In Search of Factors Negatively Affecting Vaccine Immunity to Pertussis in Preschool Children before the Administration of the First Booster
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group of Children
2.2. Serological Analysis
2.3. Statistical Analysis
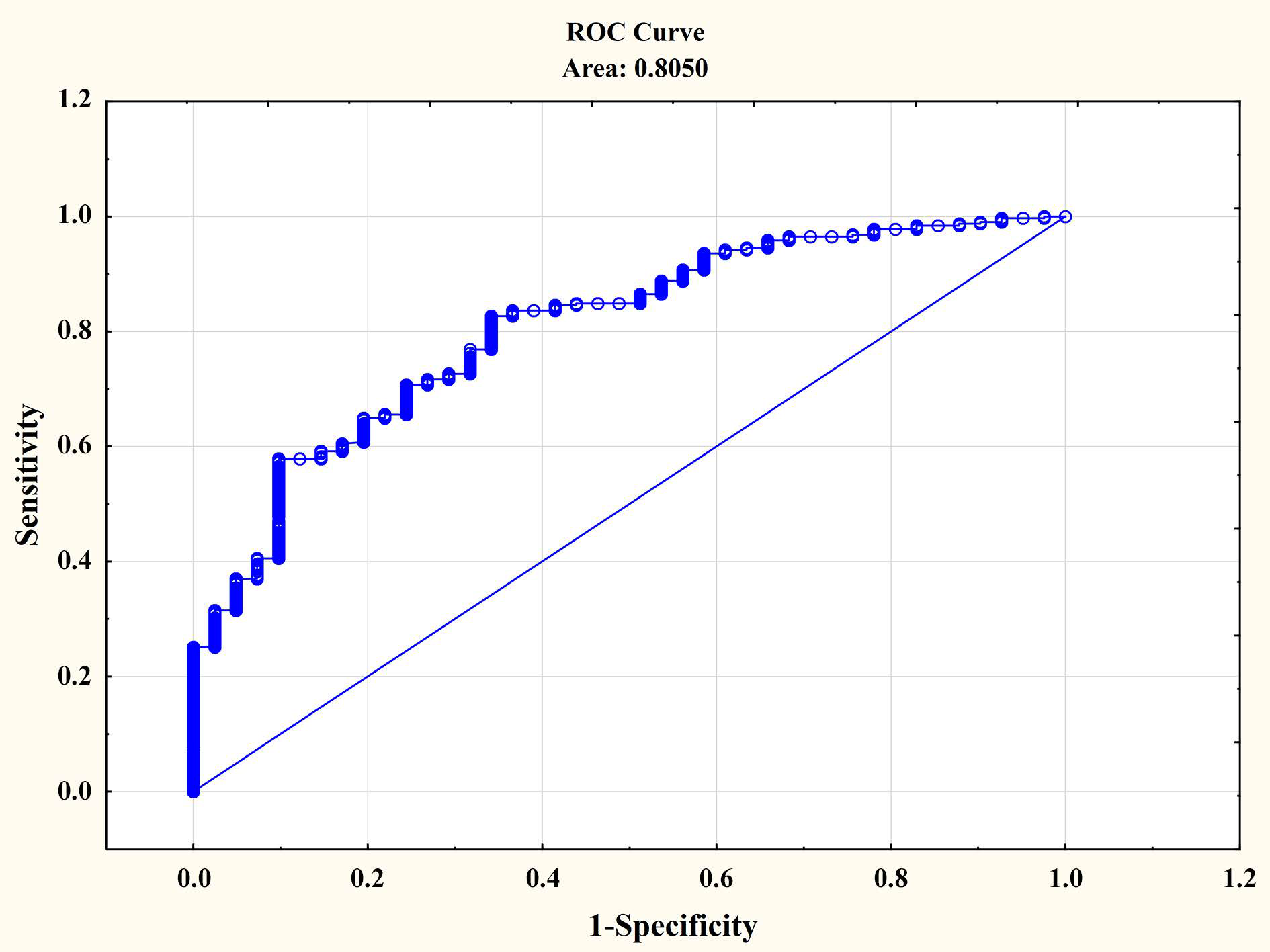
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baxter, D. Impaired functioning of immune defenses to infection in premature and term infants and their implications for vaccination. Hum. Vaccines 2010, 6, 494–505. [Google Scholar] [CrossRef]
- Ghazal, P.; Dickinson, P.; Smith, C.L. Early life response to infection. Curr. Opin. Infect. Dis. 2013, 26, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Gagneur, A.; Pinquier, D.; Quach, C. Immunization of preterm infants. Hum. Vaccines Immunother. 2015, 11, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D. Why do pertussis vaccines fail? Pediatrics 2012, 129, 968–970. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Seaton, B.L. Patterns of Bordetella parapertussis respiratory illnesses: 2008–2010. Clin. Infect. Dis. 2012, 54, 534–537. [Google Scholar] [CrossRef] [PubMed]
- De Greeff, S.C.; Mooi, F.R.; Westerhof, A.; Verbakel, J.M.; Peeters, M.F.; Heuvelman, C.J.; Heuvelman, C.J.; Notermans, D.W.; Elvers, L.H.; Schellekens, J.F.; et al. Pertussis disease burden in the household: How to protect young infants. Clin. Infect. Dis. 2010, 50, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Bartlett, J.; Rowhani-Rahbar, A.; Fireman, B.; Baxter, R. Waning protection after fifth dose of acellular pertussis vaccine in children. N. Engl. J. Med. 2012, 367, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Barlett, J.; Fireman, B.; Rowhani-Rahbar, A.; Baxter, R. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics 2013, 131, e1716–e1722. [Google Scholar] [CrossRef] [PubMed]
- Liko, J.; Robison, S.G.; Cieslak, P.R. Proming with whole-cell versus acellular pertussis vaccine. N. Engl. J. Med. 2013, 368, 581–582. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.W.; Pawloski, L.; Williams, M.; Weening, K.; DeBolt, C.; Qin, X.; Reynolds, L.; Kenyon, C.; Giambrone, G.; Kudish, K.; et al. Pertactin-negative Bordetella pertussis strains: Evidence for a possible selective advantage. Clin. Infect. Dis. 2015, 60, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.J. Research aims to boost pertussis control. JAMA 2011, 306, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, L.; Hessel, L.; Storsaeter, J.; Olin, P. Long-term follow-up of Swedish children vaccinated with acellular pertussis vaccines at 3, 5 and 12 months of age indicates the need for a booster dose at 5 to 7 years of age. Pediatrics 2006, 118, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, P.E.; Salim, A.M.; Zervos, M.J.; Schmitt, H. Pertussis: Microbiology, Disease, Treatment, and Prevention. Clin. Microbiol. Rev. 2016, 29, 449–486. [Google Scholar] [CrossRef] [PubMed]
