Chemical Characterization of the Indoor Air Quality of a University Hospital: Penetration of Outdoor Air Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source Characterization
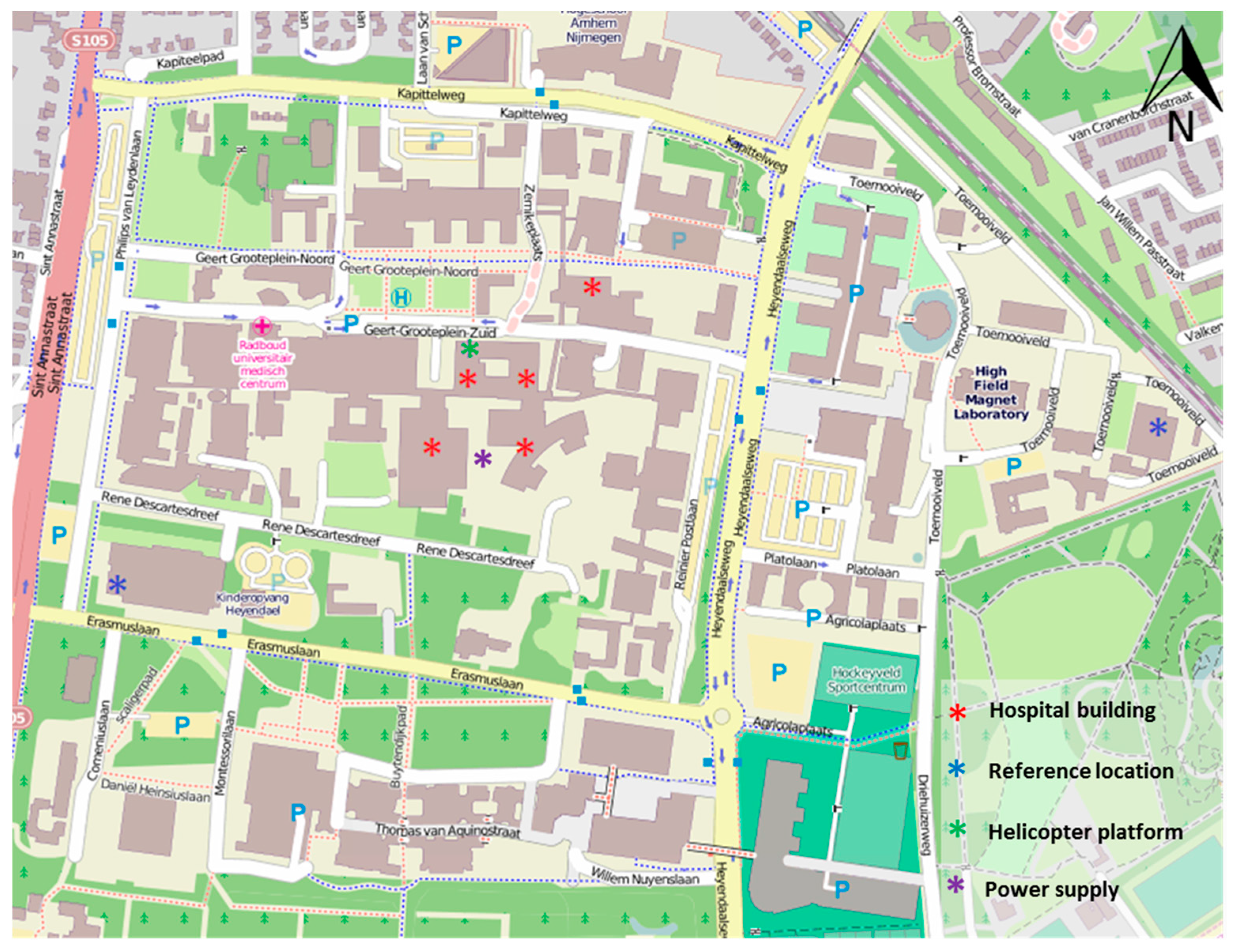
2.2. Selection of Study Locations and Air Quality Parameters
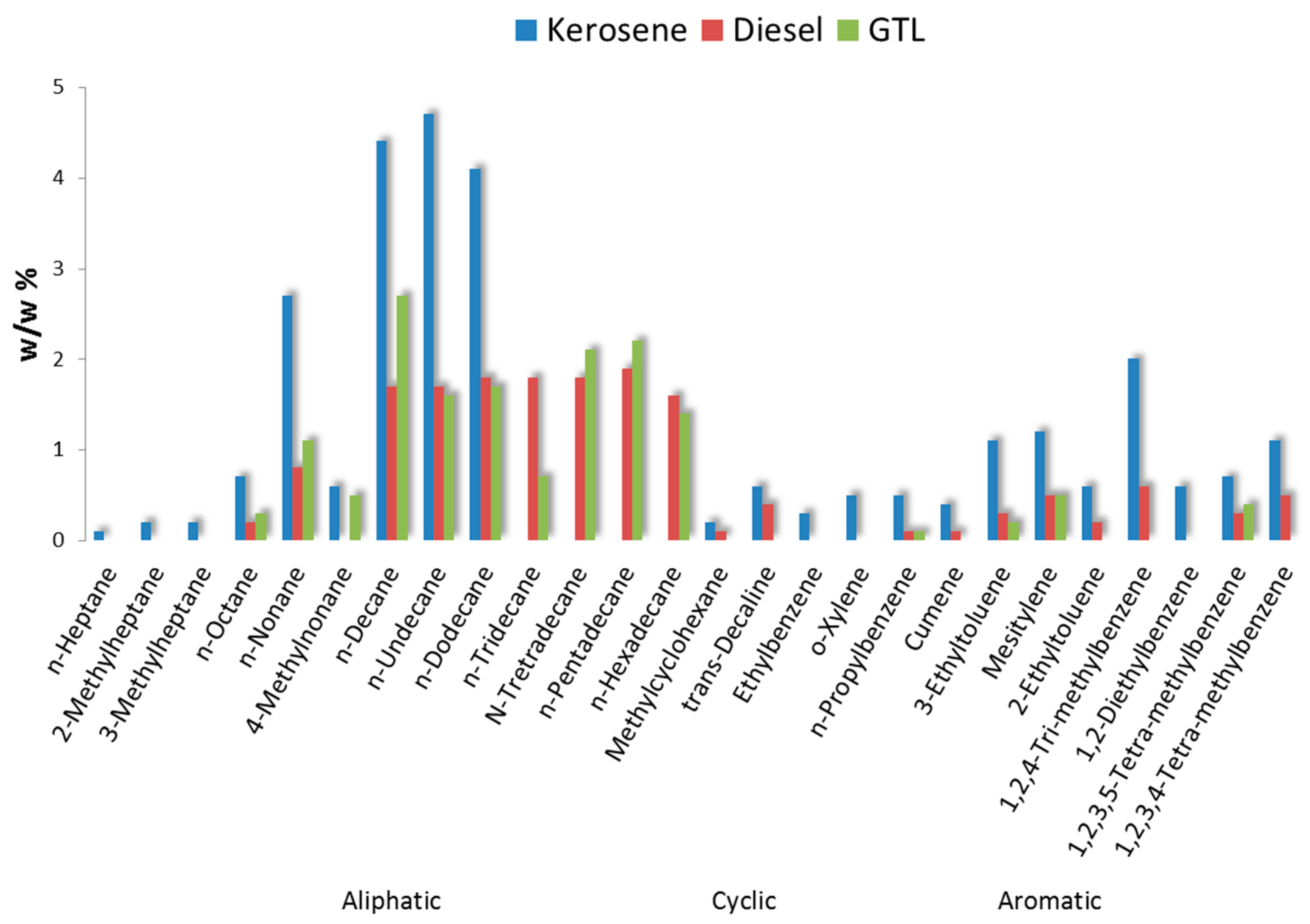
2.3. Fuel Composition
2.4. Gas Phase Air Pollutants
2.5. Particulate Matter and B(a)P Analysis
2.6. Soot Deposition
2.7. Canister Air Sampling
2.8. Calculations and Statistical Testing
3. Results
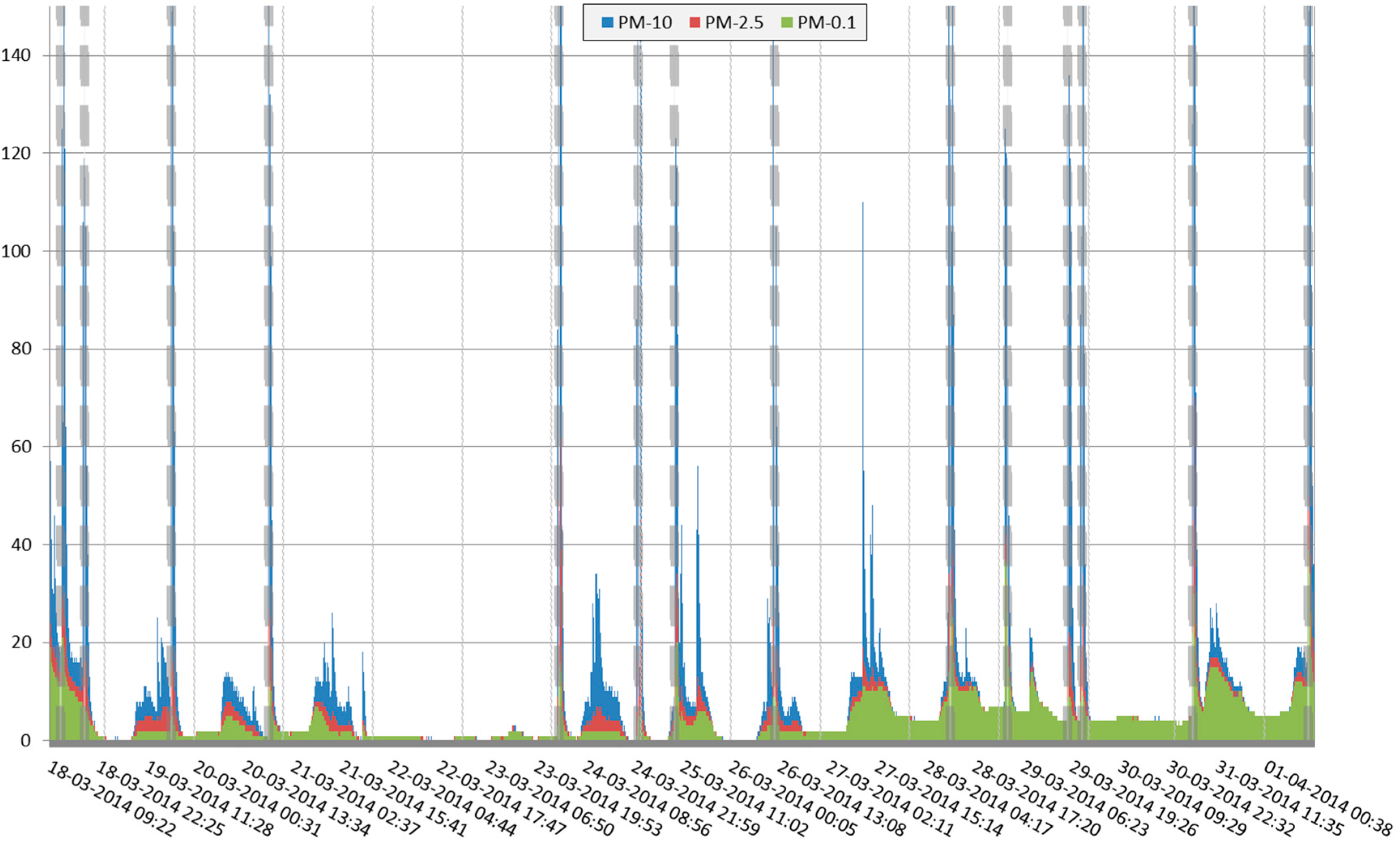
3.1. Study Period and Weather Conditions
