The Cumulative Risk of Chemical and Nonchemical Exposures on Birth Outcomes in Healthy Women: The Fetal Growth Study
Abstract
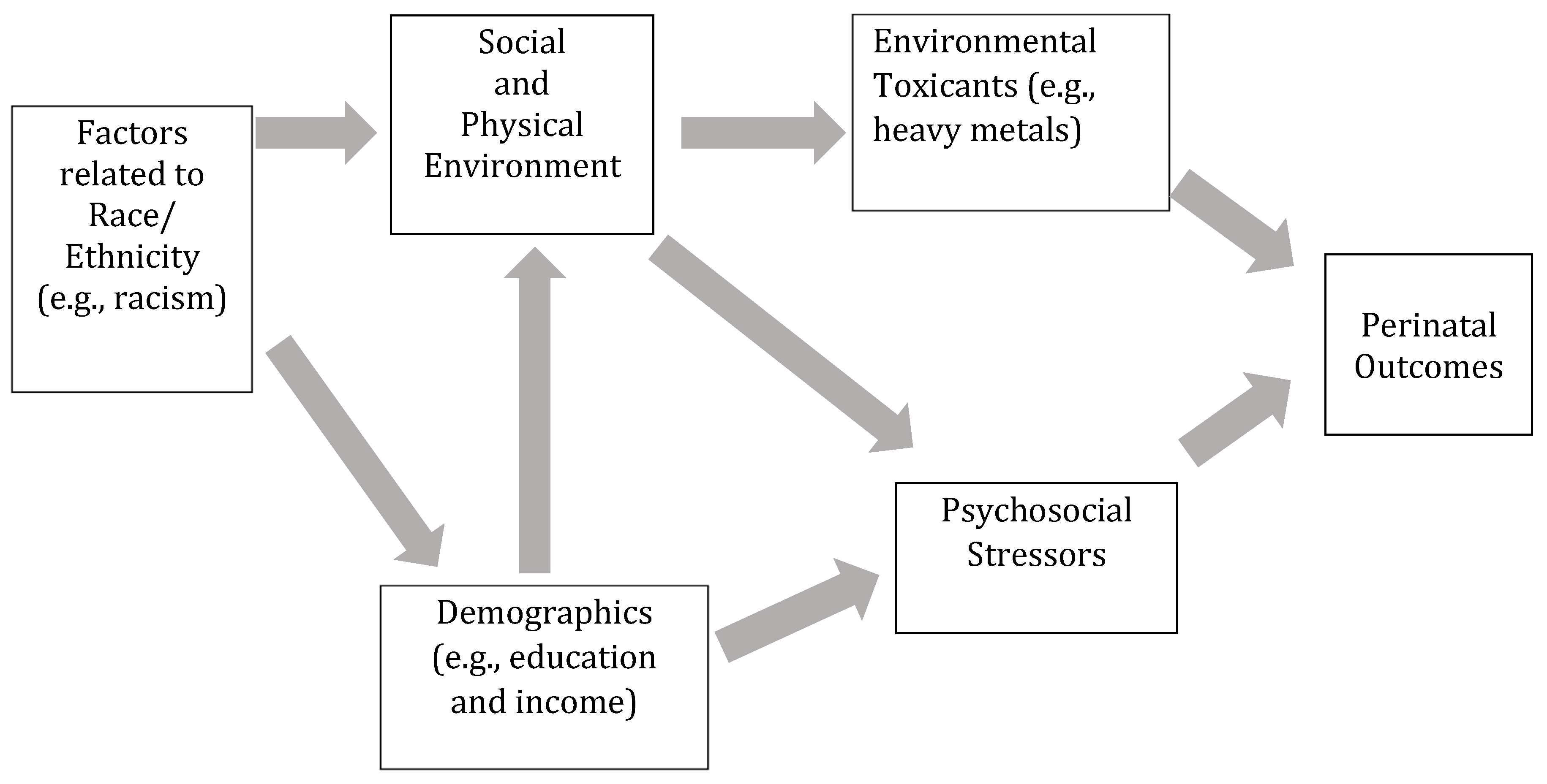
:1. Introduction
2. Material and Methods
2.1. Study Participants
2.2. Variables
2.3. Specimen Collection and Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Environmental Protection Agency. Framework for Cumulative Risk Assessment; Office of Research and Development National Center for Environmental Assessment, Ed.; Environmental Protection Agency: Washington, DC, USA, 2003.
