Validation and Assessment of Three Methods to Estimate 24-h Urinary Sodium Excretion from Spot Urine Samples in High-Risk Elder Patients of Stroke from the Rural Areas of Shaanxi Province
Abstract
:1. Background
2. Methods
2.1. Sites
2.2. Subjects
2.3. Urine Collection
2.4. Specimen Analysis
2.5. The Estimated 24-h Sodium Excretion from Spot Urine Samples
2.6. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Validity of Three Methods of Estimated versus Measured 24-h Urinary Sodium Excretion in High-Risk Elder Patients of Stroke from the Rural Areas of Shaanxi Province
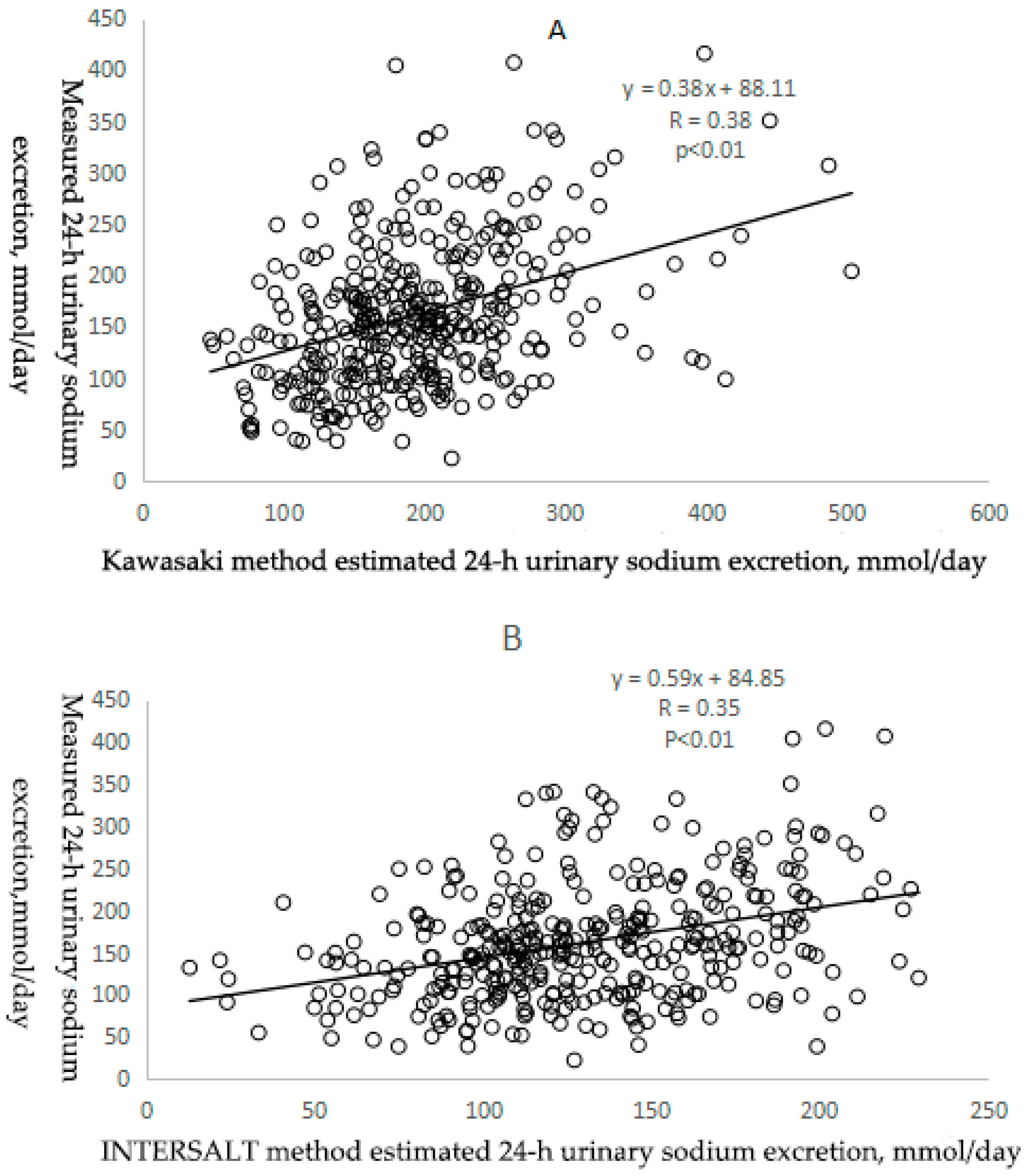
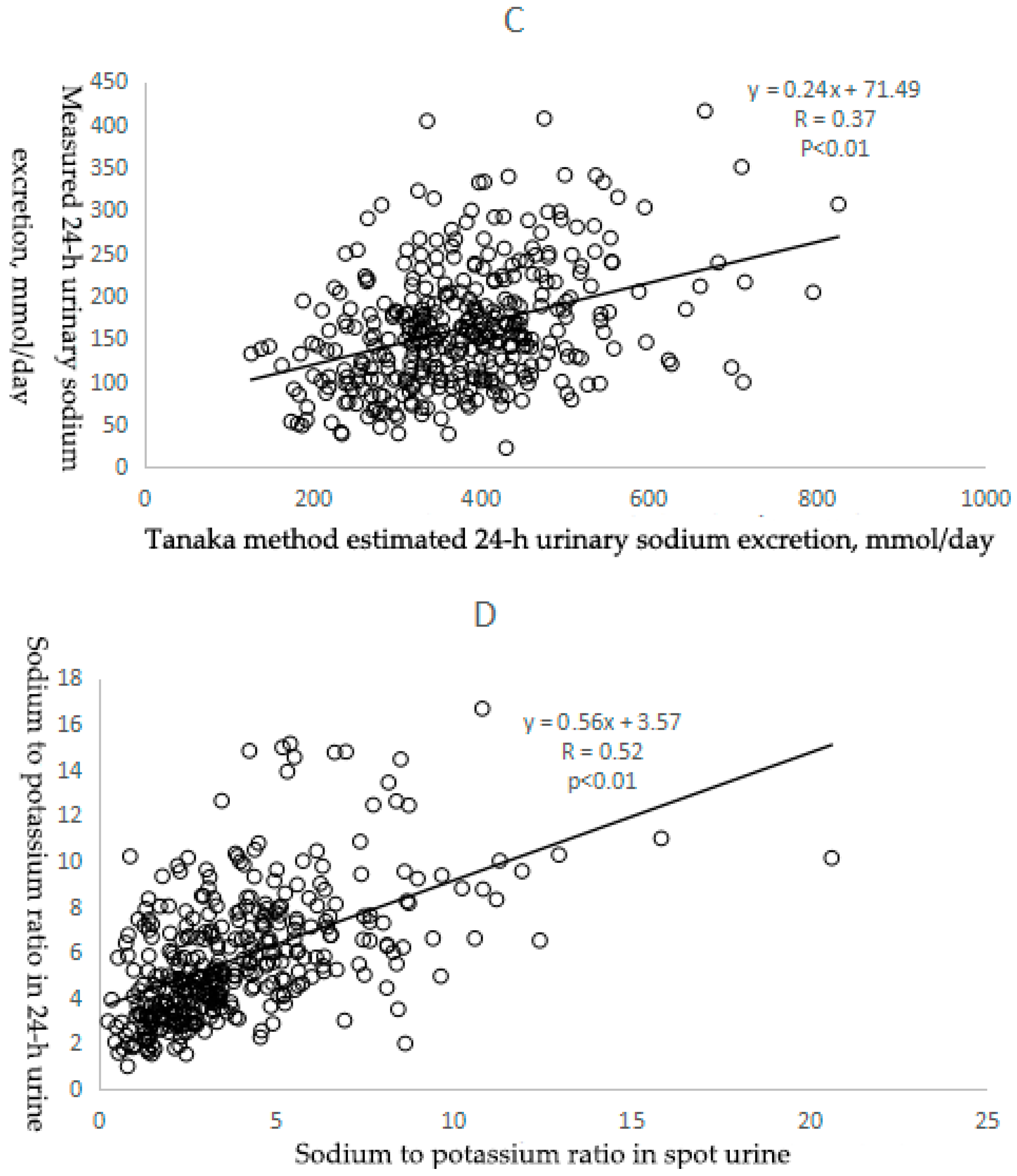
3.2.1. Correlation between Estimated and Measured Sodium Excretion
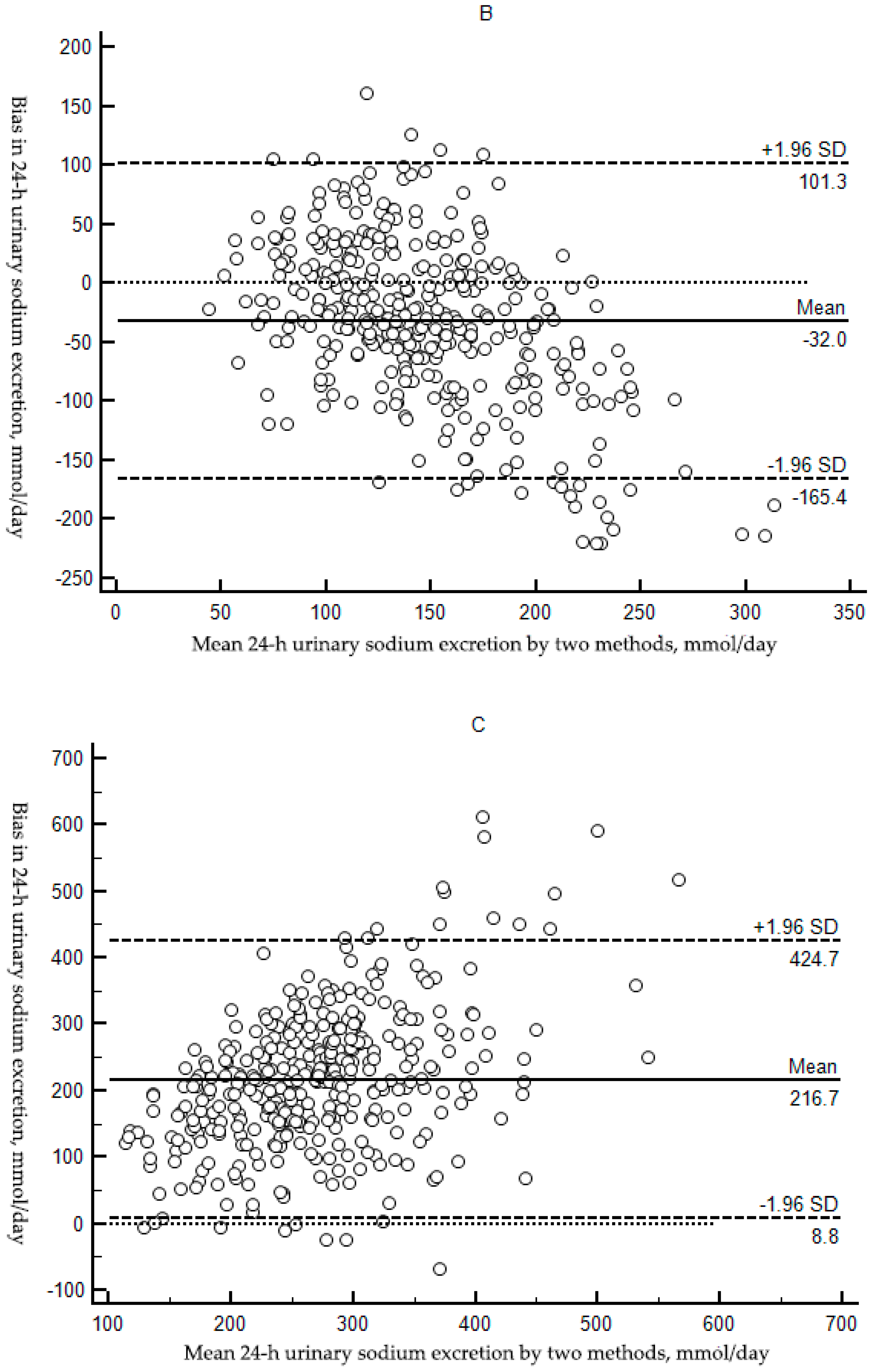
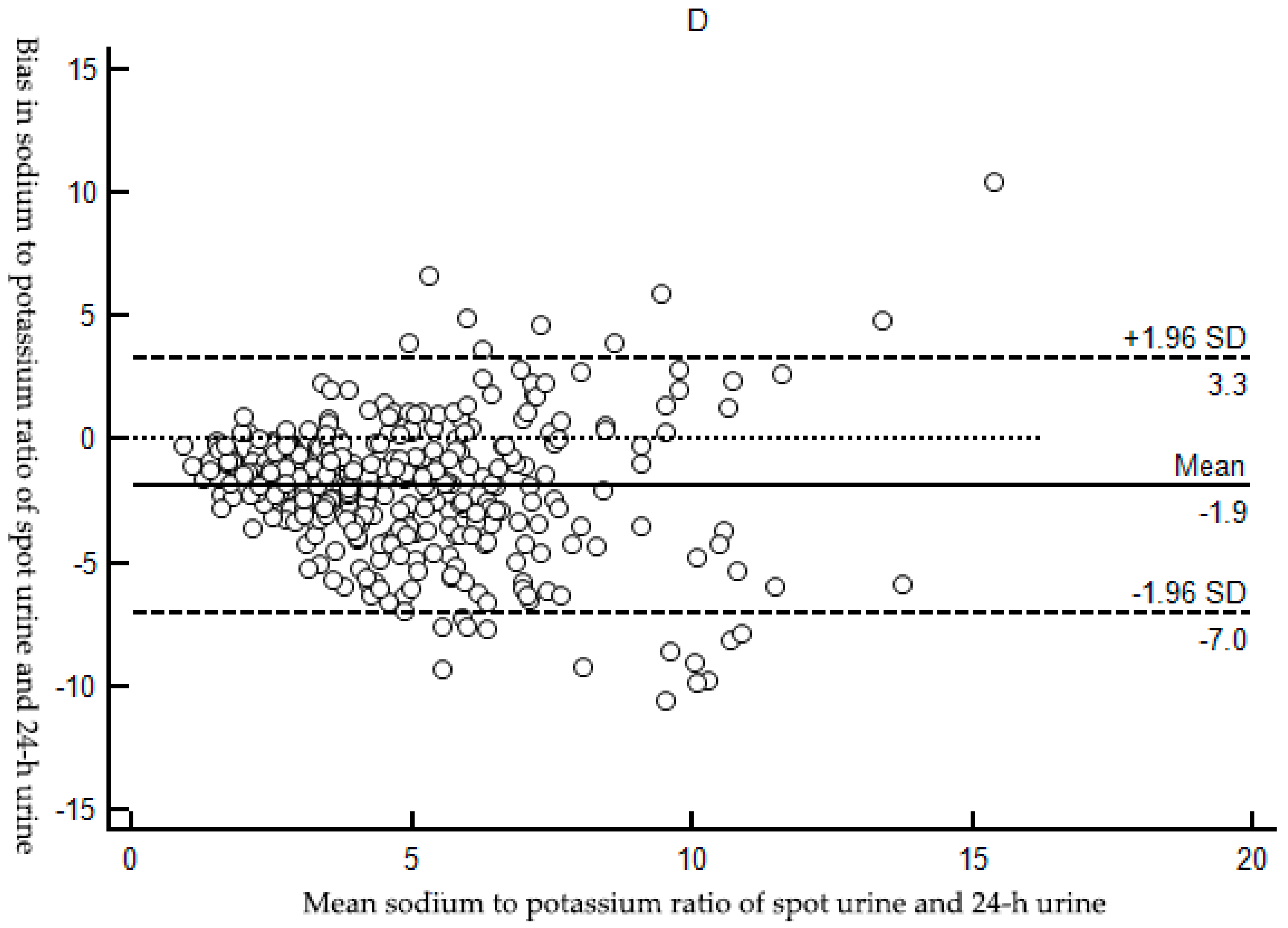
3.2.2. Bias between Estimated and Measured Sodium Excretion
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Conlin, P.R. Eat your fruits and vegetables but hold the salt. Circulation 2007, 116, 1530–1531. [Google Scholar] [CrossRef] [PubMed]
- Bibbins-Domingo, K.; Chertow, G.M.; Coxson, P.G.; Moran, A.; Lightwood, J.M.; Pletcher, M.J.; Goldman, L. Projected effect of dietary salt reductions on future cardiovascular disease. N. Engl. J. Med. 2010, 362, 590–599. [Google Scholar] [CrossRef]
- Mattes, R.D.; Donnelly, D. Relative contributions of dietary sodium sources. J. Am. Coll. Nutr. 1991, 10, 383–393. [Google Scholar] [CrossRef]
- James, W.P.; Ralph, A.; Sanchez-Castillo, C.P. The dominance of salt in manufactured food in the sodium intake of affluent societies. Lancet 1987, 1, 426–429. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- World Health Organization. Follow-up to the Political Declaration of the High-Level Meeting of the General Assembly on the Prevention and Control of Non-Communicable Diseases. In Proceedings of the Sixty-Sixth World Health Assembly (WHA66.10), Geneva, Switzerland, 27 May 2013. [Google Scholar]