3.2. Source Characteristics
3.3. Indoor and Outdoor Air Quality at the Helicopter Platform and at Reference Locations
3.4. Air Quality at the Indoor Hospital Building Locations
3.5. Impact of Power Supply Test Runs
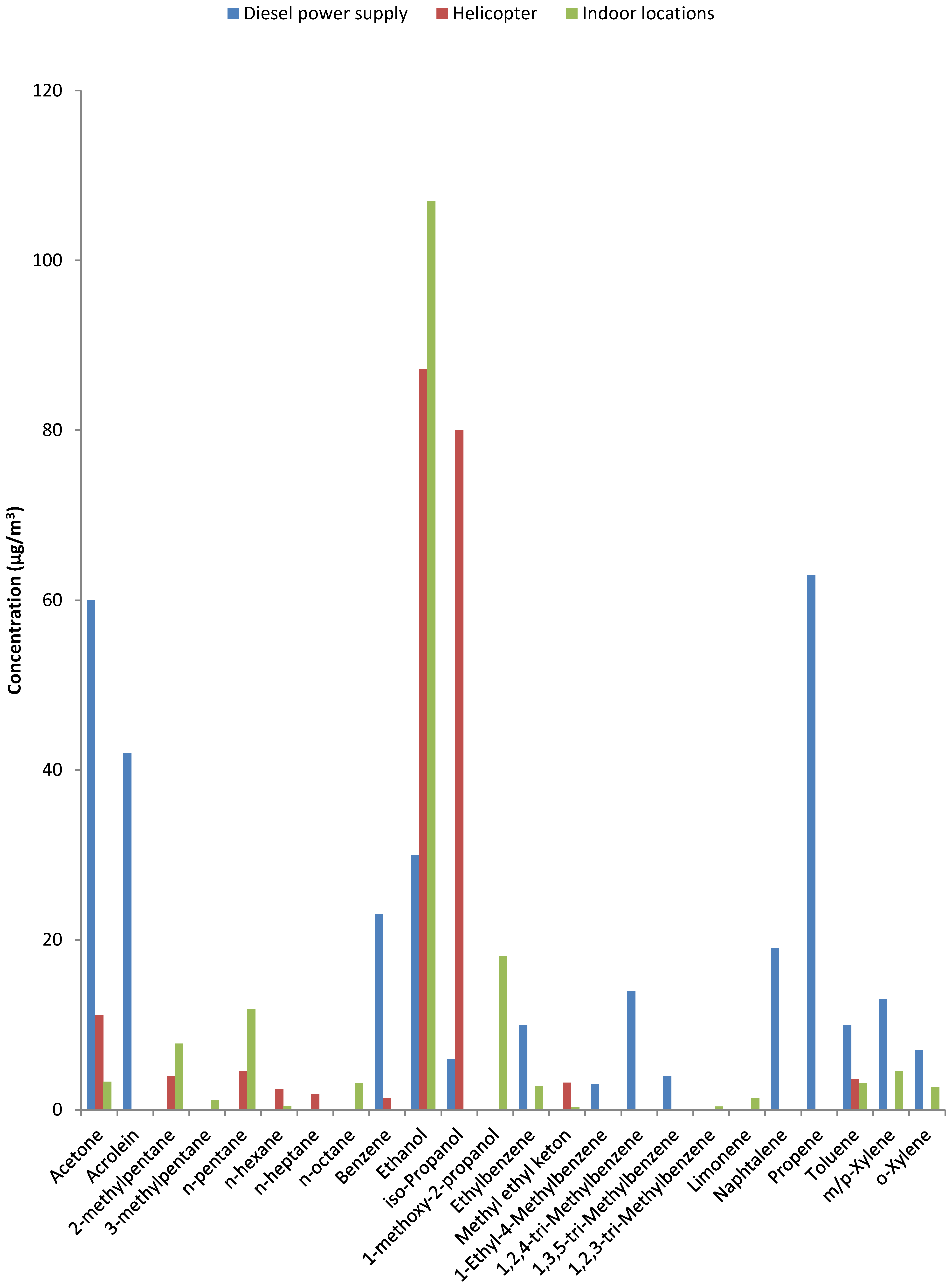
3.6. Indoor VOC Profile Compared to Source VOC Profile
- Chemical substances not normally used in professional healthcare or consumer products but not confirmed and prominent constituents of diesel exhaust. These include acrolein, benzene and naphthalene and were not observed at indoor locations in the hospital nor at the reference locations. The only location that was positive for benzene was the service building near the helicopter platform (cf. Table S5).
- The largest group consists of substances that are not exclusive to either combustion or indoor sources [3]: acetone, ethanol and isopropanol are prominent in combustion emissions and also used in large quantities to control infections. Much less abundant were n-alkanes, methylated alkanes and mono-, di- and tri-methyl-benzenes. This group includes constituents confirmed as prominent components in fossil fuels (see Figure 2). Their presence could lead to attribution of an odor as ‘diesel’ or ‘kerosene’ and originate from penetration of fuel or combustion components [19]. However, most of these constituents are not exclusive to combustion sources and have also been identified in products used in a laboratory environment (e.g., xylenes in pathology).
- A group consisting of a few chemical substances that are unlikely components of combustion emissions: limonene was observed in the hospital and also at the reference locations but not in the source samples and 1-methoxy-2-propanol was observed in the two ORs (Table S4). Limonene and 1-methoxy-2-propanol are both used in cleaning and cosmetic products.
