Curcumin, Cardiometabolic Health and Dementia
Abstract
:1. Introduction
2. Curcumin
2.1. Curcumin Safety
2.2. Curcumin Bioavailability
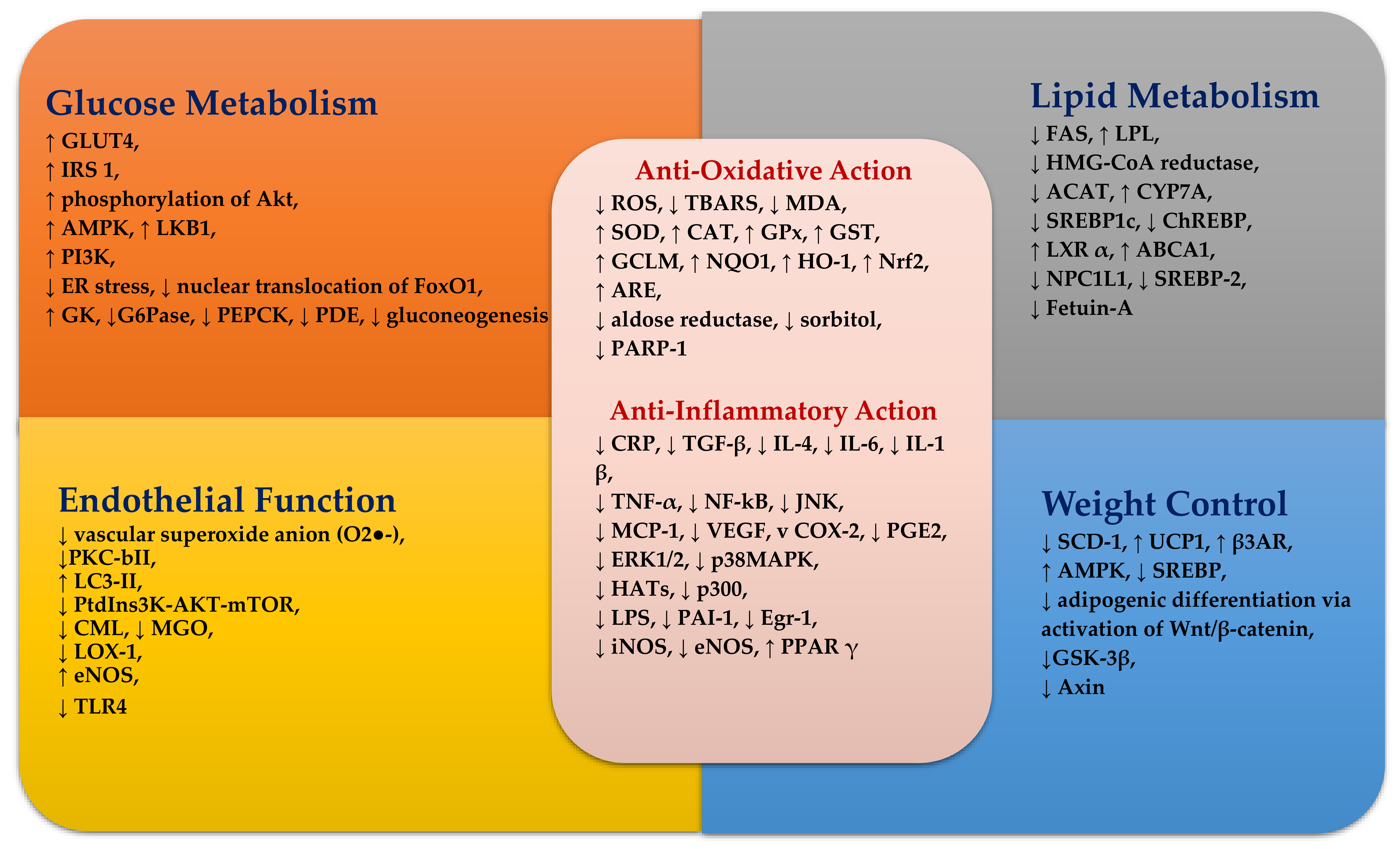
3. Effects of Curcumin on Cardiometabolic Health
3.1. Anti-Oxidative Effects
3.1.1. Human Studies
3.1.2. Several Possible Mechanisms of Curcumin Anti-Oxidant Activity Have Been Proposed [82,83,84,85,86,87,88,89,90,91,92,93]
3.2. Anti-Inflammatory Effects
Curcumin and Inflammatory Pathways
3.3. Glucose Homeostasis
Potential Mechanisms of Curcumin
3.4. Lipid Homeostasis
Potential Mechanisms of Actions of Curcumin on Lipids
3.5. Weight Control
Potential Mechanisms
3.6. Gut Microbiome Changes
3.7. Endothelial Function
3.8. Neurodegenerative Diseases
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mancini, G.B.; Cheng, A.Y.; Connelly, K.; Fitchett, D.; Goldenberg, R.; Goodman, S.G.; Leiter, L.A.; Lonn, E.; Paty, B.; Poirier, P.; et al. Diabetes for cardiologists: Practical issues in diagnosis and management. Can. J. Cardiol. 2017, 33, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Pessin, J.E.; Saltiel, A.R. Signaling pathways in insulin action: Molecular targets of insulin resistance. J. Clin. Investig. 2000, 106, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. Idf diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bommer, C.; Heesemann, E.; Sagalova, V.; Manne-Goehler, J.; Atun, R.; Barnighausen, T.; Vollmer, S. The global economic burden of diabetes in adults aged 20–79 years: A cost-of-illness study. Lancet Diabetes Endocrinol. 2017, 5, 423–430. [Google Scholar] [CrossRef]
- Thomas, T.; Pfeiffer, A.F. Foods for the prevention of diabetes: How do they work? Diabetes Metab. Res. Rev. 2012, 28, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B.; Hogger, P. Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and glycemic control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Lestari, M.L.; Indrayanto, G. Curcumin. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 113–204. [Google Scholar] [PubMed]
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sung, B.; Kim, J.H.; Prasad, S.; Li, S.; Aggarwal, B.B. Multitargeting by turmeric, the golden spice: From kitchen to clinic. Mol. Nutr. Food Res. 2013, 57, 1510–1528. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar] [PubMed]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kismali, G.; Aggarwal, B.B. Curcumin, a component of turmeric: From farm to pharmacy. BioFactors 2013, 39, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Sanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Rahimi, R.; Farzaei, M.H. Pharmacokinetic interactions of curcuminoids with conventional drugs: A review. J. Ethnopharmacol. 2017, 209, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Garcea, G.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J.; Berry, D.P. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br. J. Cancer 2004, 90, 1011–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcea, G.; Berry, D.P.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomark. Prev. 2005, 14, 120–125. [Google Scholar]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L., Jr.; et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Metzler, M.; Pfeiffer, E.; Schulz, S.I.; Dempe, J.S. Curcumin uptake and metabolism. BioFactors 2013, 39, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Burapan, S.; Kim, M.; Han, J. Curcuminoid demethylation as an alternative metabolism by human intestinal microbiota. J. Agric. Food Chem. 2017, 65, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.-S.; Wu, J.-C.; Huang, Q.; Shahidi, F.; Wang, Y.-J.; Ho, C.-T.; Pan, M.-H. Metabolic and colonic microbiota transformation may enhance the bioactivities of dietary polyphenols. J. Funct. Foods 2014, 7, 3–25. [Google Scholar] [CrossRef]
- Tan, S.; Calani, L.; Bresciani, L.; Dall’asta, M.; Faccini, A.; Augustin, M.A.; Gras, S.L.; Del Rio, D. The degradation of curcuminoids in a human faecal fermentation model. Int. J. Food Sci. Nutr. 2015, 66, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.; Rupasinghe, T.W.; Tull, D.L.; Boughton, B.; Oliver, C.; McSweeny, C.; Gras, S.L.; Augustin, M.A. Degradation of curcuminoids by in vitro pure culture fermentation. J. Agric. Food Chem. 2014, 62, 11005–11015. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Kumar, M.; Jain, M.; Dhawan, D.K. Combined effects of curcumin and piperine in ameliorating benzo(a)pyrene induced DNA damage. Food Chem. Toxicol. 2011, 49, 3002–3006. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Singh, A.T.; Xu, W.; Sulchek, T.; Gordon, L.I.; Ryan, R.O. Curcumin nanodisks: Formulation and characterization. Nanomedicine 2011, 7, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Shen, Y.; Hou, J.; Lei, L.; Guo, S.; Qian, C. Polymeric micelles for parenteral delivery of curcumin: Preparation, characterization and in vitro evaluation. Colloids Surf. A 2011, 390, 25–32. [Google Scholar] [CrossRef]
- Mulik, R.S.; Monkkonen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. Transferrin mediated solid lipid nanoparticles containing curcumin: Enhanced in vitro anticancer activity by induction of apoptosis. Int. J. Pharm. 2010, 398, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.S.; Kausar, H.; Vadhanam, M.V.; Ravoori, S.; Gupta, R.C. Controlled systemic delivery by polymeric implants enhances tissue and plasma curcumin levels compared with oral administration. Eur. J. Pharm. Biopharm. 2012, 80, 571–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boruah, B.; Saikia, P.M.; Dutta, R.K. Binding and stabilization of curcumin by mixed chitosan–surfactant systems: A spectroscopic study. J. Photochem. Photobiol. A 2012, 245, 18–27. [Google Scholar] [CrossRef]
- Ghalandarlaki, N.; Alizadeh, A.M. Nanotechnology-applied curcumin for different diseases therapy. BioMed Res. Int. 2014, 2014, 394264. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, H.R.; Nedaeinia, R.; Sepehri Shamloo, A.; Nikdoust, S.; Kazemi Oskuee, R. Novel delivery system for natural products: Nano-curcumin formulations. Avicenna J. Phytomed. 2016, 6, 383–398. [Google Scholar] [PubMed]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Reiner, Z.; Majeed, M.; Sahebkar, A. Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial. Complement. Ther. Med. 2017, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Bressan, A.; Ranaldi, D.; Rapacioli, G.; Giacomelli, L.; Bertuccioli, A. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: Preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4195–4202. [Google Scholar] [PubMed]
- Antony, B.; Merina, B.; Iyer, V.S.; Judy, N.; Lennertz, K.; Joyal, S. A pilot cross-over study to evaluate human oral bioavailability of BCM-95CG (biocurcumax), a novel bioenhanced preparation of curcumin. Indian J. Pharm. Sci. 2008, 70, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Comblain, F.; Sanchez, C.; Lesponne, I.; Balligand, M.; Serisier, S.; Henrotin, Y. Curcuminoids extract, hydrolyzed collagen and green tea extract synergically inhibit inflammatory and catabolic mediator’s synthesis by normal bovine and osteoarthritic human chondrocytes in monolayer. PLoS ONE 2015, 10, e0121654. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Narain, U.; Mishra, R.; Misra, K. Design, development and synthesis of mixed bioconjugates of piperic acid-glycine, curcumin-glycine/alanine and curcumin-glycine-piperic acid and their antibacterial and antifungal properties. Bioorg. Med. Chem. 2005, 13, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.; Mukherjee, K.; Gantait, A.; Saha, B.P.; Mukherjee, P.K. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int. J. Pharm. 2007, 330, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Singh, P.; Mishra, V.; Vyas, S.P. Mannosylated niosomes as adjuvant-carrier system for oral genetic immunization against hepatitis B. Immunol. Lett. 2005, 101, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Haddadi, A.; Molavi, O.; Lavasanifar, A.; Lai, R.; Samuel, J. Micelles of poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J. Biomed. Mater. Res. Part A 2008, 86, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, J.; Shi, K.; Huang, Q. Structure of modified epsilon-polylysine micelles and their application in improving cellular antioxidant activity of curcuminoids. Food Funct. 