- Anderson, C.A.; Cobb, L.K.; Miller, E.R.; Woodward, M.; Hottenstein, A.; Chang, A.R.; Mongraw-Chaffin, M.; White, K.; Charleston, J.; Tanaka, T.; et al. Effects of a behavioral intervention that emphasizes spices and herbs on adherence to recommended sodium intake: Results of the SPICE randomized clinical trial. Am. J. Clin. Nutr. 2015, 102, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Okamura, T.; Miura, K.; Kadowaki, T.; Ueshima, H.; Nakagawa, H.; Hashimoto, T. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J. Hum. Hypertens. 2002, 16, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Itoh, K.; Uezono, K.; Sasaki, H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin. Exp. Pharmacol. Physiol. 1993, 20, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Uezono, K.; Itoh, K.; Ueno, M. Prediction of 24-hour urinary creatinine excretion from age, body weight and height of an individual and its application. Nihon Koshu Eisei Zasshi 1991, 38, 567–574. [Google Scholar] [PubMed]
- Brown, I.J.; Dyer, A.R.; Chan, Q.; Cogswell, M.E.; Ueshima, H.; Stamler, J.; Elliott, P. Estimating 24-hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: The INTERSALT study. Am. J. Epidemiol. 2013, 177, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Tian, M.; Li, N.; Elliott, P.; Yan, L.L.; Labarthe, D.R.; Huang, L.; Yin, X.; Hao, Z.; Stepien, S.; et al. Rationale, design, and baseline characteristics of the Salt Substitute and Stroke Study (SSaSS)-A large-scale cluster randomized controlled trial. Am. Heart J. 2017, 188, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, W.; Wang, Y.; Chen, H.; Bo, J.; Wang, X.; Liu, L. Validation and Assessment of Three Methods to Estimate 24-h Urinary Sodium Excretion from Spot Urine Samples in Chinese Adults. PLoS ONE 2016. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, M.; Chen, Y.; Zhen, B.; Li, J.; Luan, W.; Ning, F.; Liu, H.; Ma, J.; Ma, G. Estimation of salt intake by 24-hour urinary sodium excretion: A cross-sectional study in Yantai, China. BMC Public Health 2014, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Sun, N.; Chen, Y.; Wang, H.; Yang, X.; Ma, Z. Validation of the Spot Urine in Evaluating 24-hour Sodium Excretion in Chinese Hypertension Patients. Am. J. Hypertens. 2015, 28, 1368. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.Y.; Kim, J.H.; Shin, S.J.; Gu, N.; Nah, D.Y.; Hong, K.S.; Cho, E.J.; Sung, K.C. Estimation of 24-hour Urinary Sodium Excretion Using Spot Urine Samples. Nutrients 2014, 6, 2360–2375. [Google Scholar] [CrossRef] [PubMed]
- Mclean, R.; Williams, S.; Mann, J. Monitoring population sodium intake using spot urine samples: Validation in a New Zealand population. J. Hum. Hypertens. 2014, 28, 657. [Google Scholar] [CrossRef] [PubMed]
- Toft, U.; Cerqueira, C.; Andreasen, A.H.; Thuesen, B.H.; Laurberg, P.; Ovesen, L.; Perrild, H.; Jørgensen, T. Estimating salt intake in a Caucasian population: Can spot urine substitute 24-hour urine samples? Eur. J. Prev. Cardiol. 2014, 21, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, M.E.; Wang, C.Y.; Chen, T.C.; Pfeiffer, C.M.; Elliott, P.; Gillespie, C.D.; Carriquiry, A.L.; Sempos, C.T.; Liu, K.; Perrine, C.G.; et al. Validity of predictive equations for 24-h urinary sodium excretion in adults aged 18–39 y. Am. J. Clin. Nutr. 2013, 98, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Pollack, H. Creatinine excretion as index for estimating urinary excretion of micronutrients or their metabolic end products. Am. J. Clin. Nutr. 1970, 23, 865–867. [Google Scholar] [PubMed]