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Morey, P.E.; Woods, J.E. Indoor air quality in health care facilities. Occup. Med. 1987, 2, 547–563. [Google Scholar] [PubMed]
- Mazzola, P.G.; Penna, T.C.; Martins, A.M. Determination of decimal reduction time (D value) of chemical agents used in hospitals for disinfection purposes. BMC Infect. Dis. 2003, 17, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, P.T.J.; Van Hout, S. Health-Related Indicators of Indoor Air Quality. In Environmental Indicators; Armon, R., Hänninen, O., Eds.; Springer: Berlin, Germany, 2015; pp. 925–944. [Google Scholar]
- El-Sharkawy, M.F.; Noweir, M.E. Indoor air quality levels in a University Hospital in the Eastern Province of Saudi Arabia. J. Fam. Community Med. 2014, 21, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.S.; Bronzaft, A.L.; Heikkinen, M.; Goodman, J.; Nádas, A. Airport-related air pollution and noise. J. Occup. Environ. Hyg. 2008, 5, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Lü, H.; Wen, S.; Feng, Y.; Wang, X.; Bi, X.; Sheng, G.; Fu, J. Indoor and outdoor carbonyl compounds and BTEX in the hospitals of Guangzhou, China. Sci. Total Environ. 2006, 368, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Nordström, K.; Norbäck, D.; Wieslander, G. Subjective indoor air quality in geriatric hospitals. Indoor Built Environ. 1999, 8, 49–57. [Google Scholar] [CrossRef]
- Hellgren, U.M.; Hyvärinen, M.; Holopainen, R.; Reijula, K. Perceived indoor air quality, air-related symptoms and ventilation in Finnish hospitals. Int. J. Occup. Med. Environ. Health 2011, 24, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, U.M.; Reijula, K. Indoor air problems in hospitals: A challenge for occupational health. AAOHNJ 2011, 59, 111–117. [Google Scholar]
- Scheepers, P.T.J.; Beckmann, G.; Van Hout, S.; Van Der Kroon, E.; Leenders, P. Determination of the filter capture efficiency for safe use of fixative solutions in the biological glove box of the International Space Station. Environ. Technol. Innov. 2015, 3, 86–96. [Google Scholar] [CrossRef]
- Busbin, D.D.; Feigley, C.E.; Underhill, D.W.; Salzberg, D. A Second look at the Palmes’ diffusive sampler. J. Air Waste Manag. Assoc. 2006, 56, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, P.T.J.; Cremers, R.; Van Hout, S.P.R.; Anzion, R.B.M. Influence of a portable air treatment unit on health-related quality indicators of indoor air in a classroom. J. Environ. Monit. 2011, 14, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, P.T.J.; De Hartog, J.J.; Reijnaerts, J.; Beckmann, G.; Anzion, R.; Poels, K.; Godderis, L. Influence of combined dust reducing carpet and compact air filtration unit on the indoor air quality of a classroom. Environ. Sci. Process. Impacts 2015, 17, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Biesterbos, J.W.H.; Beckmann, G.; Anzion, R.B.M.; Ragas, A.M.J.; Russel, F.G.M.; Scheepers, P.T.J. A sensitive method for quantification of octamethylcyclotetrasiloxane (D4) and decamethylcyclopentasi-loxane (D5) in end-exhaled air by thermal desorption gas chromatography mass spectrometry. Anal. Chem. 2014, 86, 5794–5799. [Google Scholar] [CrossRef] [PubMed]
- Wolkoff, P.; Crump, D.R.; Harrison, P.T. Pollutant exposures and health symptoms in aircrew and office workers: Is there a link? Environ. Int. 2016, 87, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Geiss, O.; Giannopoulos, G.; Tirendi, S.; Barrero-Moreno, J.; Larsen, B.R.; Kotzias, D. The AIRMEX Study—VOC Measurements in public buildings and schools/kindergartens in eleven European cities: Statistical analysis of the data. Atmos. Environ. 2011, 45, 3676–3684. [Google Scholar] [CrossRef]
- Arbeitsgemeinschaft Ökologischer Forschungsinstitute (AGÖF). Orientierungswerte für Flüchtige Organische Verbindungen in der Raumluft. Available online: http://www.agoef.de/orientierungswerte/agoef-voc-orientierungswerte.html (accessed on 21 February 2016).
