Evaluation of Drinking Water Disinfectant Byproducts Compliance Data as an Indirect Measure for Short-Term Exposure in Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Community Water System
2.2. Sampling Plan and Laboratory Analysis
2.3. Collection of Compliance Data
2.4. Data Analyses
3. Results
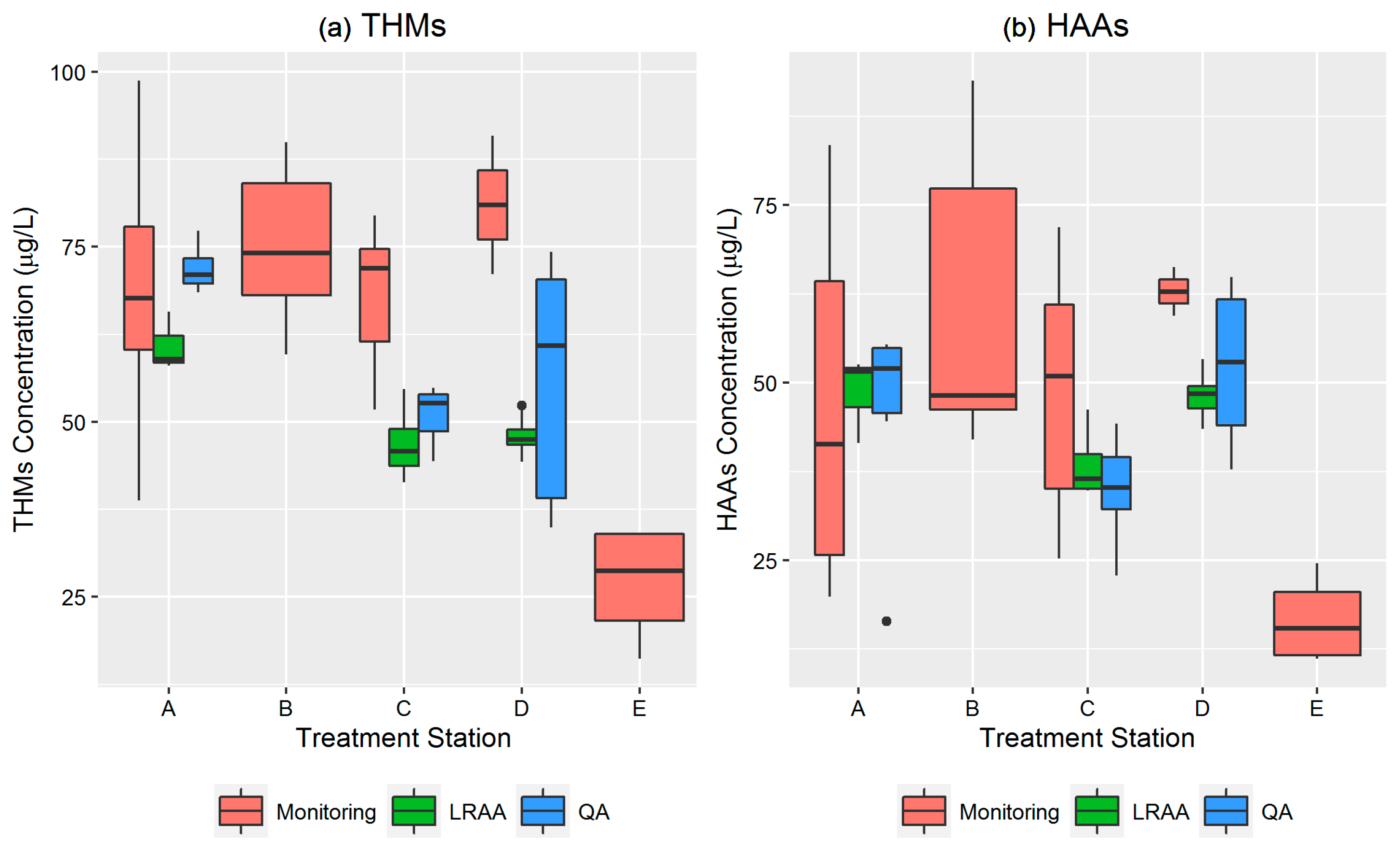
3.1. Summary of the Residential Monitoring Data
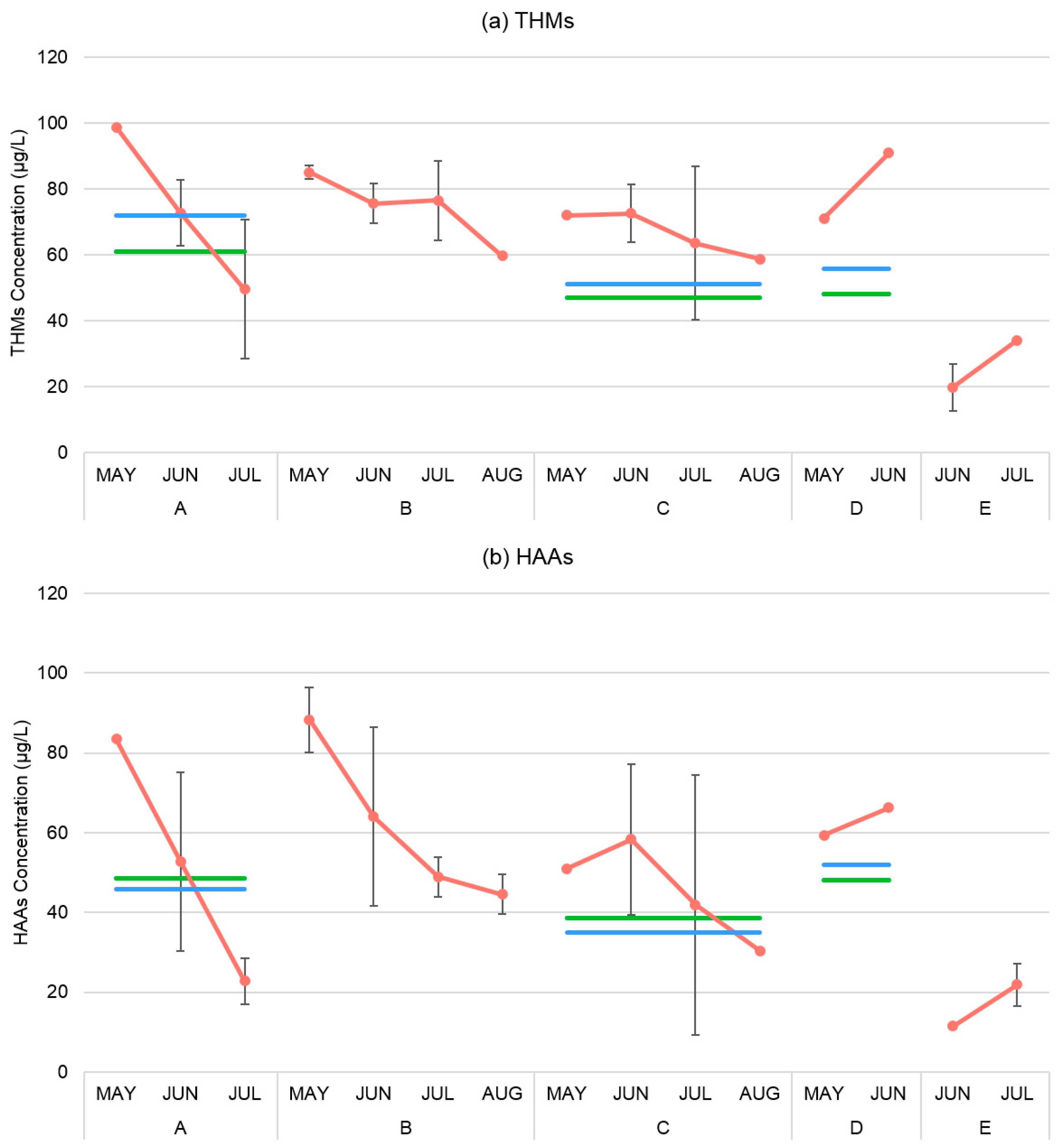
3.2. Temporal and Spatial Assessment of Total THMs and HAAs
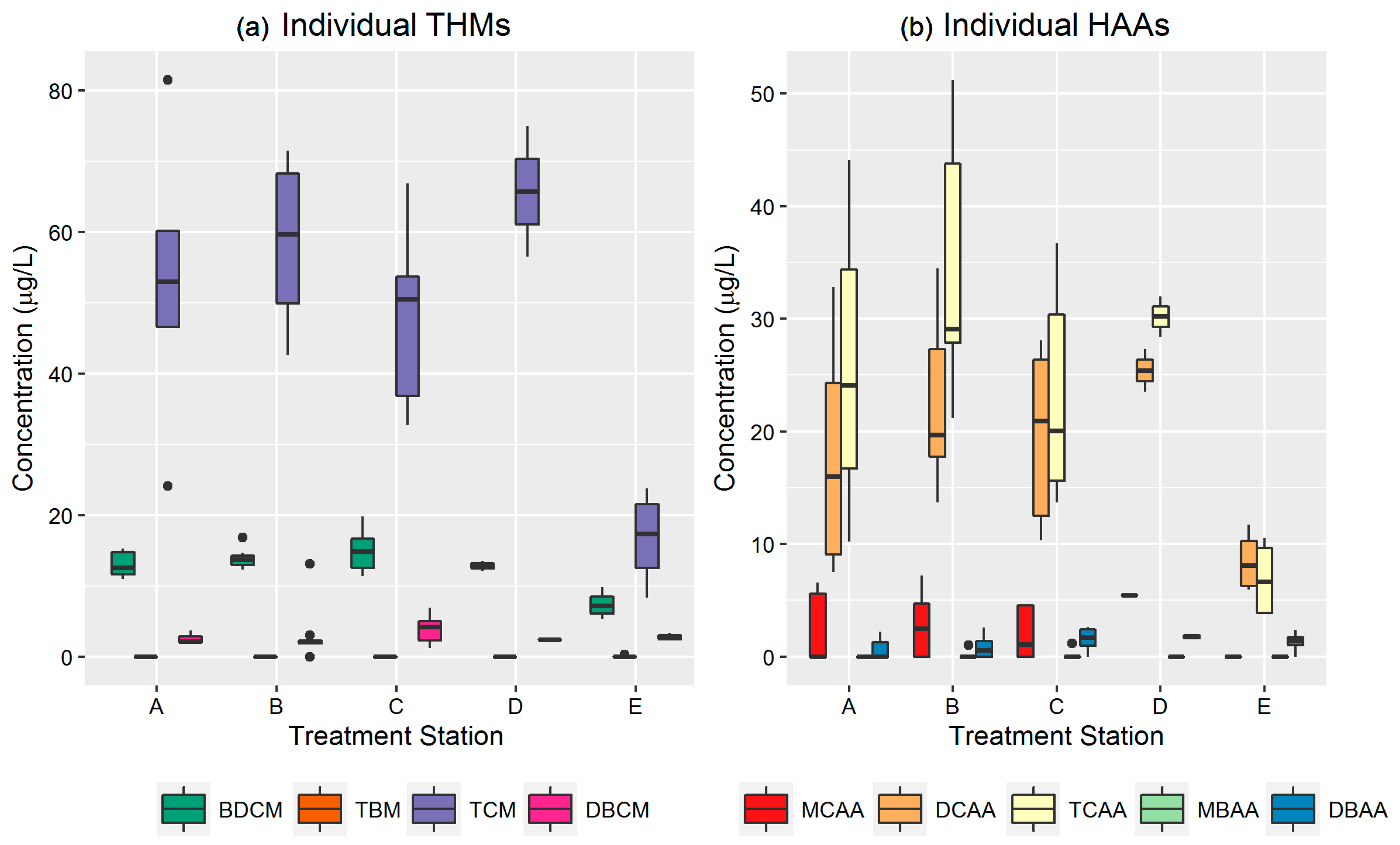
3.3. Temporal and Spatial Assessment of Individual THMs and HAAs
4. Discussion
4.1. Limitations of the Study
4.2. Strengths of the Study
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LRAA | Locational running annual average |
| QA | Quarterly running average |
| DBPs | Disinfectant Byproducts |
| THMs | Trihalomethanes |
| TOC | Total organic carbon |
| HAAs | Haloacetic acids |
| MCL | Maximum concentration limit |
| IDSE | Initial distribution system evaluation |
| BDCM | Bromodichloromethane |
| TBM | Bromoform |
| TCM | Chloroform |
| DBCM | Dibromochloromethane |
| MCAA | Monochloroacetic acid |
| DCAA | Dichloroacetic acid |
| TCAA | Trichloroacetic acid |
| MBAA | Monobromoacetic acid |
| DBAA | Dibromoacetic acid |
| HCl | Hydrochloric acid |
| SGA | Small for gestational age |
| PTB | Preterm birth |
| FGR | Fetal growth restriction |
| LBW | Low birthweight |
| IUGR | Intrauterine growth retardation |
| GM | Geometric Mean |
| SD | Standard deviation |
| CV | Coefficient of variation |
| CI | Confidence interval |
References
- CDC (Centers for Disease Control and Prevention). Achievements in Public Health. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4829a1.htm (accessed on 21 May 2016).