2011, 2, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Podaralla, S.; Averineni, R.; Alqahtani, M.; Perumal, O. Synthesis of novel biodegradable methoxy poly(ethylene glycol)-zein micelles for effective delivery of curcumin. Mol. Pharm. 2012, 9, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-loaded plga-peg-plga triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J. Colloid Interface Sci. 2011, 354, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, H.R.; Mohammadpour, A.H.; Dastani, M.; Jaafari, M.R.; Abnous, K.; Ghayour Mobarhan, M.; Kazemi Oskuee, R. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial. Avicenna J. Phytomed. 2016, 6, 567–577. [Google Scholar] [PubMed]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Water-dispersible multifunctional hybrid nanogels for combined curcumin and photothermal therapy. Biomaterials 2011, 32, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Karewicz, A.; Bielska, D.; Loboda, A.; Gzyl-Malcher, B.; Bednar, J.; Jozkowicz, A.; Dulak, J.; Nowakowska, M. Curcumin-containing liposomes stabilized by thin layers of chitosan derivatives. Colloids Surf. B Biointerfaces 2013, 109, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, Q.; Xu, X.; Li, N. Development and evaluation of a novel phytosome-loaded chitosan microsphere system for curcumin delivery. Int. J. Pharm. 2013, 448, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, K.; Indra, R.; Rajaram, A.; Sreeram, K.J.; Rajaram, R. Investigations on the interaction of gold-curcumin nanoparticles with human peripheral blood lymphocytes. J. Biomed. Nanotechnol. 2011, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Omidfar, K.; Khorsand, F.; Darziani Azizi, M. New analytical applications of gold nanoparticles as label in antibody based sensors. Biosens. Bioelectron. 2013, 43, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Sweet, M.J.; Singleton, I. Silver nanoparticles: A microbial perspective. Adv. Appl. Microbiol. 2011, 77, 115–133. [Google Scholar] [PubMed]
- Torne, S.; Darandale, S.; Vavia, P.; Trotta, F.; Cavalli, R. Cyclodextrin-based nanosponges: Effective nanocarrier for tamoxifen delivery. Pharm. Dev. Technol. 2013, 18, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, C.; Fonseca, H.B.; Jhabvala, P.; Escalon, E.A.; Melnick, S.J. Curcumin inhibits telomerase activity through human telomerase reverse transcritpase in MCF-7 breast cancer cell line. Cancer Lett. 2002, 184, 1–6. [Google Scholar] [CrossRef]
- Ekambaram, P.; Abdul, H.S. Formulation and evaluation of solid lipid nanoparticles of ramipril. J. Young Pharm. 2011, 3, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wu, X.; Xu, W.; Yang, J.; Yang, Q. Fluorescence enhancement of the silver nanoparticales—Curcumin-cetyltrimethylammonium bromide-nucleic acids system and its analytical application. J. Fluoresc. 2010, 20, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Imaizumi, A.; Otsuka, Y.; Sasaki, H.; Hashiguchi, M.; Tsujiko, K.; Matsumoto, S.; Ishiguro, H.; Chiba, T. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother. Pharmacol. 2012, 69, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.P.; Liu, J.; Merrill, D.A.; et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: A double-blind, placebo-controlled 18-month trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Durackova, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.; Rehman, K.; Chen, S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 2013, 114, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Akash, M. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2016, 23, 87. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- DiSilvestro, R.A.; Joseph, E.; Zhao, S.; Bomser, J. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr. J. 2012, 11, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Xu, W.; Zhou, Z.; Liu, J.; Li, X.; Chen, L.; Weng, J.; Yu, Z. Curcumin attenuates urinary excretion of albumin in type II diabetic patients with enhancing nuclear factor erythroid-derived 2-like 2 (Nrf2) system and repressing inflammatory signaling efficacies. Exp. Clin. Endocrinol. Diabetes 2015, 123, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Cialdella-Kam, L.; Knab, A.M.; Shanely, R.A. Influence of red pepper spice and turmeric on inflammation and oxidative stress biomarkers in overweight females: A metabolomics approach. Plant Foods Hum. Nutr. 2012, 67, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Arun, N.; Nalini, N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant Foods Hum. Nutr. 2002, 57, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Rains, J.; Jones, K. Effect of curcumin on protein glycosylation, lipid peroxidation, and oxygen radical generation in human red blood cells exposed to high glucose levels. Free Radic. Biol. Med. 2006, 41, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Muthenna, P.; Suryanarayana, P.; Gunda, S.K.; Petrash, J.M.; Reddy, G.B. Inhibition of aldose reductase by dietary antioxidant curcumin: Mechanism of inhibition, specificity and significance. FEBS Lett. 2009, 583, 3637–3642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeifer, M.A.; Schumer, M.P.; Gelber, D.A. Aldose reductase inhibitors: The end of an era or the need for different trial designs? Diabetes 1997, 46 (Suppl. 2), S82–S89. [Google Scholar] [CrossRef]
- Tomlinson, D.R.; Willars, G.B.; Carrington, A.L. Aldose reductase inhibitors and diabetic complications. Pharmacol. Ther. 1992, 54, 151. [Google Scholar] [CrossRef]
- Suryanarayana, P.; Satyanarayana, A.; Balakrishna, N.; Kumar, P.U.; Reddy, G.B. Effect of turmeric and curcumin on oxidative stress and antioxidant enzymes in streptozotocin-induced diabetic rat. Med. Sci. Monit. 2007, 13, BR286–BR292. [Google Scholar] [PubMed]
- Pari, L.; Murugan, P. Tetrahydrocurcumin prevents brain lipid peroxidation in streptozotocin-induced diabetic rats. J. Med. Food 2007, 10, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, A.N.; Akhov, L.; Selvaraj, G.; Pugazhenthi, S. Induction of antioxidant enzymes by curcumin and its analogues in human islets: Implications in transplantation. Pancreas 2009, 38, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Meghana, K.; Sanjeev, G.; Ramesh, B. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: A prophylactic and protective role. Eur. J. Pharmacol. 2007, 577, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanyam, M.; Koteswari, A.A.; Kumar, R.S.; Monickaraj, S.F.; Maheswari, J.U.; Mohan, V. Curcumin-induced inhibition of cellular reactive oxygen species generation: Novel therapeutic implications. J. Biosci. 2003, 28, 715–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, K.I.; Choi, M.S.; Jung, U.J.; Kim, H.J.; Yeo, J.; Jeon, S.M.; Lee, M.K. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol. Nutr. Food Res. 2008, 52, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Virag, L. Structure and function of poly(ADP-ribose) polymerase-1: Role in oxidative stress-related pathologies. Curr. Vasc. Pharmacol. 2005, 3, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, C.; Virag, L. Inputs and outputs of poly(ADP-ribosyl)ation: Relevance to oxidative stress. Redox Biol. 2014, 2, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Martin, K.A.; Hwa, J. Aldose reductase, oxidative stress, and diabetic mellitus. Front. Pharmacol. 2012, 3, 87. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, S.; Yang, G.; Liu, H.; Boden, G.; Li, L. Efficacy and safety of aldose reductase inhibitor for the treatment of diabetic cardiovascular autonomic neuropathy: Systematic review and meta-analysis. PLoS ONE 2014, 9, e87096. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Yu, Z.; Chiang, Y.; Yang, Y.; Chai, T.; Foltz, W.; Lu, H.; Fantus, I.G.; Jin, T. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS ONE 2012, 7, e28784. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F. Therapeutic approaches to mitochondrial dysfunction in parkinson’s disease. Parkinsonism Relat. Disord. 2009, 15 (Suppl. 3), S189–S194. [Google Scholar] [CrossRef]