- Scheepers, P.T.J.; Bos, R.P. Combustion of diesel fuel from a toxicological perspective. Int. Arch. Occup. Environ. Health 1992, 64, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Touri, L.; Marchetti, H.; Sari-Minodier, I.; Molinari, N.; Chanez, P. The airport atmospheric environment: Respiratory health at work. Eur. Respir. Rev. 2013, 128, 124–130. [Google Scholar] [CrossRef] [PubMed]
- LeBouf, R.F.; Virji, M.A.; Saito, R.; Henneberger, P.K.; Simcox, N.; Stefaniak, A.B. Exposure to volatile organic compounds in healthcare settings. Occup. Environ. Med. 2014, 71, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, T.; Horike, T.; Ohashi, Y.; Kataoka, H.; Wang, D.H.; Kira, S. Were volatile organic compounds the inducing factors for subjective symptoms of employees working in newly constructed hospitals? Environ. Toxicol. 2004, 19, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Bessonneau, V.; Mosqueron, L.; Berrubé, A.; Mukensturm, G.; Buffet-Bataillon, S.; Gangneux, J.P.; Thomas, O. VOC contamination in hospital, from stationary sampling of a large panel of compounds, in view of healthcare workers and patients exposure assessment. PLoS ONE 2013, 8, e55535. [Google Scholar] [CrossRef] [PubMed]
- Ghasemkhani, M.; Jahanpeyma, F.; Azam, K. Formaldehyde exposure in some educational hospitals of Teheran. Ind. Health 2005, 43, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Koda, S.; Kumagai, S.; Ohara, H. Environmental monitoring and assessment of short-term exposures to hazardous chemicals of a sterilization process in hospital working environments. Acta Med. Okayama 1999, 53, 217–223. [Google Scholar] [PubMed]
- Kabir, E.; Kim, K.H.; Sohn, J.R.; Kweon, B.Y.; Shin, H. Indoor air quality assessment in child care and medical facilities in Korea. Environ. Monit. Assess. 2012, 184, 6395–6409. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, T.W.; Lapin, C.A.; Bunn, W.B. A comparison of emissions from vehicles fueled with diesel or compressed natural gas. Environ. Sci. Technol. 2008, 42, 6437–6445. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Pottage, T.; Bennett, A.; Walker, J. Gaseous and air decontamination technologies for Clostridium difficile in the healthcare environment. J. Hosp. Infect. 2011, 77, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Nerandzic, M.M.; Thota, P.; Sankar, C.T.; Jencson, A.; Cadnum, J.L.; Ray, A.J.; Salata, R.A.; Watkins, R.R.; Donskey, C.J. Evaluation of a pulsed xenon ultraviolet disinfection system for reduction of healthcare-associated pathogens in hospital rooms. Infect. Control Hosp. Epidemiol. 2015, 36, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Benbrahim-Tallaa, L.; Baan, R.A.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Guha, N.; Loomis, D.; Straif, K. Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet Oncol. 2012, 13, 663–664. [Google Scholar] [CrossRef]
- Leung, M.; Chan, A.H. Control and management of hospital indoor air quality. Med. Sci. Monit. 2006, 12, 17–23. [Google Scholar]