- Richardson, S.D. Encyclopedia of Environmental Analysis and Remediation; Meyers, R., Ed.; Wiley: New York, NY, USA, 1998; Volume 3, pp. 1398–1421. [Google Scholar]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; Demarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Pressman, J.G.; Richardson, S.D.; Speth, T.F.; Miltner, R.J.; Narotsky, M.G.; Hunter, S.E.; Rice, G.E.; Teuschler, L.K.; Mcdonald, A.; Parvez, S.; et al. Concentration, chlorination, and chemical analysis of drinking water for disinfection byproduct mixtures health effects research. Environ. Sci. Technol. 2010, 44, 7184–7192. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.C. Control of disinfection by-products in drinking water. J. Environ. Eng. 1994, 120, 727–744. [Google Scholar] [CrossRef]
- EPA (U.S. Environmental Protection Agency). Stage 1 and Stage 2 Disinfectants and Disinfection Byproducts Rules; EPA: Washington, DC, USA, 2016.
- Villanueva, C.M.; Cantor, K.P.; Grimalt, J.O.; Malats, N.; Silverman, D.; Tardon, A.; Garcia-Closas, R.; Serra, C.; Carrato, A.; Castaño Vinyals, G.; et al. Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am. J. Epidemiol. 2006, 165, 148–156. [Google Scholar] [CrossRef] [PubMed]
- King, W.D.; Marrett, L.D.; Woolcott, C.G. Case-control study of colon and rectal cancers and chlorination by-products in treated water. Cancer Epidemiol. Biomark. Prev. 2009, 9, 813–818. [Google Scholar]
- Doyle, T.J.; Zheng, W.; Cerhan, J.R.; Hong, C.P.; Sellers, T.A.; Kushi, L.H.; Folsom, A.R. The association of drinking water source and chlorination by-products with cancer incidence among postmenopausal women in Iowa prospective cohort study. Am. J. Public Health 1997, 87, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Jeng, W.L.; Chang, R.M.; Chien, L.C.; Han, B.C. Estimation of potential lifetime cancer risks for trihalomethanes from consuming chlorinated drinking water in Taiwan. Environ. Res. 2001, 85, 77–82. [Google Scholar] [CrossRef] [PubMed]
- EPA (U.S. Environmental Protection Agency). National Primary Drinking Water Regulations: Stage 2 Disinfectants and Disinfection Byproducts Rule. Available online: https://www.epa.gov/dwreginfo/stage-1-and-stage-2-disinfectants-and-disinfection-byproducts-rules (accessed on 20 May 2017).
- Symanski, E.; Savitz, D.A.; Singer, P.C. Assessing spatial fluctuations, temporal variability, and measurement error in estimated levels of disinfection by-products in tap water: Implications for exposure assessment. Occup. Environ. Med. 2004, 61, 65–72. [Google Scholar] [PubMed]
- Waller, K.; Swan, S.H.; Windham, S.C.; Fenster, L. Influence of exposure assessment methods on risk estimates in an epidemiologic study of total trihalomethane exposure and spontaneous abortion. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Rivera-Nunez, Z.; Meyer, A.; Wright, J.M. Temporal variability in trihalomethane and haloacetic acid concentrations in Massachusetts public drinking water systems. Environ. Res. 2011, 111, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Cedergren, M. Chlorination byproducts and nitrate in drinking water and risk for congenital cardiac defects. Environ. Res. 2002, 89, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.M.; Schwartz, J.; Dockery, D.W. The effect of disinfection by-products and mutagenic activity on birth weight and gestational duration. Environ. Health Perspect. 2004, 112, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Nunez, Z.; Wright, J.M. Association of brominated trihalomethane and haloacetic acid exposure with fetal growth and preterm delivery in Massachusetts. J. Occup. Environ. Med. 2013, 55, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Costet, N.; Garlantezec, R.; Monfort, C.; Rouget, F.; Gagniere, B.; Chevrier, C.; Cordier, S. Environmental and urinary markers of prenatal exposure to drinking water disinfection by-products, fetal growth, and duration of gestation in the pelagie birth cohort (Brittany, France, 2002–2006). Am. J. Epidemiol. 2012, 175, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Levallois, P.; Gingras, S.; Marcoux, S.; Legay, C.; Catto, C.; Rodriguez, M.; Tardif, R. Maternal exposure to drinking-water chlorination by-products and small-for-gestational-age neonates. Epidemiology 2012, 23, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, C.S. Exposure to Drinking Water Disinfection By-Products and Pregnancy Health: Impacts on Fetal Growth and Duration of Gestation; University of North Carolina at Chapel Hill: Chapel Hill, NC, USA, 2007. [Google Scholar]
- Hoffman, C.S.; Mendola, P.; Savitz, D.A.; Herring, A.H.; Loomis, D.; Hartmann, K.E.; Singer, P.C.; Weinberg, H.S.; Olshan, A.F. Drinking water disinfection by-product exposure and fetal growth. Epidemiology 2008, 19, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Hinckley, A.F.; Bachand, A.M.; Reif, J.S. Late pregnancy exposures to disinfection by-products and growth-related birth outcomes. Environ. Health Perspect. 2005, 113, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Waller, K.; Swan, S.H.; DeLorenze, G.; Hopkins, B. Trihalomethanes in drinking water and spontaneous abortion. J. Epidemiol. 1998, 9, 134–140. [Google Scholar] [CrossRef]