- Liu, K.; Stamler, J.; Dyer, A.; McKeever, J.; McKeever, P. Statistical methods to assess and minimize the role of intra-individual variability in obscuring the relationship between dietary lipids and serum cholesterol. J. Chronic Dis. 1978, 31, 399–418. [Google Scholar] [CrossRef]
- Arroyave, G.; Wilson, D. Urinary excretion of creatinine of children under different nutritional conditions. Am. J. Clin. Nutr. 1961, 9, 170–175. [Google Scholar] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Mente, A.; O’Donnell, M.J.; Dagenais, G.; Wielgosz, A.; Lear, S.A.; Mcqueen, M.J.; Jiang, Y.; Xingyu, W.; Jian, B.; Calik, K.B.; et al. Validation and comparison of three formulae to estimate sodium and potassium excretion from a single morning fasting urine compared to 24-h measures in 11 countries. J. Hypertens. 2014, 32, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.R.; Stamler, R.; Grimm, R.; Stamler, J.; Berman, R.; Gosch, F.C.; Emidy, L.A.; Elmer, P.; Fishman, J.; Heel, N.V.; et al. Do hypertensive patients have a different diurnal pattern of electrolyte excretion? Hypertension 1987, 10, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Lerchl, K.; Rakova, N.; Dahlmann, A.; Rauh, M.; Goller, U.; Basner, M.; Dinges, D.F.; Beck, L.; Agureev, A.; Larina, I.; et al. Agreement between 24-hour salt ingestion and sodium excretion in a controlled environment. Hypertension 2015, 66, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Rakova, N.; Juttner, K.; Dahlmann, A.; Schroder, A.; Linz, P.; Kopp, C.; Rauh, M.; Goller, U.; Beck, L.; Agureev, A.; et al. Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab. 2013, 17, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Kusano, Y.; Takahashi, T.; Owada, M.; Sugawara, T. Effectiveness of a Spot Urine Method in Evaluating Daily Salt Intake in Hypertensive Patients Taking Oral Antihypertensive Drugs. Hypertens. Res. 2006, 29, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Iwahori, T.; Miura, K.; Ueshima, H.; Chan, Q.; Dyer, A.R.; Elliott, P.; Stamler, J. Estimating 24-h urinary sodium/potassium ratio from casual (‘spot’) urinary sodium/potassium ratio: The INTERSALT Study. Int. J. Epidemiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Cogswell, M.E.; Loria, C.M.; Chen, T.C.; Pfeiffer, C.M.; Swanson, C.A.; Caldwell, K.L.; Perrine, C.G.; Carriquiry, A.L.; Liu, K.; et al. Urinary excretion of sodium, potassium, and chloride, but not iodine, varies by timing of collection in a 24-hour calibration study. J. Nutr. 2013, 143, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Morise, T.; Okamoto, S.; Ikeda, M.; Takeda, R. The possible role of endogenous digitalis-like substance in the regulation of circadian changes in urinary electrolyte excretion in man. Endocrinol. Jpn. 1989, 36, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Muratani, H.; Kawasaki, T.; Ueno, M.; Kawazoe, N.; Fujishima, M. Circadian rhythms of urinary excretions of water and electrolytes in patients receiving total parenteral nutrition (TPN). Life Sci. 1985, 37, 645–649. [Google Scholar] [CrossRef]