- Mohammadi, A.; Sahebkar, A.; Iranshahi, M.; Amini, M.; Khojasteh, R.; Ghayour-Mobarhan, M.; Ferns, G.A. Effects of supplementation with curcuminoids on dyslipidemia in obese patients: A randomized crossover trial. Phytother. Res. 2013, 27, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Wongcharoen, W.; Jai-Aue, S.; Phrommintikul, A.; Nawarawong, W.; Woragidpoonpol, S.; Tepsuwan, T.; Sukonthasarn, A.; Apaijai, N.; Chattipakorn, N. Effects of curcuminoids on frequency of acute myocardial infarction after coronary artery bypass grafting. Am. J. Cardiol. 2012, 110, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Sahebkar, A.; Parvin, S.; Saadat, A. A randomized controlled trial on the anti-inflammatory effects of curcumin in patients with chronic sulphur mustard-induced cutaneous complications. Ann. Clin. Biochem. 2012, 49, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Chainani-Wu, N.; Madden, E.; Lozada-Nur, F.; Silverman, S., Jr. High-dose curcuminoids are efficacious in the reduction in symptoms and signs of oral lichen planus. J. Am. Acad. Dermatol. 2012, 66, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Product-evaluation registry of meriva(r), a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. 2010, 52, 55–62. [Google Scholar] [PubMed]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Hosseini Nouzari, S.M.; Jalalian, H.R.; Sahebkar, A. Antioxidant effects of bioavailability-enhanced curcuminoids in patients with solid tumors: A randomized double-blind placebo-controlled trial. J. Funct. Foods 2014, 6, 615–622. [Google Scholar] [CrossRef]
- Rahimnia, A.R.; Panahi, Y.; Alishiri, G.; Sharafi, M.; Sahebkar, A. Impact of supplementation with curcuminoids on systemic inflammation in patients with knee osteoarthritis: Findings from a randomized double-blind placebo-controlled trial. Drug Res. 2015, 65, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Usharani, P.; Mateen, A.A.; Naidu, M.U.; Raju, Y.S.; Chandra, N. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: A randomized, parallel-group, placebo-controlled, 8-week study. Drugs R D 2008, 9, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Na, L.X.; Yan, B.L.; Jiang, S.; Cui, H.L.; Li, Y.; Sun, C.H. Curcuminoids target decreasing serum adipocyte-fatty acid binding protein levels in their glucose-lowering effect in patients with type 2 diabetes. Biomed. Environ. Sci. 2014, 27, 902–906. [Google Scholar] [PubMed]
- Yu, J.J.; Pei, L.B.; Zhang, Y.; Wen, Z.Y.; Yang, J.L. Chronic supplementation of curcumin enhances the efficacy of antidepressants in major depressive disorder: A randomized, double-blind, placebo-controlled pilot study. J. Clin. Psychopharmacol. 2015, 35, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, S.; Sahebkar, A.; Mahdipour, E.; Jamialahmadi, K.; Torabi, S.; Akhlaghi, S.; Ferns, G.; Parizadeh, S.M.R.; Ghayour-Mobarhan, M. Investigation of the effects of curcumin on serum cytokines in obese individuals: A randomized controlled trial. Sci. World J. 2014, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendia, L.E.; Majeed, M.; Sahebkar, A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Khajehdehi, P.; Pakfetrat, M.; Javidnia, K.; Azad, F.; Malekmakan, L.; Nasab, M.H.; Dehghanzadeh, G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-beta and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: A randomized, double-blind and placebo-controlled study. Scand. J. Urol. Nephrol. 2011, 45, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Cicero, A.F.G.; Simental-Mendia, L.E.; Aggarwal, B.B.; Gupta, S.C. Curcumin downregulates human tumor necrosis factor-alpha levels: A systematic review and meta-analysis ofrandomized controlled trials. Pharmacol. Res. 2016, 107, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Ghanei, M.; Bashiri, S.; Hajihashemi, A.; Sahebkar, A. Short-term curcuminoid supplementation for chronic pulmonary complications due to sulfur mustard intoxication: Positive results of a randomized double-blind placebo-controlled trial. Drug Res. 2015, 65, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Tyagi, A.K.; Deshmukh-Taskar, P.; Hinojosa, M.; Prasad, S.; Aggarwal, B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 2014, 559, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P.; Simental-Mendia, L.E.; Bo, S.; Sahebkar, A. Effect of curcumin on circulating interleukin-6 concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016, 111, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Errede, B.; Cade, R.M.; Yashar, B.M.; Kamada, Y.; Levin, D.E.; Irie, K.; Matsumoto, K. Dynamics and organization of map kinase signal pathways. Mol. Reprod. Dev. 1995, 42, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Herlaar, E.; Brown, Z. P38 MAPK signalling cascades in inflammatory disease. Mol. Med. Today 1999, 5, 439–447. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Binion, D.G.; Heidemann, J.; Theriot, M.; Fisher, P.J.; Johnson, N.A.; Otterson, M.F.; Rafiee, P. Mechanisms of madcam-1 gene expression in human intestinal microvascular endothelial cells. Am. J. Physiol. Cell Physiol. 2005, 288, C272–C281. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, P.; Johnson, C.P.; Li, M.S.; Ogawa, H.; Heidemann, J.; Fisher, P.J.; Lamirand, T.H.; Otterson, M.F.; Wilson, K.T.; Binion, D.G. Cyclosporine a enhances leukocyte binding by human intestinal microvascular endothelial cells through inhibition of p38 MAPK and inos. Paradoxical proinflammatory effect on the microvascular endothelium. J. Biol. Chem. 2002, 277, 35605–35615. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.M.; Orlando, R.A. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr. Metab. 2008, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Li, Y.; Wen, Y.; Chen, Y.F.; Na, L.X.; Li, S.T.; Sun, C.H. Curcumin, a potential inhibitor of up-regulation of TNF-alpha and IL-6 induced by palmitate in 3T3-L1 adipocytes through Nf-kappaB and JNK pathway. Biomed. Environ. Sci. 2009, 22, 32–39. [Google Scholar] [CrossRef]
- Woo, H.-M.; Kang, J.-H.; Kawada, T.; Yoo, H.; Sung, M.-K.; Yu, R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007, 80, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Wang, S.; Zhu, W.; Xie, C.; Li, X.; Wu, J.; Zhu, J.; Jiang, Y.; Yang, X.; Li, Y.; et al. Curcumin suppresses JNK pathway to attenuate BPA-induced insulin resistance in LO2 cells. Biomed. Pharmacother. 2018, 97, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Rostamirad, A.; Seyyedebrahimi, S.; Meshkani, R. Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NF-κB pathway and ROS production. Inflammopharmacology 2018, 26, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Wang, S.; Zhu, W.; Xie, C.; Li, X.; Wu, J.; Zhu, J.; Jiang, Y.; Yang, X.; Li, Y.; et al. Curcumin attenuates bpa-induced insulin resistance in HepG2 cells through suppression of JNK/p38 pathways. Toxicol. Lett. 2017, 272, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Masamune, A.; Suzuki, N.; Kikuta, K.; Satoh, M.; Satoh, K.; Shimosegawa, T. Curcumin blocks activation of pancreatic stellate cells. J. Cell. Biochem. 2006, 97, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.M.; Jialal, I.; Devaraj, S. Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J. Nutr. Biochem. 2011, 22, 450–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.X.; Lee, J. Dietary regulation of histone acetylases and deacetylases for the prevention of metabolic diseases. Nutrients 2012, 4, 1868–1886. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of non-alcoholic fatty liver disease with curcumin: A randomized placebo-controlled trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Na, L.X.; Li, Y.; Pan, H.Z.; Zhou, X.L.; Sun, D.J.; Meng, M.; Li, X.X.; Sun, C.H. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: A double-blind, placebo-controlled trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, F. Regulation of adiponectin multimerization, signaling and function. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salahshooh, M.; Parizadeh, S.; Pasdar, A.; Saberi Karimian, M.; Safarian, H.; Javandoost, A.; Ferns, G.; Ghayour-Mobarhan, M.; Sahebkar, A. The effect of curcumin (Curcuma longa L.) on circulating levels of adiponectin in patients with metabolic syndrome. Comp. Clin. Pathol. 2017, 26, 17–23. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J. Nutr. Biochem. 2014, 25, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Larson-Meyer, D.E.; Liebman, M. Effect of cinnamon and turmeric on urinary oxalate excretion, plasma lipids, and plasma glucose in healthy subjects. Am. J. Clin. Nutr. 2008, 87, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Wickenberg, J.; Ingemansson, S.L.; Hlebowicz, J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr. J. 2010, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Franco-Robles, E.; Campos-Cervantes, A.; Murillo-Ortiz, B.O.; Segovia, J.; Lopez-Briones, S.; Vergara, P.; Perez-Vazquez, V.; Solis-Ortiz, M.S.; Ramirez-Emiliano, J. Effects of curcumin on brain-derived neurotrophic factor levels and oxidative damage in obesity and diabetes. Appl. Physiol. Nutr. Metab. 