- Quinn, M.M.; Fuller, T.P.; Bello, A.; Galligan, C.J. Pollution prevention—Occupational safety and health in hospitals: Alternatives and interventions. J. Occup. Environ. Hyg. 2006, 3, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Lee, K.; Pai, S.; Ledoux, W.R. Histomorphometric comparison after fixation with formaldehyde or glyoxal. Biotech. Histochem. 2011, 86, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Aydin, I.; Yörükoglu, K.; Cingöz, S.; Agilkaya, S. The effect of the alternative solutions to formaldehyde and xylene on tissue processing. Indian J. Pathol. Microbiol. 2013, 56, 221–230. [Google Scholar] [PubMed]
- Ho, S.S.H.; Ho, F.; Liu, W.D.; Lee, S.C.; Dai, W.T.; Cao, J.J.; Ip, H.S.S. Unsuitability of using the DNPH-coated sorbent cartridge for determination of airborne unsaturated carbonyls. Atmos. Environ. 2011, 45, 261–265. [Google Scholar] [CrossRef]

| Substance | Origin | Air Sampling Method | LOD | WHO a | The Netherlands a | EU a | WHO a | The Netherlands a |
|---|---|---|---|---|---|---|---|---|
| Classification Cancer | Public Standard Outdoor Air | EU Standard Outdoor Air | IAQ Guidance Value Indoor Air | Public Standard Workplace | ||||
| Acrolein | Oxidation | Silicagel with DNPH reagent | 0.001 e | Group 3 | 0.5–0.01 | − | − | 230 (15-min) |
| Benzene | Pyrosynthesis | Activated charcoal | 0.001 f | Group 1 | 10–1 | 5 | 17 c; 0.17 d | 3.250 (8-h) |
| Benzo(a)pyrene | Pyrosynthesis | Membrane filter | 0.000010 | Group 1 | 0.001–0.00001 | - | 0.0012 c; 0.000012 d | 550 (8-h) |
| Cumene | Fuel | Activated charcoal | 0.001 f | Group 2B | - | - | - | 100.000 (8-h) 250.000 (15-h) |
| Formaldehyde | Oxidation | Silicagel with DNPH reagent | 0.001 | Group 1 | 10–1 | - | 100 (0.5-h average) | 150 (8-h) 500 (15-min) |
| Napthalalene | Fuel/pyrosynthesis | Activated charcoal | 0.001 f | Group 3 | 10 | 10 (year average) | - | 50.000 (8-h) 80.000 (15-min) |
| Respirable dust (PM-2.5) | Fuel/pyrosynthesis | Membrane filter | 1.0 | Group 1 | − | 25 b (year average) | - | - |
| Nitrogen dioxide | Oxidation | Palmes tubes | 1.0 | - | 40–4 | 40 (year average) 200 (1-h average) | - | 400 (8-h) 1.000 (15-min) |
| Toluene | Fuel | Activated charcoal | 0.001 f | - | 400–4 | - | - | 150.000 (8-h) 384.000 (15-min) |
| Xylene | Fuel | Activated charcoal | 0.001 f | - | 870 | - | - | 210.000 (8-h) 442.000 (15-min) |
| Sampling Location a | PM-2.5 (μg/m3) | VOC (μg/m3) | Formaldehyde (μg/m3) | |||
|---|---|---|---|---|---|---|
| Helicopter | Diesel-Fueled Power Supply | Helicopter | Diesel-Fueled Power Supply b | Helicopter | Diesel-Fueled Power Supply | |
| Reference | 29 | 13.7 | <0.10 | 9.7 b | 4.5 | 26.5 |
| A | 86 | 67.2 | <0.21 | 154 c | 85 | 81.6 d |
| B | 66 | 1141 | <0.16 | 28.5 c | 66 | 135 |
| C | 36 | 2586 | <0.16 | 29.3 c | 36 | 71.2 |
| Substance | Week No. | Helicopter Platform | Dentistry Building | Kindergarten | |||
|---|---|---|---|---|---|---|---|
| Type | Indoor | Outdoor | Indoor | Outdoor | Indoor | Outdoor | |
| Description | Seventh Floor | Under Platform | Front Desk | Court Yard | Office FirstFloor | Terrace First Floor | |
| Acrolein | 1 | 0.17 | 0.14 | 0.24 | <0.001 | 0.17 | - a |
| 2 | 0.15 | 0.08 | 0.32 | 0.09 | 0.19 | - a | |
| Formaldehyde | 1 | 9.7 | 2.6 | 7.7 | 2.0 | 8.3 | - a |
| 2 | 9.9 | 2.9 | 10.2 | 2.6 | 8.4 | - a | |
| Nitrogen dioxide | 1 + 2 | 11.8 | 15.5 | 15.3 | 17.4 | 8.63 | 15.6 |