- Sexton, K.; Linder, S.H. The role of cumulative risk assessment in decisions about environmental justice. Int. J. Environ. Res. Public Health 2010, 7, 4037–4049. [Google Scholar] [CrossRef] [PubMed]
- Sexton, K.; Linder, S.H. Cumulative risk assessment for combined health effects from chemical and nonchemical stressors. Am. J. Public Health 2011, 101 (Suppl. 1), S81–S88. [Google Scholar] [CrossRef]
- Sexton, K. Cumulative risk assessment: An overview of methodological approaches for evaluating combined health effects from exposure to multiple environmental stressors. Int. J. Environ. Res. Public Health 2012, 9, 370–390. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.D.; Luben, T.J.; Herring, A.H.; Sacks, J.D. Current approaches used in epidemiologic studies to examine short-term multipollutant air pollution exposures. Ann. Epidemiol. 2017, 27, 145–153.e141. [Google Scholar] [CrossRef]
- Brunst, K.J.; Sanchez Guerra, M.; Gennings, C.; Hacker, M.; Jara, C.; Bosquet Enlow, M.; Wright, R.O.; Baccarelli, A.; Wright, R.J. Maternal lifetime stress and prenatal psychological functioning are associated with decreased placental mitochondrial DNA copy number in the PRISM study. Am. J. Epidemiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Perlin, S.A.; Sexton, K.; Wong, D.W. An examination of race and poverty for populations living near industrial sources of air pollution. J. Expo. Anal. Environ. Epidemiol. 1999, 9, 29–48. [Google Scholar] [CrossRef] [Green Version]
- Prochaska, J.D.; Nolen, A.B.; Kelley, H.; Sexton, K.; Linder, S.H.; Sullivan, J. Social Determinants of Health in Environmental Justice Communities: Examining Cumulative Risk in Terms of Environmental Exposures and Social Determinants of Health. Hum. Ecol. Risk Assess. 2014, 20, 980–994. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.L.; Weisskopf, M.G.; Spiro, A.; Schwartz, J.; Sparrow, D.; Nie, H.; Hu, H.; Wright, R.O.; Wright, R.J. Interaction of stress, lead burden, and age on cognition in older men: The VA Normative Aging Study. Environ. Health Perspect. 2010, 118, 505–510. [Google Scholar] [CrossRef]
- Chen, D.; Cho, S.I.; Chen, C.; Wang, X.; Damokosh, A.I.; Ryan, L.; Smitha, T.J.; Christiania, D.C.; Xua, X. Exposure to benzene, occupational stress, and reduced birth weight. Occup. Environ. Med. 2000, 57, 661–667. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.L.; Fabian, M.P.; Levy, J.I. Combined impact of lead, cadmium, polychlorinated biphenyls and non-chemical risk factors on blood pressure in NHANES. Environ. Res. 2014, 132, 93–99. [Google Scholar] [CrossRef]
- Lau, C.; Rogers, J.M.; Desai, M.; Ross, M.G. Fetal programming of adult disease: Implications for prenatal care. Obstet. Gynecol. 2011, 117, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Wigle, D.T.; Arbuckle, T.E.; Turner, M.C.; Bérubé, A.; Yang, Q.; Liu, S.; Krewski, D. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J. Toxicol. Environ. Health B Crit. Rev. 2008, 11, 373–517. [Google Scholar] [CrossRef] [PubMed]