2014, 39, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Su, Y.F.; Yang, H.W.; Lee, Y.H.; Chou, J.I.; Ueng, K.C. Lipid-lowering effects of curcumin in patients with metabolic syndrome: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2014, 28, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Santos-Parker, J.R.; Strahler, T.R.; Bassett, C.J.; Bispham, N.Z.; Chonchol, M.B.; Seals, D.R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging 2017, 9, 187–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alwi, I.; Santoso, T.; Suyono, S.; Sutrisna, B.; Suyatna, F.D.; Kresno, S.B.; Ernie, S. The effect of curcumin on lipid level in patients with acute coronary syndrome. Acta Med. Indones. 2008, 40, 201–210. [Google Scholar] [PubMed]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendia, L.E.; Sahebkar, A. Curcumin lowers serum lipids and uric acid in subjects with nonalcoholic fatty liver disease: A randomized controlled trial. J. Cardiovasc. Pharmacol. 2016, 68, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Na, L.X.; Zhang, Y.L.; Li, Y.; Liu, L.Y.; Li, R.; Kong, T.; Sun, C.H. Curcumin improves insulin resistance in skeletal muscle of rats. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK: A key regulator of energy balance in the single cell and the whole organism. Int. J. Obes. 2008, 32 (Suppl. 4), S7–S12. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.T.; Chang, T.W.; Lee, M.S.; Lin, J.K. Suppression of free fatty acid-induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J. Agric. Food Chem. 2012, 60, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, J.M.; Kim, E.K.; Lee, J.O.; Lee, S.K.; Jung, J.H.; You, G.Y.; Park, S.H.; Suh, P.G.; Kim, H.S. Curcumin stimulates glucose uptake through AMPK-p38 MAPK pathways in l6 myotube cells. J. Cell. Physiol. 2010, 223, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Li, Y.C.; Kong, L.D.; Hu, Q.H. Curcumin inhibits hepatic protein-tyrosine phosphatase 1B and prevents hypertriglyceridemia and hepatic steatosis in fructose-fed rats. Hepatology 2010, 51, 1555–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, F.; Kang, J.; Cao, Y.; Fan, S.; Yang, H.; An, Y.; Pan, Y.; Tie, L.; Li, X. Curcumin attenuates palmitate-induced apoptosis in min6 pancreatic β-cells through PI3K/Akt/FOxO1 and mitochondrial survival pathways. Apoptosis 2015, 20, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Best, L.; Elliott, A.C.; Brown, P.D. Curcumin induces electrical activity in rat pancreatic beta-cells by activating the volume-regulated anion channel. Biochem. Pharmacol. 2007, 73, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Rouse, M.; Younes, A.; Egan, J.M. Resveratrol and curcumin enhance pancreatic beta-cell function by inhibiting phosphodiesterase activity. J. Endocrinol. 2014, 223, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Leibel, R.; Tortoriello, D.V. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Shao, J. SIRT1 regulates adiponectin gene expression through foxo1-C/enhancer-binding protein alpha transcriptional complex. J. Biol. Chem. 2006, 281, 39915–39924. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Auwerx, J. Ppar(gamma) and glucose homeostasis. Annu. Rev. Nutr. 2002, 22, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Cheung, S.K.; Mok, V.C.; Lam, L.C.; Leung, V.P.; Hui, E.; Ng, C.C.; Chow, M.; Ho, P.C.; Lam, S.; et al. Curcumin effects on blood lipid profile in a 6-month human study. Pharmacol. Res. 2007, 56, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.J.A.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, M.L. Curcumin potentiates cholesterol-lowering effects of phytosterols in hypercholesterolaemic individuals. A randomised controlled trial. Metabolism 2018, 82, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. A systematic review and meta-analysis of randomized controlled trials investigating the effects of curcumin on blood lipid levels. Clin. Nutr. 2014, 33, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Khalili, N.; Hosseini, M.S.; Abbasinazari, M.; Sahebkar, A. Lipid-modifying effects of adjunctive therapy with curcuminoids-piperine combination in patients with metabolic syndrome: Results of a randomized controlled trial. Complement. Ther. Med. 2014, 22, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid lowering nutraceuticals in clinical practice: Position paper from an international lipid expert panel. Arch. Med. Sci. 2017, 13, 965–1005. [Google Scholar] [CrossRef] [PubMed]
- Saenz, J.; Alba, G.; Reyes-Quiroz, M.E.; Geniz, I.; Jimenez, J.; Sobrino, F.; Santa-Maria, C. Curcumin enhances LXRalpha in an AMP-activated protein kinase-dependent manner in human macrophages. J. Nutr. Biochem. 2018, 54, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Peschel, D.; Koerting, R.; Nass, N. Curcumin induces changes in expression of genes involved in cholesterol homeostasis. J. Nutr. Biochem. 2007, 18, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, J.; Song, B.; Xiao, X.; Zhang, B.; Qi, M.; Huang, W.; Yang, L.; Wang, Z. Curcumin rescues high fat diet-induced obesity and insulin sensitivity in mice through regulating srebp pathway. Toxicol. Appl. Pharmacol. 2016, 304, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Um, M.Y.; Hwang, K.H.; Ahn, J.; Ha, T.Y. Curcumin attenuates diet-induced hepatic steatosis by activating AMP-activated protein kinase. Basic Clin. Pharmacol. Toxicol. 2013, 113, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef] [PubMed]
- El-Moselhy, M.A.; Taye, A.; Sharkawi, S.S.; El-Sisi, S.F.; Ahmed, A.F. The antihyperglycemic effect of curcumin in high fat diet fed rats. Role of TNF-alpha and free fatty acids. Food Chem. Toxicol. 2011, 49, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.S.; Srinivasan, K. Hypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa) in streptozotocin induced diabetic rats. Mol. Cell. Biochem. 1997, 166, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.M.; Choi, M.S.; Jung, U.J.; Kim, M.J.; Kim, H.J.; Jeon, S.M.; Shin, S.K.; Seong, C.N.; Lee, M.K. Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat-fed hamsters. Metabolism 2008, 57, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Maithilikarpagaselvi, N.; Sridhar, M.G.; Swaminathan, R.P.; Sripradha, R.; Badhe, B. Curcumin inhibits hyperlipidemia and hepatic fat accumulation in high-fructose-fed male wistar rats. Pharm. Biol. 2016, 54, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- Oner-Iyidogan, Y.; Kocak, H.; Seyidhanoglu, M.; Gurdol, F.; Gulcubuk, A.; Yildirim, F.; Cevik, A.; Uysal, M. Curcumin prevents liver fat accumulation and serum fetuin-a increase in rats fed a high-fat diet. J. Physiol. Biochem. 2013, 69, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.K.; Ha, T.Y.; McGregor, R.A.; Choi, M.S. Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol. Nutr. Food Res. 2011, 55, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Prakash, U.N.; Srinivasan, K. Fat digestion and absorption in spice-pretreated rats. J. Sci. Food Agric. 2012, 92, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, Y. Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7a-hydroxylase in rats fed a high fat diet. Nutr. Res. Pract. 2010, 4, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Yap, F.; Craddock, L.; Yang, J. Mechanism of AMPK suppression of LXR-dependent Srebp-1c transcription. Int. J. Biol. Sci. 2011, 7, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Denechaud, P.D.; Dentin, R.; Girard, J.; Postic, C. Role of chrebp in hepatic steatosis and insulin resistance. FEBS Lett. 2008, 582, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.Z.; Zhao, S.P.; Wu, Z.H.; Yang, J.; Xie, X.Z.; Yu, B.L.; Nie, S. Curcumin promotes cholesterol efflux from adipocytes related to ppargamma-lxralpha-abca1 passway. Mol. Cell. Biochem. 2011, 358, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Malhotra, P.; Ma, K.; Singla, A.; Hedroug, O.; Saksena, S.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Srebp2 mediates the modulation of intestinal NPC1L1 expression by curcumin. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G148–G155. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Ohlsson, L.; Duan, R.D. Curcumin inhibits cholesterol uptake in Caco-2 cells by down-regulation of NPC1L1 expression. Lipids Health Dis. 2010, 9, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefan, N.; Hennige, A.M.; Staiger, H.; Machann, J.; Schick, F.; Krober, S.M.; Machicao, F.; Fritsche, A.; Haring, H.U. Alpha2-heremans-schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 2006, 29, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Emoto, M.; Araki, T.; Yokoyama, H.; Lee, E.; Teramura, M.; Koyama, H.; Shoji, T.; Inaba, M.; Nishizawa, Y. Effects of pioglitazone on serum fetuin-a levels in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Hariri, M.; Haghighatdoost, F. Effect of curcumin on anthropometric measures: A systematic review on randomized clinical trials. J. Am. Coll. Nutr. 2018, 37, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Esmaily, H.; Sahebkar, A.; Iranshahi, M.; Ganjali, S.; Mohammadi, A.; Ferns, G.; Ghayour-Mobarhan, M. An investigation of the effects of curcumin on anxiety and depression in obese individuals: A randomized controlled trial. Chin. J. Integr. Med. 2015, 21, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Ikonomov, O.C.; Sbrissa, D.; Mlak, K.; Shisheva, A. Requirement for PIKfyve enzymatic activity in acute and long-term insulin cellular effects. Endocrinology 2002, 143, 4742–4754. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Krause, J.; Rumberger, J.M. Curcumin is a direct inhibitor of glucose transport in adipocytes. Phytomedicine 2014, 21, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Y.; Ye, M.; Ding, Y.; Tang, Z.; Li, M.; Zhou, Y.; Wang, C. Interference with Akt signaling pathway contributes curcumin-induced adipocyte insulin resistance. Mol. Cell. Endocrinol. 2016, 429, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mar-Heyming, R.; Miyazaki, M.; Weissglas-Volkov, D.; Kolaitis, N.A.; Sadaat, N.; Plaisier, C.; Pajukanta, P.; Cantor, R.M.; de Bruin, T.W.; Ntambi, J.M.; et al. Association of stearoyl-coa desaturase 1 activity with familial combined hyperlipidemia. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Ntambi, J.M. The role of stearoyl-coa desaturase in obesity, insulin resistance, and inflammation. Ann. N. Y. Acad. Sci. 2011, 1243, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Ye, Z.; Xu, C.; Zhang, M.; Ruan, B.; Wei, M.; Jiang, Y.; Zhang, Y.; Wang, L.; et al. Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem. Biophys. Res. Commun. 2015, 466, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Choi, J.H.; Mukherjee, R.; Hwang, K.C.; Yun, J.W. Proteomic identification of fat-browning markers in cultured white adipocytes treated with curcumin. Mol. Cell. Biochem. 2016, 415, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Vitali, A.; Murano, I.; Zingaretti, M.C.; Frontini, A.; Ricquier, D.; Cinti, S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J. Lipid Res. 2012, 53, 619–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenzl, A.; Kiefer, F.W. Brown adipose tissue and thermogenesis. Horm. Mol. Biol. Clin. Investig. 2014, 19, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Mottillo, E.P.; Granneman, J.G. Adipose tissue plasticity from wat to bat and in between. Biochim. Biophys. Acta 2014, 1842, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Christian, M. Hormonal factors in the control of the browning of white adipose tissue. Horm. Mol. Biol. Clin. Investig. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, H.; Zeng, W. Whole-tissue 3D imaging reveals intra-adipose sympathetic plasticity regulated by NGF-TrkA signal in cold-induced beiging. Protein Cell 2018, 9, 527–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidossis, L.S.; Porter, C.; Saraf, M.K.; Borsheim, E.; Radhakrishnan, R.S.; Chao, T.; Ali, A.; Chondronikola, M.; Mlcak, R.; Finnerty, C.C.; et al. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. 2015, 22, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Kim, S.; Ha, T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. Am. J. Physiol. Cell Physiol. 2010, 298, C1510–C1516. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.C.; Jacka, F.N.; Prescott, S.L. Immune-microbiota interactions: Dysbiosis as a global health issue. Curr. Allergy Asthma Rep. 2016, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise modifies the gut microbiota with positive health effects. Oxid. Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef] [PubMed]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef] [PubMed]
- Brahe, L.; Astrup, A.; Larsen, L. Can we prevent obesity-related metabolic diseases by dietary modulation of the gut microbiota? Adv. Nutr. 2016, 7, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.; Tremaroli, V.; Nielsen, J.; Backhed, F. Assessing the human gut microbiota in metabolic diseases. Diabetes 2013, 62, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Srivatsav, V.; Rizwan, A.; Nashed, A.; Liu, R.; Shen, R.; Akhtar, M. Bridging the gap between gut microbial dysbiosis and cardiovascular diseases. Nutrients 2017, 9, 859. [Google Scholar] [CrossRef] [PubMed]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in parkinson’s disease. World J Gastroenterol. 2015, 21, 10609–10620. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The gut microbiota and alzheimer’s disease. J. Alzheimer’s Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef] [PubMed]
- McFadden, R.M.; Larmonier, C.B.; Shehab, K.W.; Midura-Kiela, M.; Ramalingam, R.; Harrison, C.A.; Besselsen, D.G.; Chase, J.H.; Caporaso, J.G.; Jobin, C.; et al. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm. Bowel Dis. 2015, 21, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Bereswill, S.; Munoz, M.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kuhl, A.A.; Loddenkemper, C.; Gobel, U.B.; Heimesaat, M.M. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS ONE 2010, 5, e15099. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lillehoj, H.S.; Hong, Y.H.; Kim, G.B.; Lee, S.H.; Lillehoj, E.P.; Bravo, D.M. Dietary capsicum and Curcuma longa oleoresins increase intestinal microbiome and necrotic enteritis in three commercial broiler breeds. Res. Vet. Sci. 2015, 102, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Shimouchi, A.; Nose, K.; Takaoka, M.; Hayashi, H.; Kondo, T. Effect of dietary turmeric on breath hydrogen. Dig. Dis. Sci. 2009, 54, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, H.; Zhang, P.; Gao, C.; Tao, J.; Ge, Z.; Zhu, D.; Bi, Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim. Biophys. Acta 2017, 1861, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.F.; Collado, M.C.; Ferreira, C.L.; Bressan, J.; Peluzio Mdo, C. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr. Res. 2012, 32, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.P.; Texeira, T.F.; Ferreira, A.B.; Peluzio Mdo, C.; Alfenas Rde, C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin improves intestinal barrier function: Modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017, 312, C438–C445. [Google Scholar] [CrossRef] [PubMed]
- Song, W.B.; Wang, Y.Y.; Meng, F.S.; Zhang, Q.H.; Zeng, J.Y.; Xiao, L.P.; Yu, X.P.; Peng, D.D.; Su, L.; Xiao, B.; et al. Curcumin protects intestinal mucosal barrier function of rat enteritis via activation of MKP-1 and attenuation of p38 and NF-κB activation. PLoS ONE 2010, 5, e12969. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Guo, R.; Wei, S.; Kong, Y.; Wei, X.; Wang, W.; Shi, X.; Jiang, H. Curcumin protects against the intestinal ischemia-reperfusion injury: Involvement of the tight junction protein ZO-1 and TNF-alpha related mechanism. Korean J. Physiol. Pharmacol. 2016, 20, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Bie, J.; Wang, J.; Ghosh, S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in ldlr−/− mice—Role of intestinal permeability and macrophage activation. PLoS ONE 2014, 9, e108577. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.T.; Qiu, Y.M.; Zhao, H.W.; Li, D.H.; Liu, Y.T.; Wang, Y.Z.; Su, S.H. Effect of curcumin on intestinal mucosal mechanical barrier in rats with non-alcoholic fatty liver disease. Chin. J. Hepatol. 2017, 25, 134–138. [Google Scholar]
- Wu, J.J.; Bennett, A.M. Essential role for mitogen-activated protein (map) kinase phosphatase-1 in stress-responsive map kinase and cell survival signaling. J. Biol. Chem. 2005, 280, 16461–16466. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Ding, M.; Zhang, X.; Rojanasakul, Y.; Shi, X. On the role of hydroxyl radical and the effect of tetrandrine on nuclear factor—κB activation by phorbol 12-myristate 13-acetate. Ann. Clin. Lab. Sci. 2000, 30, 65–71. [Google Scholar] [PubMed]
