.M.; Fujii, Y.; Matsumoto, R.; Yasumitsu, H.; Ozeki, Y. Protein R-phycoerythrin from marine red alga Amphiroa anceps: Extraction, purification and characterization. Phytol. Balc. 2011, 17, 347–354. [Google Scholar]
- Nguyen, H.P.T.; Morançais, M.; Déléris, P.; Fleurence, J.; Nguyen-Le, C.T.; Vo, K.H.; Dumay, J. Purification of R-phycoerythrin from a marine macroalga Gracilaria gracilis by anion-exchange chromatography. J. Appl. Phycol. 2020, 32, 553–561. [Google Scholar] [CrossRef]
- Gu, D.; Lazo-Portugal, R.; Fang, C.; Wang, Z.; Ma, Y.; Knight, M.; Ito, Y. Purification of R-phycoerythrin from Gracilaria lemaneiformis by centrifugal precipitation chromatography. J. Chromatogr. B 2018, 1087–1088, 138–141. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, X.; Niu, J.; He, L.; Gu, W.; Xie, X.; Wu, M.; Wang, G. Agar extraction and purification of R-phycoerythrin from Gracilaria tenuistipitata, and subsequent wastewater treatment by Ulva prolifera. Algal Res. 2020, 47, 101862. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Gong, X.; Zhao, M.; Fu, X.; Wang, L. Isolation, purification and characteristics of R-phycoerythrin from a marine macroalga Heterosiphonia japonica. Protein Expr. Purif. 2009, 64, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.T.; Morancais, M.; Fleurence, J.; Tran, T.N.L.; Dumay, J. Extracting and Purifying Pigment R-phycoerythrin from the Red alga Mastocarpus stellatus. In Proceedings of the 2018 4th International Conference on Green Technology and Sustainable Development (GTSD), Ho Chi Minh City, Vietnam, 23–24 November 2018; IEEE: New York, NY, USA; pp. 573–577. [Google Scholar] [CrossRef]
- Afreen, S.; Fatma, T. Extraction, purification and characterization of phycoerythrin from Michrochaete and its biological activities. Biocatal. Agric. Biotechnol. 2018, 13, 84–89. [Google Scholar] [CrossRef]
- Liu, L.-N.; Chen, X.-L.; Zhang, X.-Y.; Zhang, Y.-Z.; Zhou, B.-C. One-step chromatography method for efficient separation and purification of R-phycoerythrin from Polysiphonia urceolata. J. Biotechnol. 2005, 116, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Wang, Y.; Li, C.; Guo, Z.; Jia, R.; Wu, W.; Hu, Y.; He, P. Purification and photodynamic bioactivity of phycoerythrin and phycocyanin from Porphyra yezoensis Ueda. J. Ocean Univ. China 2014, 13, 479–484. [Google Scholar] [CrossRef]
- Cai, C.; Li, C.; Wu, S.; Wang, Q.; Guo, Z.; He, P. Large scale preparation of phycobiliproteins from Porphyra yezoensis using co-precipitation with ammonium sulfate. Nat. Sci. 2012, 04, 536–543. [Google Scholar] [CrossRef]
- Niu, J.-F.; Chen, Z.-F.; Wang, G.-C.; Zhou, B.-C. Purification of phycoerythrin from Porphyra yezoensis Ueda (Bangiales, Rhodophyta) using expanded bed absorption. J. Appl. Phycol. 2010, 22, 25–31. [Google Scholar] [CrossRef]
- Sakai, S.; Komura, Y.; Nishimura, Y.; Sugawara, T.; Hirata, T. Inhibition of Mast Cell Degranulation by Phycoerythrin and Its Pigment Moiety Phycoerythrobilin, Prepared from Porphyra yezoensis. Food Sci. Technol. Res. 2011, 17, 171–177. [Google Scholar] [CrossRef]
- Dumay, J.; Morançais, M.; Nguyen, H.P.T.; Fleurence, J. Extraction and Purification of R-phycoerythrin from Marine Red Algae. In Natural Products From Marine Algae; Methods in Molecular Biology; Stengel, D., Connan, S., Eds.; Humana Press: New York, NY, USA, 2015; Volume 1308, pp. 109–117. [Google Scholar] [CrossRef]
- Galland-Irmouli, A.V.; Pons, L.; Lucon, M.; Villaume, C.; Mrabet, N.T.; Guéant, J.L.; Fleurence, J. One-step purification of R-phycoerythrin from the red macroalga Palmaria palmata using preparative polyacrylamide gel electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 2000, 739, 117–123. [Google Scholar] [CrossRef]
- Hardy, R. Purification and coupling of fluorescent proteins for use in flow cytometry. In Handbook of Experimental Immunology; Weir, D., Herzenberg, L., Blackell, C., Herzenberg, L., Eds.; Blackwell Scientific Publications: Hoboken, NJ, USA, 1986; pp. 31.1–31.12. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Han, T.; Park, J. Purified Pyropia yezoensis Pigment Extract-Based Tandem Dye Synthesis. Mar. Drugs 2024, 22, 197. https://doi.org/10.3390/md22050197
Lee H, Han T, Park J. Purified Pyropia yezoensis Pigment Extract-Based Tandem Dye Synthesis. Marine Drugs. 2024; 22(5):197. https://doi.org/10.3390/md22050197
Chicago/Turabian StyleLee, Hojun, Taejun Han, and Jihae Park. 2024. "Purified Pyropia yezoensis Pigment Extract-Based Tandem Dye Synthesis" Marine Drugs 22, no. 5: 197. https://doi.org/10.3390/md22050197