- Fisman, D.N.; Tang, P.; Hauck, T.; Richardson, S.; Drews, S.J.; Low, D.E.; Jamieson, F. Pertussis resurgence in Toronto, Canada: A population-based study including test-incidence feedback modeling. BMC Public Health 2011, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.M.; Rench, M.A.; Wootton, S.H.; Castagnini, L.A. Evaluation of the impact of a pertussis cocooning program on infant pertussis infection. Pediatr. Infect. Dis. J. 2015, 34, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Locht, C.; Mielcarek, N. New pertussis vaccination approaches: En route to protect newborns? FEMS Immunol. Med. Microbiol. 2012, 66, 121–133. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M. Acellular vaccines provided less protection during California pertussis outbreak. BMJ 2013, 346, f3325. [Google Scholar] [CrossRef] [PubMed]
- Zeddeman, A.; van Gent, M.; Heuvelman, C.J.; van der Heide, H.G.; Bart, M.J.; Advani, A.; Hallander, H.O.; von Konig, C.H.W.; Riffelman, M.; Storsaeter, J.; et al. Investigations into the emergence of pertactin deficient Bordetella pertussis isolated in six European countries, 1996 to 2012. Euro Surveill. 2014, 19, 20881. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global routine vaccination coverage, 2011. Wkly. Epidemiol. Rec. 2012, 44, 432–435. [Google Scholar]
- Libster, R.; Edwards, K.M. Re-emergence of pertussis: What are the solutions? Expert Rev. Vaccines 2012, 11, 1331–1346. [Google Scholar] [CrossRef] [PubMed]
- Souder, E.; Long, S.S. Pertussis in the Era of New Strains of Bordetella pertussis. Infect. Dis. Clin. N. Am. 2015, 29, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Van Gent, M.; Heuvelman, K.J.; van der Heide, H.G.J.; Hallander, H.O.; Advani, A.; Guiso, N.; von Konig, C.H.W.; Vestrheim, D.F.; Dalby, T.; Fry, N.K.; et al. Analysis of Bordetella pertussis clinical isolates circulating in European countries from 1998–2012. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Vickers, D.; Ross, A.G.; Mainar-Jaime, R.C.; Neudorf, C.; Shah, S. Whole-cell and acellular pertussis vaccination programs and rates of pertussis among infants and young children. CMAJ 2006, 175, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Rumbo, M.; Hozbor, D. Development of improved pertussis vaccine. Hum. Vaccines Immunother. 2014, 10, 2450–2453. [Google Scholar] [CrossRef] [PubMed]
- From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2012. Available online: http://www.ginasthma.org/ (accessed on 5 August 2013).