- Horton, B.J.; Luben, T.J.; Herring, A.H.; Savitz, D.A.; Singer, P.C.; Weinberg, H.S.; Hartmann, K.E. The effect of water disinfection by-products on pregnancy outcomes in two southeastern U.S. communities. J. Occup. Environ. Med. 2011, 53, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Dodds, L.; King, W.D. Relation between trihalomethane compounds and birth defects. J. Occup. Environ. Med. 2001, 58, 443–446. [Google Scholar] [CrossRef]
- Savitz, D.A.; Andrews, K.W.; Pastore, L.M. Drinking water and pregnancy outcome in Central North Carolina: Source, amount, and trihalomethane levels. Environ. Health Prospect. 1995, 103, 592–596. [Google Scholar] [CrossRef]
- Savitz, D.A.; Singer, P.C.; Herring, A.H.; Hartmann, K.E.; Weinberg, H.S.; Makarushka, C. Exposure to drinking water disinfection by-products and pregnancy loss. Am. J. Epidemiol. 2006, 164, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Patelarou, E.; Kargaki, S.; Stephanou, E.G.; Nieuwenhuijsen, M.; Sourtzi, P.; Gracia, E.; Chatzi, L.; Koutis, A.; Kogevinas, M. Exposure to brominated trihalomethanes in drinking water and reproductive outcomes. Occup. Environ. Med. 2011, 68, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Grazuleviciene, R.; Nieuwenhuijsen, M.J.; Vencloviene, J.; Kostopoulou-Karadanelli, M.; Krasner, S.W.; Danileviciute, A.; Balcius, G.; Kapustinskiene, V. Individual exposures to drinking water trihalomethanes, low birth weight and small for gestational age risk: A prospective kaunas cohort study. Environ. Health 2011, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.K.; Putnam, S.D.; Hunting, K.L.; Riddle, M.R. The effect of trihalomethane and haloacetic acid exposure on fetal growth in a Maryland county. Am. J. Epidemiol. 2005, 162, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Luben, T.J.; Nuckols, J.R.; Mosley, B.S.; Hobbs, C.; Reif, J.S. Maternal exposure to water disinfection by-products during gestation and risk of hypospadias. Occup. Environ. Med. 2008, 65, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Plewa, M.J.; Kargalioglu, Y.; Vankerk, D.; Minear, R.A.; Wagner, E.D. Mammalian cell cytotoxicity and genotoxicity analysis of drinking water disinfection by-products. Environ. Mol. Mutagen. 2002, 40, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Plewa, M.J.; Wagner, E.D.; Jazwierska, P.; Richardson, S.D.; Chen, P.H.; McKague, A.B. Halonitromethane drinking water disinfection byproducts: Chemical characterization and mammalian cell cytotoxicity and genotoxicity. Environ. Sci. Technol. 2004, 38, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Plewa, M.J.; Muellner, M.G.; Richardson, S.D.; Fasanot, F.; Buettner, K.M.; Woo, Y.; McKague, A.B.; Wagner, E.D. Occurrence, synthesis, and mammalian cell cytotoxicity and genotoxicity of haloacetamides: An emerging class of nitrogenous drinking water disinfection byproducts. Environ. Sci. Technol. 2008, 42, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Plewa, M.J.; Simmons, J.E.; Richardson, S.D.; Wagner, E.D. Mammalian cell cytotoxicity and genotoxicity of the haloacetic acids, a major class of drinking water disinfection by-products. Environ. Mol. Mutagen. 2010, 51, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Wagner, E.D.; Siebert, V.R.; Anduri, S.; Richardson, S.D.; Daiber, E.J.; McKague, A.B.; Kogevinas, M.; Villanueva, C.M.; Goslan, E.H.; et al. Occurrence and toxicity of disinfection byproducts in European drinking waters in relation with the hiwate epidemiology study. Environ. Sci. Technol. 2012, 46, 12120–12128. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.M.; Grayman, W.M. Modeling Water Quality in Drinking Water Distribution Systems; American Water Works Association: Denver, CO, USA, 1998. [Google Scholar]
- American Water Works Association. Water Industry Database: Utility Profiles; American Water Works Association: Denver, CO, USA, 1992. [Google Scholar]
- American Water Works Association. Effects of Water Age on Distribution System Water Quality; American Water Works Association: Denver, CO, USA, 2002; p. 19. [Google Scholar]
- Squillace, P.J.; Pankow, J.F.; Barbash, J.E.; Price, C.V.; Zogorski, J.S. Preserving ground water samples with hydrochloric acid does not result in the formation of chloroform. Ground Water Monit. Remediat. 1999, 19, 67–74. [Google Scholar] [CrossRef]
- EPA (U.S. Environmental Protection Agency). 1995a. Method 551.1: Determination of chlorination disinfection byproducts, chlorinated solvents, and halogenated pesticides/herbicides in drinking water by liquid-liquid extraction and gas chromatography with electron capture detection. In Methods for the Determination of Organic Compounds in Drinking Water, Supplement iii. Report Epa-600/r-95/131; Usepa Office of Research and Development, National Exposure Research Laboratory: Cincinnati, OH, USA, 1992. [Google Scholar]
- EPA (U.S. Environmental Protection Agency). 1995b Method 552.2: Determination of Haloacetic Acids and Dalapon in Drinking Water by Liquid–Liquid Extraction, Derivatization, and Gas Chromatography with Electron Capture Detection; U.S. EPA: Cincinnati, OH, USA, 1995.