- Field, T.; Diego, M.; Hernandez-Reif, M. Prenatal depression effects on the fetus and newborn: A review. Infant Behav. Dev. 2006, 29, 445–455. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, M.J.; Harvey, J.M.; Tudehope, D.I.; Gray, P.H. Aetiology and classification of small for gestational age infants. J. Paediatr. Child Health 1997, 33, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.M.; Irgens, L.M.; Rasmussen, S.; Daltveit, A.K. Secular trends in socio-economic status and the implications for preterm birth. Paediatr. Perinat. Epidemiol. 2006, 20, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; Draper, E.S.; Manktelow, B.N.; Dorling, J.S.; Field, D.J. Socioeconomic inequalities in very preterm birth rates. Arch. Dis. Child. Fetal Neonatal. Ed. 2007, 92, F11–F14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, J.W.; Wambach, J.; David, R.J.; Rankin, K.M. Women’s lifelong exposure to neighborhood poverty and low birth weight: A population-based study. Matern Child. Health J. 2009, 13, 326–333. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, J.P.; Steegers, E.A.; Bonsel, G.J. Inequalities in perinatal and maternal health. Curr. Opin. Obstet. Gynecol. 2013, 25, 98–108. [Google Scholar] [CrossRef]
- Caito, S.; Aschner, M. Neurotoxicity of metals. Handb. Clin. Neurol. 2015, 131, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Xu, C.; Lin, N.; Liu, K.; Zhang, Y.; Yu, X.; Liu, W. Lead, mercury, and cadmium in umbilical cord serum and birth outcomes in Chinese fish consumers. Chemosphere 2016, 148, 270–275. [Google Scholar] [CrossRef]
- Murcia, M.; Ballester, F.; Enning, A.M.; Iñiguez, C.; Valvi, D.; Basterrechea, M.; Rebagliato, M.; Vioque, J.; Maruri, M.; Tardon, A.; et al. Prenatal mercury exposure and birth outcomes. Environ. Res. 2016, 151, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Llop, S.; Ballester, F.; Murcia, M.; Forns, J.; Tardon, A.; Andiarena, A.; Vioque, J.; Ibarluzea, J.; Fernández-Somoano, A.; Sunyer, J.; et al. Prenatal exposure to mercury and neuropsychological development in young children: The role of fish consumption. Int. J. Epidemiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M.S.; Buck Louis, G.M.; Sundaram, R.; Maisog, J.M.; Steuerwald, A.J.; Parsons, P.J. Birth outcomes and background exposures to select elements, the Longitudinal Investigation of Fertility and the Environment (LIFE). Environ. Res. 2015, 138, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagiv, S.K.; Thurston, S.W.; Bellinger, D.C.; Amarasiriwardena, C.; Korrick, S.A. Prenatal exposure to mercury and fish consumption during pregnancy and attention-deficit/hyperactivity disorder-related behavior in children. Arch. Pediatr. Adolesc. Med. 2012, 166, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, M.C.; Burke, T.A.; Navas-Acien, A.; Breysse, P.N.; McGready, J.; Fox, M.A. Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: A systematic review. Bull. World Health Organ. 2014, 92, 254–269F. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Khoury, J.C.; Sucharew, H.; Dietrich, K.; Yolton, K. Low-level gestational exposure to mercury and maternal fish consumption: Associations with neurobehavior in early infancy. Neurotoxicol. Teratol. 2016, 54, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Arbuckle, T.E.; Fisher, M.; Fraser, W.D.; Ettinger, A.; King, W. Metals exposure and risk of small-for-gestational age birth in a Canadian birth cohort: The MIREC study. Environ. Res. 2015, 140, 430–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaRocca, J.; Binder, A.M.; McElrath, T.F.; Michels, K.B. The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes. Environ. Res. 2014, 133, 396–406. [Google Scholar] [CrossRef] [Green Version]