- Pinto, M.V.; Merkel, T.J. Pertussis disease and transmission and host responses: Insights from the baboon model of pertussis. J. Infect. 2017, 74, S114–S119. [Google Scholar] [CrossRef]
- Nieves, D.J.; Heininger, U. Bordetella pertussis. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Salmaso, S.; Mastrantonio, P.; Tozzi, A.E. Sustained efficacy during the first 6 years of life of 3-component acellular pertussis vaccines administered in infancy: The Italian experience. Pediatrics 2001, 108, E81. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.L.; Kwong, J.C.; Deeks, S.L.; Campitelli, M.A.; Jamieson, F.B.; Marchand-Austin, A.; Stukel, T.A.; Rosella, L.; Daneman, N.; Bolotin, S.; et al. Effectiveness of pertussis vaccination and duration of immunity. CMAJ 2016, 188, E399–E406. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, S.L.; Ware, R.S.; Grimwood, K.; Lamberst, S.B. Number and order of whole cel pertussis vaccines in infancy and disease protection. JAMA 2012, 308, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Dalby, T.; Forsythe, K.; Halperin, S.A.; Heininger, U.; Hozbor, D.; Plotkin, S.; Ulloa-Gutierrez, R.; von König, C.H.W. Pertussis across the globe. Pediatr. Infect. Dis. J. 2015, 34, e222–e232. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; Cherry, J.D.; Harrman, K. Effectiveness of prenatal versus postpartum tetanus, diphtheria, and acellular pertussis vaccination in preventing infant pertussis. Clin. Infect. Dis. 2017, 64, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Misegades, L.K.; Martin, S.W.; Messonnier, N.E.; Clark, T.A. Estimating the effectiveness of acellular pertussis vaccines. Clin. Infect. Dis. 2012, 55, 1432–1433. [Google Scholar] [CrossRef] [PubMed]
- Witt, M.A.; Katz, P.H.; Witt, D.J. Unexpectedly limited durability of immunity following acellular pertussis vaccinationin preadolescents in a North American outbreak. Clin. Infect. Dis. 2012, 54, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, B.; Gröndahl-Yli-Hannuksila, K.; Tan, Y.; Feng, L.; Kallonen, T.; Wang, L.; Peng, D.; He, Q.; Wang, L.; et al. Whole-genome sequencing reveals the effect of vaccination on the evolution of Bordetella pertussis. Sci. Rep. 2015, 5, 12888. [Google Scholar] [CrossRef] [PubMed]
- Bart, M.J.; Harris, S.R.; Advani, A.; Arakawa, Y.; Bottero, D.; Bouchez, V.; Cassiday, P.K.; Chiang, C.-S.; Dalby, T.; Fry, N.K.; et al. Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. mBio 2014, 5, e01074-14. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, C.S.; Blanchard-Rohner, G.; Lemaitre, B.; Boukrid, M.; Combescure, C.; Othenin-Girard, V.; Chilin, A.; Petre, J.; de Tejada, B.M.; Siegrist, C.A. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin. Infect. Dis. 2016, 62, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Castagnini, L.A.; Healy, C.M.; Rench, M.A.; Wooton, S.H.; Munoz, F.M.; Baker, C.L. Impact of maternal postpartum tetanus and diphtheria toxoids and acellular pertussis immunization on infant pertussis infection. Clin. Infect. Dis. 2012, 54, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.L.; Meade, B.D.; Messionnier, N.E. Pertussis resurgence: Perspectives from the working group meeting on pertussis on the causes, possible paths forward, and gaps in our knowledge. J. Infect. Dis. 2014, 209, S32–S35. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Aguado, M.T.; Dutel, C.; Goronzy, J.; Louis, J.; Grubeck-Loebenstein, B.; Rappuoli, R.; Del Giudice, G. The gracefully aging immune system. Sci. Transl. Med. 2013, 5, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Bodilis, H.; Guiso, N. Virulence of pertactin-negative Bordetella pertussis isolates from infants, France. Emerg. Infect. Dis. 2013, 19, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, G.; Andrews, N.; Campbell, H.; Ribeiro, S.; Kara, E.; Donegan, K.; Fry, N.K.; Miller, E.; Ramsay, M. Effectiveness of maternal pertussis vaccination in England: An observational study. Lancet 2014, 384, 1521–1528. [Google Scholar] [CrossRef]
- Clark, T.A. Changing pertussis epidemiology: Everything old is new again. J. Infect. Dis. 2014, 209, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D. Epidemic pertussis in 2012—The resurgence of a vaccine-preventable Disease. N. Engl. J. Med. 2012, 367, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Q. Bordetella pertussis isolates circulating in China where whole cell vaccines have been used for 50 years. Clin. Infect. Dis. 2015, 61, 1028–1029. [Google Scholar] [CrossRef] [PubMed]
- Locht, C.; Mielcarek, N. Live attenuated vaccines against pertussis. Expert Rev. Vaccines 2014, 13, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.S. Gender differences in asthma development and progression. Gend. Med. 2007, 4, S133–S146. [Google Scholar] [CrossRef]
- Lai, X.; Li, J.; Xiao, X.; Liu, E.; Zhang, C.; Wang, H.; Gjesing, B.; Zhong, N.; Spangfort, M.D. Specific IgG4 production during house dust mite immunotherapy among age, gender and allergic disease populations. Int. Arch. Allergy Immunol. 2013, 160, 37–46. [Google Scholar] [CrossRef] [PubMed]

| Feature | Pertussis ≥10 U/mL (+) * | Pertussis <10 U/mL (−) | p | ||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||
| Birth weight (g) | 311 | 3416.05 | 462.72 | 41 | 3432.75 | 465.81 | 0.7699 |
| Current weight (kg) | 311 | 20.73 | 3.55 | 41 | 20.61 | 4.35 | 0.8034 |
| Birth body length (cm) | 311 | 55.13 | 3.75 | 41 | 54.55 | 4.38 | 0.3288 |
| Current height (cm) | 311 | 117.86 | 4.54 | 41 | 118.46 | 5.29 | 0.5468 |
| Feature | Pertussis ≥10 U/mL (+) n = 311 | Pertussis <10 U/mL (−) n = 41 | p | OR | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||||
| Gender | Male | 172 | 55.31 | 27 | 65.85 | 0.2003 | 1.56 | 0.79 | 3.09 |
| Female | 139 | 44.69 | 14 | 34.15 | |||||
| Birth age (week) | <37 (32–36) | 41 | 13.18 | 5 | 12.20 | 0.5466 | 0.91 | 0.34 | 2.47 |
| ≥37 | 270 | 86.82 | 36 | 87.80 | |||||
| First child in family | Yes | 86 | 27.65 | 9 | 21.95 | 0.2823 | 1.36 | 0.62 | 2.97 |
| No | 225 | 72.35 | 32 | 78.05 | |||||
| Age * | <Me | 133 | 42.77 | 17 | 41.46 | 0.8741 | 0.95 | 0.49 | 1.84 |
| >Me | 178 | 57.23 | 24 | 58.54 | |||||
| Place of residence | Town | 221 | 71.06 | 27 | 65.85 | 0.4921 | 0.79 | 0.39 | 1.57 |
| Countryside | 90 | 28.94 | 14 | 34.15 | |||||
| Suspected asthma | Yes | 142 | 45.66 | 34 | 82.93 | <0.001 | 5.78 | 2.49 | 1.44 |
| No | 169 | 54.34 | 7 | 17.07 | |||||
| Suffered infectious diseases ** | Yes | 51 | 16.40 | 6 | 14.63 | 0.4909 | 1.14 | 0.46 | 2.86 |
| No | 260 | 83.60 | 35 | 85.37 | |||||
| Taking immunostimulating drugs *** | Yes | 150 | 48.23 | 29 | 70.73 | <0.01 | 0.39 | 0.19 | 0.78 |
| No | 161 | 51.77 | 12 | 29.27 | |||||
| Type of nutrition at the age of 0–6 months old | Breast milk + evaporated milk formula | 33 | 10.61 | 5 | 12.20 | 0.7195 | |||
| Breast milk | 200 | 64.31 | 28 | 68.29 | |||||
| evaporated milk formula | 78 | 25.08 | 8 | 19.51 | |||||