- Rodriguez, M.J.; Serodes, J.-B. Spatial and temporal evolution of trihalomethanes in three water distribution systems. Water Res. 2001, 35, 1572–1586. [Google Scholar] [CrossRef]
- Serodes, J.B.; Rodriguez, M.J.; Hanmei, L.; Bouchard, C. Occurence of thms and haas in experimental chlorinated waters of the Quebec City area (Canada). Chemosphere 2003, 51, 253–263. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Serodes, J.-B.; Levallois, P. Behavior of trihalomethanes and haloacetic acids in a drinking water distribution system. Water Res. 2004, 38, 4367–4382. [Google Scholar] [CrossRef] [PubMed]
- Toroz, I.; Uyak, V. Seasonal variations of trihalomethans (thms) in water distribution networks of Istanbul city. Desalination 2004, 176, 127–141. [Google Scholar] [CrossRef]
- Hinckley, A.F.; Bachand, A.M.; Nuckols, J.R.; Reif, J.S. Identifying public water facilities with low spatial variability of disinfection by-products for epidemiological investigations. Occup. Environ. Med. 2005, 62, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; Wright, J.M.; Meyer, A.; Rivera-Nunez, Z. Spatial variation of disinfection by-product concentrations: Exposure assessment implications. Water Res. 2013, 47, 6130–6140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Yang, H.W.; Wang, X.M.; Fu, J.; Xie, Y.F. Formation of disinfection by-products: Effect of temperature and kinetic modeling. Chemosphere 2013, 90, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-S.; Wang, C.-L.; Zhang, J.; Zhu, X.-W.; Li, W.-Y. Combined toxicity of pesticide mixtures on green algae and photobacteria. Ecotoxicol. Environ. Saf. 2013, 95, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Nunez, Z.; Wright, J.M.; Blount, B.C.; Silva, L.K.; Jones, E.; Chan, R.L.; Pegram, R.A.; Singer, P.C.; Savitz, D.A. Comparison of trihalomethanes in tap water and blood: A case study in the United States. Environ. Health Prospect. 2012, 120, 661–667. [Google Scholar] [CrossRef] [PubMed]

| Study | DBPs Type | Birth Outcomes Examined | Findings | Study Location |
|---|---|---|---|---|
| Dodds and King, 2001 [25] | THMs, TCM, BDCM | Neural tube defects, cardiovascular defects, cleft defects, chromosomal abnormalities | No statistically significant association was found with any of the congenital anomalies | Nova Scotia, Canada |
| Cedergren, 2002 [15] | THMs | Cardiac defects | Statistically significant association | Sweden |
| Waller et al., 1998 [23] | THMs | Spontaneous abortion | Only high BDCM exposure (>18 µg/L) was associated with spontaneous abortion | USA |
| Savitz et al., 1995, 2006 [26,27] | THMs | Miscarriage, preterm birth (PTB), low birth weight (LBW) | No statistically significant association | North Carolina, USA |
| Wright et al., 2004 [16] | THMs and HAAs | Mean birth weight (MBW), mean gestational age, small for gestational age (SGA), PTB | Elevated mutagenic activity was associated with SGA (odd ratio = 1.25; 95% confidence interval (CI), 1.04 to 1.51) and MBW (−27 g; 95% CI) | Massachusetts, USA |
| Hoffman et al., 2008 [21] | THMs and HAAs | SGA | Analysis did not show a consistent association | USA |