| TVOC | 1 | 159 | 12.8 | 311 | 1.6 | 312 | <0.1 |
| 2 | 166 | 11.2 | 281 | <0.1 | 21.5 | <0.1 | |
| PM-4.0 | 1 | 6.4 | 3.7 | 8.4 | 13.3 | 6.4 | 5.9 |
| 2 | 5.4 | 28.3 | 12.4 | 25.3 | 14.2 | 5.6 | |
| PM-2.5 | 1 | - | - | 4.6 | 11.1 | - | - |
| 2 | - | - | 9.8 | 25.7 | - | - | |
| Benz(a)pyrene b | 1 | 2.3 × 10−5 | 2.5 × 10−5 | 7.3 × 10−5 | 7.7 × 10−5 | 3.0 × 10−5 | 1.9 × 10−5 |
| 2 | 5.5 × 10−5 | 1.5 × 10−4 | 1.1 × 10−4 | 1.4 × 10−4 | 1.2 × 10−4 | 2.7 × 10−5 | |
| Substance | Week No. | High Efficiency PM-Filters a (n = 5) | Standard PM-Filters b (n = 6) | p-Value | ||
|---|---|---|---|---|---|---|
| Median | Range | Median | Range | |||
| Acrolein | 1 | 0.09 | <0.001–0.13 | 0.12 | <0.001–0.14 | 0.41 |
| 2 | 0.09 | <0.001–0.13 | 0.11 | 0.10–0.19 | 0.09 | |
| Formaldehyde | 1 | 2.9 | 2.7–4.7 | 3.65 | 3.1–15.5 | 0.31 |
| 2 | 3.5 | 3.4–5.2 | 4.05 | 2.2–21.7 | 0.42 | |
| NO2 | 1 + 2 | 16.20 | 4.9–17.0 | 16.55 | 13.6–19.6 | 0.13 |
| TVOC | 1 | 0.15 | 50.9–2418 | 0.34 | 56.2–2928 | 0.64 |
| 2 | 0.30 | 33.1–2449 | 0.46 | 58.0–1142 | 0.61 | |
| PM-4.0 | 1 | 0.50 | <0.01–2.7 | 3.05 | 1.5–4.4 | <0.05 |
| 2 | 0.05 | <0.01–1.0 | 6.90 | 3.9–9.4 | <0.01 | |
| Benz(a)pyrene | 1 | <0.3 × 10−6 | <0.3 × 10−6 | 3.5 × 10−5 | 2.4–6.1 × 10−5 | <0.01 × 10−6 |
| 2 | <0.3 × 10−6 | <0.3 × 10−6 | 5.7 × 10−5 | 3.7–6.8 × 10−5 | <0.01 × 10−6 | |
| Substance | Outdoor Air | Indoor Air | |
|---|---|---|---|
| Helicopter (n = 3) a | Power Supply (n = 3) a | Location of Complaint (n = 2) b | |
| Acetone | 6.9 ± 0.9 | <0.1 | 9.18 ± 2.7 |
| Benzene | <0.1 | 10.2 ± 5.2 | <0.1 |
| Ethanol | 8.3 ± 2.0 | <0.1 | 73.05 ± 51.3 |
| Ethylbenzene | <0.1 | 4.4 ± 1.0 | 0.35 ± 0.30 |
| Isopropanol | 75.6 ± 17.0 | <0.1 | 73.73 ± 42.8 |
| Toluene | 0.2 ± 0.01 | 39.0 ± 12.2 | 0.20 ± 0.01 |
| m-Xylene | <0.1 | 5.7 ± 1.1 | 0.43 ± 0.38 |
| p-Xylene | <0.1 | 0.43 ± 0.38 | |
| o-Xylene | <0.1 | 6.2 ± 2.0 | 0.43 ± 0.38 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheepers, P.T.J.; Van Wel, L.; Beckmann, G.; Anzion, R.B.M. Chemical Characterization of the Indoor Air Quality of a University Hospital: Penetration of Outdoor Air Pollutants. Int. J. Environ. Res. Public Health 2017, 14, 497. https://doi.org/10.3390/ijerph14050497
Scheepers PTJ, Van Wel L, Beckmann G, Anzion RBM. Chemical Characterization of the Indoor Air Quality of a University Hospital: Penetration of Outdoor Air Pollutants. International Journal of Environmental Research and Public Health. 2017; 14(5):497. https://doi.org/10.3390/ijerph14050497
Chicago/Turabian StyleScheepers, Paul T. J., Luuk Van Wel, Gwendolyn Beckmann, and Rob B. M. Anzion. 2017. "Chemical Characterization of the Indoor Air Quality of a University Hospital: Penetration of Outdoor Air Pollutants" International Journal of Environmental Research and Public Health 14, no. 5: 497. https://doi.org/10.3390/ijerph14050497
APA StyleScheepers, P. T. J., Van Wel, L., Beckmann, G., & Anzion, R. B. M. (2017). Chemical Characterization of the Indoor Air Quality of a University Hospital: Penetration of Outdoor Air Pollutants. International Journal of Environmental Research and Public Health, 14(5), 497. https://doi.org/10.3390/ijerph14050497