| Type of immunisation (vaccine) from birth to 2–2.5 years old | Uncombined (DTwP, HBV, IPV, Hib, MMR) | 147 | 47.27 | 29 | 70.73 | < 0.01 | 2.70 | 1.33 | 5.48 |
| Combined (DTaP-HBV-IPV-Hib, MMR) | 164 | 52.73 | 12 | 29.27 | |||||
| Promptness of vaccinations | Yes | 230 | 73.95 | 29 | 70.73 | 0.6599 | 1.17 | 0.57 | 2.41 |
| No | 81 | 26.05 | 12 | 29.27 | |||||
| Type of Vaccination and Protective Antibody Titre | Pertussis ≥10 U/mL (+) n = 311 | Pertussis <10 U/mL (−) n = 41 | p | OR | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||||
| Diphtheria | ≥1 IU/mL (+) | 140 | 45.02 | 13 | 31.71 | 0.1061 | 0.57 | 0.28 | 1.14 |
| <1 IU/mL | 171 | 54.98 | 28 | 68.29 | |||||
| Tetanus | ≥1 IU/mL (+) | 221 | 71.06 | 23 | 56.10 | 0.0508 | 0.52 | 0.27 | 1.01 |
| <1 IU/mL | 90 | 28.94 | 18 | 43.90 | |||||
| HBV | >12.5 mlU/mL (+) | 249 | 80.06 | 30 | 73.17 | 0.3061 | 0.68 | 0.32 | 1.43 |
| <12.5 mlU/mL | 62 | 19.94 | 11 | 26.83 | |||||
| Hib | ≥1 µg/mL (+) | 284 | 91.32 | 31 | 75.61 | <0.01 | 0.29 | 0.13 | 0.67 |
| <1 µg/mL | 27 | 8.68 | 10 | 24.39 | |||||
| Mumps | ≥12 U/mL (+) | 253 | 81.35 | 31 | 75.61 | 0.381 | 0.71 | 0.33 | 1.53 |
| <12 U/mL | 58 | 18.65 | 10 | 24.39 | |||||
| Rubella | ≥12 lU/mL (+) | 277 | 89.07 | 35 | 85.37 | 0.3151 | 0.72 | 0.28 | 1.83 |
| <12 lU/mL | 34 | 10.93 | 6 | 14.63 | |||||
| Category of Analysis | Wald | p |
|---|---|---|
| Age A | 0.64 | 0.4253 |
| Gender | 3.99 | 0.0459 |
| Place of residence | 1.86 | 0.1729 |
| Childhood infectious diseases undergone | 0.08 | 0.7716 |
| Taking immunestimulating preparations | 0.00 | 0.9643 |
| Birth age | 0.02 | 0.8840 |
| Type of nutrition at the age of 0-6 months old | 0.49 | 0.4826 |
| Type of vaccine | 8.22 | 0.0041 |
| Promptness of vaccinations | 0.30 | 0.5853 |
| Group (children tentatively diagnosed with asthma) | 12.93 | 0.0003 |
| Hepatitis B | 1.56 | 0.2113 |
| Diphtheria | 1.70 | 0.1924 |
| Tetanus | 0.08 | 0.7817 |
| Hib | 6.17 | 0.0130 |
| Mumps | 4.78 | 0.0288 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarek, A.; Bodajko-Grochowska, A.; Hasiec, B.; Klepacz, R.; Szczekala, K.; Zarzycka, D.; Emeryk, A. In Search of Factors Negatively Affecting Vaccine Immunity to Pertussis in Preschool Children before the Administration of the First Booster. Int. J. Environ. Res. Public Health 2018, 15, 1432. https://doi.org/10.3390/ijerph15071432
Bednarek A, Bodajko-Grochowska A, Hasiec B, Klepacz R, Szczekala K, Zarzycka D, Emeryk A. In Search of Factors Negatively Affecting Vaccine Immunity to Pertussis in Preschool Children before the Administration of the First Booster. International Journal of Environmental Research and Public Health. 2018; 15(7):1432. https://doi.org/10.3390/ijerph15071432
Chicago/Turabian StyleBednarek, Anna, Anna Bodajko-Grochowska, Barbara Hasiec, Robert Klepacz, Katarzyna Szczekala, Danuta Zarzycka, and Andrzej Emeryk. 2018. "In Search of Factors Negatively Affecting Vaccine Immunity to Pertussis in Preschool Children before the Administration of the First Booster" International Journal of Environmental Research and Public Health 15, no. 7: 1432. https://doi.org/10.3390/ijerph15071432
APA StyleBednarek, A., Bodajko-Grochowska, A., Hasiec, B., Klepacz, R., Szczekala, K., Zarzycka, D., & Emeryk, A. (2018). In Search of Factors Negatively Affecting Vaccine Immunity to Pertussis in Preschool Children before the Administration of the First Booster. International Journal of Environmental Research and Public Health, 15(7), 1432. https://doi.org/10.3390/ijerph15071432