| Patelarou et al., 2011 [28] | THMs | LBW, SGA, PTB | No significant association was found | Crete |
| Grazuleviciene et al., 2011 [29] | THMs | Congenital anomalies | No significant association was found | Lithuania |
| Rivera-Nunez and Wright, 2013 [17] | THMs, HAAs, brominated THMs (BrTHMs) | Mean birth weight, SGA, PTB | Statistical association was found between BrTHMs and mean birth weight | Massachusetts, USA |
| Costet et al., 2012 [18] | THMs and TCAA | Fetal growth restriction (FGR), PTB | Higher uptake BrTHMs was associated with FGR | France |
| Porter et al., 2005 [30] | THMs and HAAs | Intrauterine growth retardation (IUGR) | No statistically significant association | Maryland, USA |
| Levallois et al., 2012 [19] | THMs and HAAs | SGA | Increased risk was observed | Quebec, Canada |
| Horton et al., 2011 [24] | THMs and HAAs | SGA and PTB | No association was observed | North Carolina, USA |
| Luben et al., 2008 [31] | THMs and HAA | Hypospadias | No association was found | Arkansas, USA |
| Hinckley et al., 2005 [22] | THMs and HAAs | LBW and IUGR | Dibromoacetic acid and dichloroacetic acid show association with LBW | Colorado, USA |
| Hoffman et al., 2007, 2008 [20,21] | THMs and HAAs | SGA | Only THMs were associated SGA | USA |
| Treatment Station | Water Type | pH | % UV Transmittance | TOC mg/L | Temperature Degree F | Chlorine mg/L | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Finished | Raw | Finished | Raw | Finished | Raw | Finished | Raw | Finished | ||
| A | SW | 7.94 | 7.37 | 75.76 | 91.49 | 4.25 | 2.46 | 74.64 | 74.94 | 7.0 | 1.9 |
| B | SW | 8.07 | 7.46 | 81.12 | 90.57 | 3.48 | 2.41 | 71.43 | 75.49 | 5.5 | 1.5 |
| C | SW | 8.11 | 7.55 | 69.03 | 90.53 | 3.63 | 2.59 | 74.92 | 75.03 | 5.0 | 2.2 |
| D | SW | 8.22 | 7.63 | 77.42 | 90.84 | 3.63 | 2.10 | 72.78 | 68.59 | 5.6 | 2.0 |
| E | GW | 7.34 | 7.66 | N/A | N/A | 58.32 | N/A | 1.6 | 1.5 | ||
| Treatment Station | Monitoring Location | Number of Samples | Mean | Min | Max | Median | GM | SD | CV | 95% CI | Number of Samples >MCL | % Samples >MCL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total THMs (µg/L) | ||||||||||||
| A | 1 | 5 | 68.65 | 38.77 | 98.71 | 67.63 | 65.60 | 22.09 | 0.57 | 42.65–100.90 | 1 | 20 |
| B | 2,3,4 | 12 | 74.86 | 59.60 | 89.90 | 74.08 | 74.14 | 10.66 | 0.33 | 67.59–81.33 | 5 | 42 |
| C | 7,8 | 7 | 67.93 | 51.74 | 79.45 | 71.95 | 67.26 | 10.02 | 0.36 | 58.28–77.62 | 0 | 0 |
| D | 5 | 2 | 80.98 | 71.10 | 90.86 | 80.98 | 80.38 | 13.97 | 0.22 | 16.92–381.72 | 1 | 50 |
| E | 6 | 4 | 26.93 | 16.19 | 34.07 | 28.74 | 26.76 | 8.73 | 0.38 | 14.62–45.38 | 0 | 0 |
| Total HAAs (µg/L) | ||||||||||||
| A | 1 | 5 | 46.95 | 19.88 | 83.46 | 41.38 | 40.85 | 26.67 | 0.32 | 19.36–86.20 | 2 | 40 |
| B | 2,3,4 | 12 | 59.76 | 42.00 | 92.49 | 48.21 | 57.05 | 19.86 | 0.14 | 46.84–69.49 | 4 | 33 |
| C | 7,8 | 7 | 48.60 | 25.29 | 71.86 | 50.93 | 45.68 | 17.42 | 0.15 | 31.79–65.64 | 2 | 29 |
| D | 5 | 2 | 62.82 | 71.10 | 90.86 | 62.82 | 62.72 | 13.97 | 0.17 | 31.41–125.23 | 1 | 50 |