- Ghosh, S.S.; He, H.; Wang, J.; Gehr, T.W.; Ghosh, S. Curcumin-mediated regulation of intestinal barrier function: The mechanism underlying its beneficial effects. Tissue Barriers 2018, 6, e1425085. [Google Scholar] [CrossRef] [PubMed]
- Patumraj, S.; Wongeakin, N.; Sridulyakul, P.; Jariyapongskul, A.; Futrakul, N.; Bunnag, S. Combined effects of curcumin and vitamin C to protect endothelial dysfunction in the iris tissue of STZ-induced diabetic rats. Clin. Hemorheol. Microcirc. 2006, 35, 481–489. [Google Scholar] [PubMed]
- Han, J.; Pan, X.Y.; Xu, Y.; Xiao, Y.; An, Y.; Tie, L.; Pan, Y.; Li, X.J. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy 2012, 8, 812–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.Y.; Liu, C.L.; Chyau, C.C.; Hu, M.L. Trapping of methylglyoxal by curcumin in cell-free systems and in human umbilical vein endothelial cells. J. Agric. Food Chem. 2012, 60, 8190–8196. [Google Scholar] [CrossRef] [PubMed]
- Rungseesantivanon, S.; Thenchaisri, N.; Ruangvejvorachai, P.; Patumraj, S. Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and pkc inhibition. BMC Complement. Altern. Med. 2010, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ahn, Y.; Hong, M.H.; Joo, S.Y.; Kim, K.H.; Sohn, I.S.; Park, H.W.; Hong, Y.J.; Kim, J.H.; Kim, W.; et al. Curcumin attenuates inflammatory responses of TNF-alpha-stimulated human endothelial cells. J. Cardiovasc. Pharmacol. 2007, 50, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Pendurthi, U.R.; Rao, L.V. Suppression of transcription factor Egr-1 by curcumin. Thromb. Res. 2000, 97, 179–189. [Google Scholar] [CrossRef]
- Li, L.; Sawamura, T.; Renier, G. Glucose enhances human macrophage LOX-1 expression: Role for LOX-1 in glucose-induced macrophage foam cell formation. Circ. Res. 2004, 94, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.M.; Stoner, L.; Rowlands, D.S.; Caldwell, A.R.; Sanders, E.; Kreutzer, A.; Mitchell, J.B.; Purpura, M.; Jager, R. Novel form of curcumin improves endothelial function in young, healthy individuals: A double-blind placebo controlled study. J. Nutr. Metab. 2016, 2016, 1089653. [Google Scholar] [CrossRef] [PubMed]
- Margina, D.; Gradinaru, D.; Manda, G.; Neagoe, I.; Ilie, M. Membranar effects exerted in vitro by polyphenols—Quercetin, epigallocatechin gallate and curcumin—On huvec and jurkat cells, relevant for diabetes mellitus. Food Chem. Toxicol. 2013, 61, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Masaki, T.; Sawamura, T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: Implications in endothelial dysfunction and atherosclerosis. Pharmacol. Ther. 2002, 95, 89–100. [Google Scholar] [CrossRef]
- Naito, T.; Oikawa, S.; Kotake, H.; Hayasaka, K.; Toyota, T. Effect of glucose concentration on foam cell formation in THP-1 cells. J. Atheroscler. Thromb. 2001, 8, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Liu, Z.; Zandi, E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997, 9, 240–246. [Google Scholar] [CrossRef]
- Laight, D.; Carrier, M.; Anggard, E. Antioxidants, diabetes and endothelial dysfunction. Cardiovasc. Res. 2000, 47, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Boonla, O.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P.; Prachaney, P.; Greenwald, S.E. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide 2014, 42, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, X.; Hu, G.; Xu, C.; Jiang, H. Curcumin attenuates hydrogen peroxide-induced premature senescence via the activation of Sirt1 in human umbilical vein endothelial cells. Biol. Pharm. Bull. 2015, 38, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, W.; Li, M.; Ren, H.; Chen, C.; Wang, J.; Wang, W.E.; Yang, J.; Zeng, C. Curcumin exerts its anti-hypertensive effect by down-regulating the AT1 receptor in vascular smooth muscle cells. Sci. Rep. 2016, 6, 25579. [Google Scholar] [CrossRef] [PubMed]
- Farhangkhoee, H.; Khan, Z.A.; Chen, S.; Chakrabarti, S. Differential effects of curcumin on vasoactive factors in the diabetic rat heart. Nutr. Metab. 2006, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.; Mehta, J.L. Currying the heart: Curcumin and cardioprotection. J. Cardiovasc. Pharmacol. Ther. 2009, 14, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Yan, C.; Deng, Q.; Gao, D.F.; Niu, X.L. Curcumin inhibits lps-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta Pharmacol. Sin. 2013, 34, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, R.; Bolshette, N.; Gadhave, K.; Ahire, A.; Ahmed, S.; Cassano, T.; Gupta, V.B.; Lahkar, M. Insulin signaling: An opportunistic target to minify the risk of Alzheimer’s disease. Psychoneuroendocrinology 2017, 83, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Deng, Y.; Zhang, B.; Gong, C.X. Deregulation of brain insulin signaling in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 282–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Monte, S.M. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 35–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mucke, L. Alzheimer mechanisms and therapeutic strategies. Cell 2012, 148, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Price, J.L.; Ko, A.I.; Wade, M.J.; Tsou, S.K.; McKeel, D.W.; Morris, J.C. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch. Neurol. 2001, 58, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Isla, T.; Price, J.L.; McKeel, D.W., Jr.; Morris, J.C.; Growdon, J.H.; Hyman, B.T. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J. Neurosci. 1996, 16, 4491–4500. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Tiwari, S. Therapeutic interventions for advanced glycation-end products and its receptor-mediated cardiovascular disease. Curr. Pharm. Des. 2017, 23, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Luevano-Contreras, C.; Gomez-Ojeda, A.; Macias-Cervantes, M.H.; Garay-Sevilla, M.E. Dietary advanced glycation end products and cardiometabolic risk. Curr. Diabetes Rep. 2017, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Clifton, P.; Keogh, J. Role of dietary advanced glycation end products. Curr. Opin. Lipidol. 2017, 28, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Matsui, T. Pathologic role of dietary advanced glycation end products in cardiometabolic disorders, and therapeutic intervention. Nutrition 2016, 32, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.D.; Chen, X.; Fu, J.; Chen, M.; Zhu, H.; Roher, A.; Slattery, T.; Zhao, L.; Nagashima, M.; Morser, J.; et al. Rage and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 1996, 382, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Donahue, J.E.; Flaherty, S.L.; Johanson, C.E.; Duncan, J.A., III; Silverberg, G.D.; Miller, M.C.; Tavares, R.; Yang, W.; Wu, Q.; Sabo, E.; et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006, 112, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Yang, F.; Rosario, E.R.; Ubeda, O.J.; Beech, W.; Gant, D.J.; Chen, P.P.; Hudspeth, B.; Chen, C.; Zhao, Y.; et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-jun N-terminal kinase signaling: Suppression by omega-3 fatty acids and curcumin. J. Neurosci. 2009, 29, 9078–9089. [Google Scholar] [CrossRef] [PubMed]