- Soundararajan, R.; Pearce, D.; Hughey, R.P.; Kleyman, T.R. Role of Epithelial Sodium Channels and Their Regulators in Hypertension. J. Biol. Chem. 2010, 285, 30363–30369. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.M.; Perrier, R.; Wang, Q.; Zuber, A.M.; Maillard, M.; Mordasini, D.; Malsure, S.; Ronzaud, C.; Stehle, J.C.; Rossier, B.C.; et al. Sodium and potassium balance depends on αENaC expression in connecting tubule. J. Am. Soc. Nephrol. 2010, 21, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Cugini, P.; Scavo, D.; Cornelissen, G.; Lee, J.Y.; Meucci, T.; Halberg, F. Circadian rhythms of plasma renin, aldosterone and cortisol on habitual and low dietary sodium intake. Horm. Res. Paediat. 1981, 15, 7–27. [Google Scholar] [CrossRef]
- Liu, K.; Cooper, R.; Mckeever, J.; Mckeever, P.; Byington, R.; Soltero, I.; Stamler, R.; Gosch, F.; Stevens, E.; Stamler, J. Assessment of the association between habitual salt intake and high blood pressure: Methodological problems. Am. J. Epidemiol. 1979, 110, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.; Stamler, J.; Stamler, R.; Elliott, P.; Marmot, M.; Pyorala, K.; Kesteloot, H.; Joossens, J.; Hansson, L.; Mancia, G.; et al. Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 1988, 297, 319–328. [Google Scholar]
- Calles-Escandon, J.; Cunningham, J.J.; Snyder, P.; Jacob, R.; Huszar, G.; Loke, J.; Felig, P. Influence of exercise on urea, creatinine, and 3-methylhistidine excretion in normal human subjects. Am. J. Physiol. 1984, 246, 334–338. [Google Scholar]

| Method | Urine Sample | Formula to Estimate 24-h Urinary Sodium Excretion (mmol/day) |
|---|---|---|
| Kawasaki [9,10] | Second morning urine * | 16.3 × (Naspot (mmol/L)/Crspot (mg/dL) × 1/10 × PrUCr24h (mg/day)) 0.5 |
| PrUCr24h = 15.12 × Weight (kg)+7.39 × Height (cm) − 12.63 × Age (year) − 79.90 (Male) | ||
| PrUCr24h = 8.58 × Weight (kg) + 5.09 × Height (cm) − 4.72 × Age (year) − 74.50 (Female) | ||