| E | 6 | 4 | 16.69 | 11.17 | 24.63 | 15.47 | 15.79 | 6.42 | 0.32 | 8.58–29.05 | 0 | 0 |
| Chemical | A | B | C | D | E | % Deviation | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May | Jun | Jul | Aug | May | Jun | Jul | Aug | May | Jun | Jul | Aug | May | Jun | Jul | Aug | May | Jun | Jul | Aug | ||
| TCM | 81.5 | 56.6 (5.1) | 24.1 (15.9) | NA | 70 (2.1) | 60.3 (6.0) | 57.6 (9.9) | 43.2 (0.8) | NA | 58.6 (8.5) | 41.9 (12.9) | 33.6 | 56.5 | 75.0 | NA | NA | NA | 11.6 (4.0) | 22.3 (2.1) | NA | 75.0 |
| BDCM | 16.30 | 13.6 (1.6) | 11 (0.5) | NA | 14.6 (0.7) | 13.2 (0.8) | 14.0 (1.9) | 14.4 (0.4) | NA | 12.1 (0.6) | 17 (4.0) | 18.3 | 12.1 | 13.6 (1.6) | NA | NA | NA | 5.8 (0.7) | 8.9 (1.3) | NA | 37.5 |
| DBCM | 1.91 | 2.5 (5.1) | 2.9 (1.3) | NA | 0.9 (1.3) | 2.0 (0.5) | 5.0 (5.4) | 2.1 (0.1) | NA | 1.8 (0.6) | 4.8 (0.2) | 6.9 | 2.5 | 2.3 | NA | NA | NA | 2.7 (0.26) | 2.8 (0.8) | NA | 37.5 |
| TBM | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | NA |
| MCAA | 6.56 | 2.8 (3.9) | NA | NA | 6.9 (0.4) | 3.9 (1.3) | 0.5 (1.1) | <LOD | NA | 3.7 (1.4) | <LOD | <LOD | 5.5 | 5.4 | NA | NA | NA | <LOD | <LOD | NA | NA |
| DCAA | 32.80 | 20.1 (5.9) | 8.2 (1.1) | NA | 32.4 (3.0) | 23.2 (7.0) | 19.1 (2.5) | 16.9 (4.5) | 22.2 | 25.2 (4.9) | 18.0 (11.0) | 12.7 | 23.5 | 27.3 | NA | NA | NA | 6.2 (0.2) | 10.8 (1.3) | NA | 87.5 |
| TCAA | 44.10 | 29.2 (7.3) | 13.5 (4.6) | NA | 46.5 (1.1) | 36.0 (13.7) | 28.9 (1.5) | 27 (1.9) | 22.1 | 28.6 (10) | 22.0 (11.7) | 15.3 | 28.4 | 32 | NA | NA | NA | 3.9 (0.1) | 10.0 (0.8) | NA | 100.0 |
| MBAA | <LOD | <LOD | <LOD | NA | 0.5 (0.7) | <LOD | 0.3 (0.5) | <LOD | <LOD | 0.4(0.7) | <LOD | <LOD | <LOD | <LOD | NA | NA | NA | <LOD | <LOD | NA | NA |
| DBAA | <LOD | 0.6 (0.9) | 1.1 (1.5) | NA | 2.1 (0.7) | 0.6 (0.7) | 0.4 (0.7) | 0.7 (1.0) | 2.1 | 0.5 (0.8) | 1.9 (0.8) | 2.4 | 2 | 1.5 | NA | NA | NA | 1.5 (0.1) | 1.2 (1.7) | NA | 0.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvez, S.; Frost, K.; Sundararajan, M. Evaluation of Drinking Water Disinfectant Byproducts Compliance Data as an Indirect Measure for Short-Term Exposure in Humans. Int. J. Environ. Res. Public Health 2017, 14, 548. https://doi.org/10.3390/ijerph14050548
Parvez S, Frost K, Sundararajan M. Evaluation of Drinking Water Disinfectant Byproducts Compliance Data as an Indirect Measure for Short-Term Exposure in Humans. International Journal of Environmental Research and Public Health. 2017; 14(5):548. https://doi.org/10.3390/ijerph14050548
Chicago/Turabian StyleParvez, Shahid, Kali Frost, and Madhura Sundararajan. 2017. "Evaluation of Drinking Water Disinfectant Byproducts Compliance Data as an Indirect Measure for Short-Term Exposure in Humans" International Journal of Environmental Research and Public Health 14, no. 5: 548. https://doi.org/10.3390/ijerph14050548
APA StyleParvez, S., Frost, K., & Sundararajan, M. (2017). Evaluation of Drinking Water Disinfectant Byproducts Compliance Data as an Indirect Measure for Short-Term Exposure in Humans. International Journal of Environmental Research and Public Health, 14(5), 548. https://doi.org/10.3390/ijerph14050548