- Zraika, S.; Hull, R.L.; Verchere, C.B.; Clark, A.; Potter, K.J.; Fraser, P.E.; Raleigh, D.P.; Kahn, S.E. Toxic oligomers and islet beta cell death: Guilty by association or convicted by circumstantial evidence? Diabetologia 2010, 53, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Morales-Scheihing, D.; Butler, P.C.; Soto, C. Type 2 diabetes as a protein misfolding disease. Trends Mol. Med. 2015, 21, 439–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartl, F.U. Protein misfolding diseases. Annu. Rev. Biochem. 2017, 86, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Sparks, S.; Liu, G.; Robbins, K.J.; Lazo, N.D. Curcumin modulates the self-assembly of the islet amyloid polypeptide by disassembling alpha-helix. Biochem. Biophys. Res. Commun. 2012, 422, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Daval, M.; Bedrood, S.; Gurlo, T.; Huang, C.J.; Costes, S.; Butler, P.C.; Langen, R. The effect of curcumin on human islet amyloid polypeptide misfolding and toxicity. Amyloid 2010, 17, 118–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Su, C.; Feng, H.; Chen, X.; Dong, Y.; Rao, Y.; Ren, Y.; Yang, J.; Shi, J.; Tian, J.; et al. Curcumin regulates insulin pathways and glucose metabolism in the brains of APPswe/PS1dE9 mice. Int. J. Immunopathol. Pharmacol. 2017, 30, 25–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Su, C.; Li, R.; Wang, H.; Ren, Y.; Sun, H.; Yang, J.; Sun, J.; Shi, J.; Tian, J.; et al. Mechanisms and effects of curcumin on spatial learning and memory improvement in APPswe/PS1dE9 mice. J. Neurosci. Res. 2014, 92, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chang, L.; Viola, K.L.; Lacor, P.N.; Lambert, M.P.; Finch, C.E.; Krafft, G.A.; Klein, W.L. Alzheimer’s disease-affected brain: Presence of oligomeric a beta ligands (addls) suggests a molecular basis for reversible memory loss. Proc. Natl. Acad. Sci. USA 2003, 100, 10417–10422. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Q.; de Felice, F.G.; Fernandez, S.; Chen, H.; Lambert, M.P.; Quon, M.J.; Krafft, G.A.; Klein, W.L. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008, 22, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Lublin, A.L.; Gandy, S. Amyloid-beta oligomers: Possible roles as key neurotoxins in alzheimer’s disease. Mt. Sinai J. Med. 2010, 77, 43–49. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C. PI3-kinase/Akt/mTOR signaling: Impaired on/off switches in aging, cognitive decline and alzheimer’s disease. Exp. Gerontol. 2013, 48, 647–653. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.; Kiely, A.P.; Coakley, M.F.; Manning, S.; Long-Smith, C.M. Insulin and IGF-1 signalling: Longevity, protein homoeostasis and alzheimer’s disease. Biochem. Soc. Trans. 2012, 40, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.; Loewith, R.; Hall, M.N. Tor signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Teng, Z.; Zhang, T.; Li, Y. Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur. J. Pharmacol. 2014, 740, 312–320. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, P.; Wei, P.; Feng, H.; Ren, Y.; Yang, J.; Rao, Y.; Shi, J.; Tian, J. Effects of curcumin on synapses in APPswe/PS1dE9 mice. Int. J. Immunopathol. Pharmacol. 2016, 29, 217–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Son, T.G.; Park, H.R.; Park, M.; Kim, M.S.; Kim, H.S.; Chung, H.Y.; Mattson, M.P.; Lee, J. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J. Biol. Chem. 2008, 283, 14497–14505. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zeng, Q.; Mitchell, E.S.; Xiu, J.; Duan, Y.; Li, C.; Tiwari, J.K.; Hu, Y.; Cao, X.; Zhao, Z. Curcumin enhances neurogenesis and cognition in aged rats: Implications for transcriptional interactions related to growth and synaptic plasticity. PLoS ONE 2012, 7, e31211. [Google Scholar] [CrossRef] [PubMed]
- Belviranli, M.; Okudan, N.; Atalik, K.E.; Oz, M. Curcumin improves spatial memory and decreases oxidative damage in aged female rats. Biogerontology 2013, 14, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Bekinschtein, P.; Cammarota, M.; Katche, C.; Slipczuk, L.; Rossato, J.I.; Goldin, A.; Izquierdo, I.; Medina, J.H. BDNF is essential to promote persistence of long-term memory storage. Proc. Natl. Acad. Sci. USA 2008, 105, 2711–2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.P.; Lindegaard, B.; Petersen, A.M.; Taudorf, S.; et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Navaratna, D.; Guo, S.Z.; Hayakawa, K.; Wang, X.; Gerhardinger, C.; Lo, E.H. Decreased cerebrovascular brain-derived neurotrophic factor-mediated neuroprotection in the diabetic brain. Diabetes 2011, 60, 1789–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreassen, C.S.; Jakobsen, J.; Flyvbjerg, A.; Andersen, H. Expression of neurotrophic factors in diabetic muscle—Relation to neuropathy and muscle strength. Brain 2009, 132, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Karczewska-Kupczewska, M.; Kowalska, I.; Nikolajuk, A.; Adamska, A.; Zielinska, M.; Kaminska, N.; Otziomek, E.; Gorska, M.; Straczkowski, M. Circulating brain-derived neurotrophic factor concentration is downregulated by intralipid/heparin infusion or high-fat meal in young healthy male subjects. Diabetes Care 2012, 35, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Bazinet, R.P.; Sinclair, A.J. Fat intake and cns functioning: Ageing and disease. Ann. Nutr. Metab. 2009, 55, 202–228. [Google Scholar] [CrossRef] [PubMed]
- Marszalek, J.R.; Lodish, H.F. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: Breastmilk and fish are good for you. Annu. Rev. Cell Dev. Biol. 2005, 21, 633–657. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Noble, E.E.; Tyagi, E.; Ying, Z.; Zhuang, Y.; Gomez-Pinilla, F. Curcumin boosts dha in the brain: Implications for the prevention of anxiety disorders. Biochim. Biophys. Acta 2015, 1852, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Pinkaew, D.; Changtam, C.; Tocharus, C.; Thummayot, S.; Suksamrarn, A.; Tocharus, J. Di-O-demethylcurcumin protects SK-N-SH cells against mitochondrial and endoplasmic reticulum-mediated apoptotic cell death induced by Abeta25-35. Neurochem. Int. 2015, 80, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W. Inflammation and neurodegenerative diseases. Am. J. Clin. Nutr. 2006, 83, S470–S474. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J. Microglia as neuroprotective, immunocompetent cells of the cns. Glia 2002, 40, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.K. Microglia as a source and target of cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; Garavito, R.M.; DeWitt, D.L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 1996, 271, 33157–33160. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.K.; Lieb, K.; Schulze-Osthoff, K.; Berger, M.; Gebicke-Haerter, P.J.; Bauer, J.; Fiebich, B.L. Expression and regulation of cyclooxygenase-2 in rat microglia. Eur.J. Biochem. 1997, 243, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Kong, P.J.; Yuh, Y.J.; Lim, S.Y.; Yim, S.V.; Chun, W.; Kim, S.S. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappab bindings in BV2 microglial cells. J. Pharmacol. Sci. 2004, 94, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.Y.; Lee, J.D.; Park, C.; Choi, Y.H.; Kim, G.Y. Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta Pharmacol. Sin. 2007, 28, 1645–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlstetter, M.; Lippe, E.; Walczak, Y.; Moehle, C.; Aslanidis, A.; Mirza, M.; Langmann, T. Curcumin is a potent modulator of microglial gene expression and migration. J. Neuroinflamm. 2011, 8, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Bhattacharyya, S.; Rashid, K.; Sil, P.C. Curcumin protects rat liver from streptozotocin-induced diabetic pathophysiology by counteracting reactive oxygen species and inhibiting the activation of p53 and MAPKs mediated stress response pathways. Toxicol. Rep. 2015, 2, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ros-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]

| Dose | Design | Subjects | Period | Effects | No Effects | Ref |
|---|---|---|---|---|---|---|
| 1 g/day of curcuminoids (curcumin C3 Complex® Sami Labs LTD, Bangalore, Karnataka, India) combined with 10 mg/day of piperine | randomised double-blind, placebo-controlled | 100 subjects with T2DM aged 18–65 years | 12 weeks | ↓ body weight, ↓ BMI, ↓ TC, ↓ Lp(a), ↑ HDL-C compared with the placebo | TG, LDL-C compared with the placebo | Panahi et al. 2017 [45] |
| 800 mg × 2/day of curcumin-based product (enteric-coated and containing 800 mg/dose/die of 95% curcumin complexed with 20% phosphatidylserine and blended with 8 mg/dose/die of piperine) + lifestyle intervention, or 400 mg × 2 day of phosphatidylserine + lifestyle intervention | randomised parallel | 44 overweight subjects with metabolic syndrome (mean BMI 25–29.9 kg/m2; mean age 39.1 ± 16.8 years) | 4 weeks | ↓ body weight, ↓ body fat, ↓ waistline, ↓ BMI compared with the phosphatidylserine group | Di Pierro et al. 2015 [46] | |
| 80 mg/day of nano- micelle curcumin (SinaCurcumin® Exir Nano Sina Co., Tehran, Tehran province, Iran) | randomised, double-blind, placebo-controlled | 70 subjects with T2DM aged over 18 years | 3 months | ↓ HbA1C, ↓ fasting blood glucose, ↓ BMI compared with the placebo | TC, LDL-C, HDL-C compared with the placebo | Rahimi et al. 2016 [57] |
| 400 mg /day of Longvida® (containing ~ 80 mg curcumin in a solid lipid formulation) | randomised, double-blind, placebo-controlled, parallel | 60 elderly subjects (mean age: 68.5 years) | 4 weeks | ↓ TC, ↓ LDL-C, ↑ memory, ↑ mood compared with the placebo | Cox et al. 2015 [68] | |