| INTERSALT [11] | Casual spot urine | (25.46 + 0.46 × Naspot (mmol/L)) − 2.75 × Crspot (mmol/L) − 0.13 × Kspot (mmol/L) + 4.10 × BMI (kg/m2) + 0.26 × Age (year) (Male) |
| (5.07 + 0.34 × Naspot (mmol/L)) − 2.16 × Crspot (mmol/L) − 0.09 × Kspot (mmol/L) + 2.39 × BMI (kg/m2) + 2.35 × Age (year) − 0.03 × Age 2 (year)) (Female) | ||
| Tanaka [8] | Casual spot urine | 21.98 × (Naspot (mmol/L)/Crspot (mg/dL) × PrUCr24h (mg/day)) 0.392 |
| PrUCr24h = 14.89 × Weight (kg) + 16.14 × Height (cm) − 2.04 × Age (year) − 2244.45 |
| Selected Characteristics | Mean (SD) 1/n (%) |
|---|---|
| Age (years) | 67.5 ± 6.8 |
| Female | 210 (57.5) |
| Weight (kg) | 61.3 ± 10.5 |
| Height (cm) | 158.4 ± 7.8 |
| Body mass index (kg/m2) | 24.4 ± 3.5 |
| Systolic blood pressure (mm Hg) | 169.6 ± 24.0 |
| Diastolic blood pressure (mm Hg) | 95.5 ± 15.5 |
| Hypertension 2 | 354 (97.0) |
| Spot Urine | |
| Sodium concentration (mmol/L) | 130.4 ± 55.0 |
| Potassium concentration (mmol/L) | 44.9 ± 27.1 |
| Creatinine concentration (mg/L) | 1171.9 ± 677.4 |
| 24-h urine | |
| Sodium concentration (mmol/L) | 122.4 ± 47.9 |
| Potassium concentration (mmol/L) | 24.7 ± 12.4 |
| Creatinine concentration (mg/L) | 730.5 ± 312.5 |
| 24-h urine volume (mL) | 1419.3 ± 547.3 |
| The duration of the 24-h urine collection (hour) | 23.7 ± 0.5 |
| Variables | Measured 24-h Urinary Sodium Excretion | Estimated 24-h Urinary Sodium Excretion | ||
|---|---|---|---|---|
| Kawasaki Method | INTERSALT Method | Tanaka Method | ||
| Sodium excretion (mmol/day) | 162.02 ± 70.35 | 193.92 ± 70.66 | 129.97 ± 41.49 | 378.74 ± 109.90 |
| Range of sodium excretion (mmol/day) | 22.08–415.80 | 47.96–502.91 | 12.69–229.39 | 126.45–825.49 |