| 80 mg/day of lipidated curcumin (Longvida®) | 38 healthy middle-aged subjects (40–60 years old) | 4 weeks | TC, LDL, HDL-C, CRP, TG, salivary amylase, salivary radical scavenging capacities, catalase, beta amyloid protein, sICAM, myeloperoxidase, nitric oxide, ALT | Disilvestro et al. 2012 [79] | ||
| 500 mg/day of curcumin | open uncontrolled | 14 subjects with T2DM | 15 days | fasting glucose, insulin, C-peptide, TG, TC, HDL-C, LDL-C, ALT, AST, BUN | Yang et al. 2015 [80] | |
| 2.8 g/day of turmeric (~112 mg/day of curcumin) | randomised, double-blind, placebo-controlled, crossover | 62 overweight/obese women aged over 40–75 years [mean (BMI) ≥ 34.5 ± 0.8 kg/m2] with CRP = 8.05 ± 1.33 mg/L | 4 weeks | F2-iso-prostanes, oxidised LDL-C, CRP, IL-6, IL-8, IL-10, TNFα, IFNγ, IL-1β, IL-12p70, glucose, body weight, percent body fat, SBP, augmentation index | Nieman et al. 2012 [81] | |
| 1 g/day of curcuminoids (500 mg C3Complex® + 5 mg bioperine®) | randomised double-blind placebo-controlled crossover | 30 subjects aged 18–65 years who were not taking lipid-lowering agent, as well as who had any conditions including BMI ≥ 30 kg/m2 or 2 risk factors (except for T2DM) for CHD or ≥ 2 risk factors (except for T2DM) for CHD and 130 mg/dL < LDL-C <160 mg/dL | 30 days | ↓ TG compared with the placebo | LDL-C, HDL-C, CRP. body weight, BMI, waist circumference, arm circumference, fat percentage | Mohammadi et al. 2013 [100] |
| 300 mg/day of curcumin (NCB-02) | randomised, placebo-controlled, parallel | 67 subjects with T2DM aged 21–80 years | 8 weeks | fasting glucose, HbA1c, TC, LDL-C, HDL-C and TG endothelial function, MDA, ET-1, IL-6, TNF-α | Usharani et al. 2008 [108] | |
| 1 g/day of curcuminoids (500 mg C3Complex® + 5 mg bioperine®) | randomised, double-blind, crossover | 30 obese subjects with BMI ≥ 30 | 4 weeks | ↓ IL-4, ↓ IL-1 β, ↓ VEGF compared with the placebo | IL-2, IL-6, IL-8, IL-10, IFN γ, EGF, MCP-1 | Ganjali et al. 2014 [111] |
| 1 g/day of curcumin | 117 subjects with metabolic syndrome | 8 weeks | ↓ TNF-α, ↓ IL-6, ↓ TGF-β, ↓MCP-1 compared with placebo | Panahi et al. 2016 [112] | ||
| 500 mg/day of an amorphous dispersion curcumin formulation (comprising 70-mg curcuminoids) | randomised double-blind, placebo-controlled | 77 subjects with NAFLD (mean age 46.37 ± 11.57 years; mean BMI 31.35 ± 5.67 kg/m2) | 8 weeks | ↓ glucose, ↓ HbA1c, ↓ TC, ↓ LDL, ↓ TG, ↓ liver fat, ↓ BMI, ↓ AST, ↓ ALT compared with the placebo | Rahmani et al. 2016 [134] | |
| 300 mg/day of curcuminoids | randomised double-blind, placebo-controlled | 100 overweight/obese subjects with T2DM (average age: 54.72 ± 8.34 years; BMI ≥ 24.0 | 12 weeks | ↓ fasting glucose, ↓ HbA1c, ↓ HOMA-IR, ↓ FFAs, ↓ TG, ↑ LPL compared with a placebo | TC, LDL- C, HDL- C, Apo A-I or Apo B, body weight, waist and hip circumferences | Na et al. 2013 [135] |
| 1.5 g/day of curcuminoid | randomised, double-blind, placebo-controlled | 237 prediabetic subjects | 3, 6, 9 months | ↓ HbA1c, ↓ fasting glucose, ↓ OGTT at 3, 6, and 9 months, ↓ diagnosis of T2DM, ↑ HOMA-β at 6 & 9 months, ↑ adiponectin at 9 months, ↓ C-peptide at 9 months, ↓ insulin at 9 months, ↓ body weight at 9 months compared with the placebo | AST, ALT, creatinine, bone mineral density, waist circumference | Chuengsamarn et al. 2012 [136] |
| 1 g/day of phospholipidated curcumin (n = 40) equivalent to 200 mg/day of pure curcumin, or 1 g/day of unformulated curcumin (n = 40), or placebo (n = 40) | Randomised, double-blind, placebo-controlled | 120 subjects with metabolic syndrome aged 18–65 years | 6 weeks | ↑ adiponectin in the unformulated curcumin group compared with the curcumin-phospholipid complex group or the placebo group | BMI, body weight, waist circumference, fasting blood glucose, fat (%) compared with a curcumin-phospholipid complex group or a placebo group | Salahshooh et al. 2017 [138] |
| 750 mg/day of curcuminoid | randomised double-blind, placebo-controlled | subjects with T2DM (mean age 59 ± 10.6 years; n = 107 | 6 months | ↑ adiponectin, ↓ leptin, ↓ PWV, ↓ HOMA-IR, ↓ TG, ↓ uric acid, ↓ visceral fat, ↓ total body fat compared with the placebo | Chuengsamarn et al. 2014 [139] | |
| 2.8 g/day of turmeric | randomised crossover | 11 healthy subjects aged 21–38 years | 4 weeks | fasting glucose, TC, TG | Tang et al. 2008 [140] | |
| 6 g of curcuma C. longa (turmeric) | crossover, acute | 14 healthy subjects (mean age: 29 ± 1 years, BMI: 23.9 ± 2.7 kg/m2) | ↑ insulin responses compared with the placebo | postprandial glucose responses | Wickenberg et al. 2010 [141] | |
| 500 and 750 mg/day of curcumin | randomised, single-blind, placebo-controlled | nondiabetic obese subjects | 12 weeks | BDNF, body weight, BMI, fat, glucose, TC, LDL, TG, HDL-C, VLDL, uric acid, oxLDL, protein carbonyls | Franco-Robles et al. 2014 [142] | |
| 1890 mg/day of curcumin extract | randomised, double-blind, placebo-controlled | 33 subjects aged over 40–60 years with metabolic syndrome (mean BMI: 30.06 ± 4.12 kg/m2) | 12 weeks | ↓ LDL-C compared with the placebo | weight, BMI, fasting glucose, HbA1C, TG, TC, VLDL, HDL-C, Non-HDL-C and T-Chol/HDL-C ratio | Yang et al. 2014 [143] |
| 2 g/day of curcumin (Longvida®) | double-blind, parallel, randomised | 39 healthy middle-aged and older adults (45–74 years) | 12 weeks | Nil compared with placebo | adiponectin, leptin, insulin, HOMA-IR, oxidised LDL-C, total antioxidant status, GPx, IL-6, TNF-α, cortisol, ET-1 FMD resistance artery endothelial function (FBFACh) | Santos-Parker et al. 2017 [144] |
| 45–180 mg/day of curcumin | randomised double-blind controlled | 75 subjects with acute coronary syndrome | 1 year | TC, LDL-C, HDL-C, TG, fasting glucose, 2-hour glucose | Alwi et al. 2008 [145] | |
| 1000 mg/day of curcumin + dietary and lifestyle intervention | Randomised, placebo-controlled | 87 subjects with NAFLD | 8 weeks | ↓ TC, ↓ non–HDL-C, ↓ LDL-C, ↓ TG ↓ uric acid compared with the placebo. | HbA1c, fasting glucose, insulin, HOMA-IR, HOMA-β, quantitative insulin sensitivity check index (QUICKI) compared with the placebo. insulin, HOMA-IR, HOMA-β. QUICKI within the group. | Panahi et al. 2016 [146] |
| 4 g/day or 1 g/day of curcumin | randomised, double-blind, placebo-controlled | 36 elderly subjects (mean age: 73.4 ± 8.8 years) | over 1 month or 6 months | TG, LDL-C, HDL-C | Baum et al. 2007 [160] | |
| 200 mg/day of curcumin (Meriva ®, Indena SpA, Viale Ortles, Milan, Italy) + 2 g/day of phytosterols (n = 17), or 200 mg/day of curcumin (n = 18), or 2 g/day of phytosterols (n = 17), or placebo (n = 18) | double-blind, randomised, placebo-controlled, 2 × 2 factorial trial | 70 hypercholesterolemia subjects (mean fasting TC: 6.57 ± 0.13 mM/L) aged 18–70 years | 4 weeks | ↓ TC, ↓LDL-C and↓ TC: HDL-C within phytosterol group and within curcumin plus phytosterol group. No change with curcumin alone | Ferguson et al. 2018 [161] | |
| 1000 mg/day of curcuminoids and piperine (bioperine®) (100: 1 ratio combination) | randomised double-blind, placebo-controlled parallel | 117 subjects with metabolic syndrome (aged 25–75 years) | 8 weeks | ↓ LDL-C, ↓non-HDL-C, ↓ TC, ↓ TG, ↓ Lp(a), ↑ HDL-C compared with the placebo | sdLDL | Panahi et al. 2014 [163] |
| 1 g/day of curcuminoids (500 mg C3Complex® + 5 mg bioperine® Sami Labs LTD, Bangalore, Karnataka, India) | randomised, double-blind, placebo-controlled, crossover | 30 obese subjects (mean BMI 33.95 ± 3.81) | 30 days | BMI and weight compared with the placebo | Esmaily et al. 2015 [186] | |
| 50 mg/day of curcumin (250 mgCurcuWIN) or 200 mg/day of curcumin (1000 mg CurcuWIN) | randomised controlled double-blind, parallel | 59 healthy subjects aged 19–29 years | 8 weeks | 200 mg/ day of curcumin: ↑ endothelial function compared with placebo | Oliver et al. 2016 [237] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Clifton, P. Curcumin, Cardiometabolic Health and Dementia. Int. J. Environ. Res. Public Health 2018, 15, 2093. https://doi.org/10.3390/ijerph15102093
Kim Y, Clifton P. Curcumin, Cardiometabolic Health and Dementia. International Journal of Environmental Research and Public Health. 2018; 15(10):2093. https://doi.org/10.3390/ijerph15102093
Chicago/Turabian StyleKim, Yoona, and Peter Clifton. 2018. "Curcumin, Cardiometabolic Health and Dementia" International Journal of Environmental Research and Public Health 15, no. 10: 2093. https://doi.org/10.3390/ijerph15102093