| ICC (95% Cl) 1 | Reference | 0.38 (0.29, 0.47) | 0.31 (0.21, 0.40) | 0.34 (0.25, 0.43) |
| Correlation coefficient 2 | Reference | 0.38 | 0.35 | 0.37 |
| Bias (mg/day, 95% Cl) 3 | Reference | 31.90 (23.84,39.97) | −32.04 (−39.04,−25.04) | 216.72 (205.81,227.65) |
| Variables | Na/K Ratio in 24-h Urine | Na/K Ratio in Spot Urine |
|---|---|---|
| Mean ± SD 1 | 5.73 ± 2.79 | 3.88 ± 2.62 |
| ICC (95%Cl) 2 | Reference | 0.52 (0.44,0.59) |
| Correlation coefficient 3 | Reference | 0.52 |
| Bias( 95%Cl) 4 | Reference | −1.85 (−2.12,−1.58) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Yin, X.; Zhang, R.; Liu, F.; Yang, D.; Fan, Y.; Rong, J.; Tian, M.; Yu, Y. Validation and Assessment of Three Methods to Estimate 24-h Urinary Sodium Excretion from Spot Urine Samples in High-Risk Elder Patients of Stroke from the Rural Areas of Shaanxi Province. Int. J. Environ. Res. Public Health 2017, 14, 1211. https://doi.org/10.3390/ijerph14101211
Ma W, Yin X, Zhang R, Liu F, Yang D, Fan Y, Rong J, Tian M, Yu Y. Validation and Assessment of Three Methods to Estimate 24-h Urinary Sodium Excretion from Spot Urine Samples in High-Risk Elder Patients of Stroke from the Rural Areas of Shaanxi Province. International Journal of Environmental Research and Public Health. 2017; 14(10):1211. https://doi.org/10.3390/ijerph14101211
Chicago/Turabian StyleMa, Wenxia, Xuejun Yin, Ruijuan Zhang, Furong Liu, Danrong Yang, Yameng Fan, Jie Rong, Maoyi Tian, and Yan Yu. 2017. "Validation and Assessment of Three Methods to Estimate 24-h Urinary Sodium Excretion from Spot Urine Samples in High-Risk Elder Patients of Stroke from the Rural Areas of Shaanxi Province" International Journal of Environmental Research and Public Health 14, no. 10: 1211. https://doi.org/10.3390/ijerph14101211