- Makelarski, J.A.; Romitti, P.A.; Rocheleau, C.M.; Burns, T.L.; Stewart, P.A.; Waters, M.A.; Lawson, C.C.; Bell, E.M.; Lin, S.; Shaw, G.M.; et al. Maternal periconceptional occupational pesticide exposure and neural tube defects. Birth Defects Res. Part A Clin. Mol. Teratol. 2014, 100, 877–886. [Google Scholar] [CrossRef] [Green Version]
- Criswell, R.; Lenters, V.; Mandal, S.; Stigum, H.; Iszatt, N.; Eggesbo, M. Persistent Environmental Toxicants in Breast Milk and Rapid Infant Growth. Ann. Nutr. Metab. 2017, 70, 210–216. [Google Scholar] [CrossRef]
- Lenters, V.; Portengen, L.; Rignell-Hydbom, A.; Jonsson, B.A.; Lindh, C.H.; Piersma, A.H.; Toft, G.; Bonde, J.P.; Heederik, D.; Rylander, L.; et al. Prenatal Phthalate, Perfluoroalkyl Acid, and Organochlorine Exposures and Term Birth Weight in Three Birth Cohorts: Multi-Pollutant Models Based on Elastic Net Regression. Environ. Health Perspect. 2016, 124, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Hass, U.; Christiansen, S.; Axelstad, M.; Scholze, M.; Boberg, J. Combined exposure to low doses of pesticides causes decreased birth weights in rats. Reprod. Toxicol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hass, U.; Boberg, J.; Christiansen, S.; Jacobsen, P.R.; Vinggaard, A.M.; Taxvig, C.; Poulsen, M.E.; Herrmann, S.S.; Jensen, B.H.; Petersen, A.; et al. Adverse effects on sexual development in rat offspring after low dose exposure to a mixture of endocrine disrupting pesticides. Reprod. Toxicol. 2012, 34, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.; Scholze, M.; Dalgaard, M.; Vinggaard, A.M.; Axelstad, M.; Kortenkamp, A.; Hass, U. Synergistic disruption of external male sex organ development by a mixture of four antiandrogens. Environ. Health Perspect. 2009, 117, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Jarde, A.; Morais, M.; Kingston, D.; Giallo, R.; MacQueen, G.M.; Giglia, L.; Beyene, J.; Wang, Y.; McDonald, S.D. Neonatal Outcomes in Women With Untreated Antenatal Depression Compared With Women Without Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry 2016, 73, 826–837. [Google Scholar] [CrossRef]
- Alder, J.; Fink, N.; Bitzer, J.; Hösli, I.; Holzgreve, W. Depression and anxiety during pregnancy: A risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J. Matern. Fetal Neonatal Med. 2007, 20, 189–209. [Google Scholar] [CrossRef]
- Szegda, K.; Bertone-Johnson, E.R.; Pekow, P.; Powers, S.; Markenson, G.; Dole, N.; Chasan-Taber, L. Depression During Pregnancy and Adverse Birth Outcomes Among Predominantly Puerto Rican Women. Matern. Child. Health J. 2017, 21, 942–952. [Google Scholar] [CrossRef]
- Cook, N.; Ayers, S.; Horsch, A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: A systematic review. J. Affect. Disord. 2017, 225, 18–31. [Google Scholar] [CrossRef]
- Staneva, A.; Bogossian, F.; Pritchard, M.; Wittkowski, A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: A systematic review. Women Birth 2015, 28, 179–193. [Google Scholar] [CrossRef]
- Cardwell, M.S. Stress: Pregnancy considerations. Obstet. Gynecol. Surv. 2013, 68, 119–129. [Google Scholar] [CrossRef]
- Dipietro, J.A. Maternal stress in pregnancy: Considerations for fetal development. J. Adolesc. Health 2012, 51, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes. The National Academies Collection: Reports funded by National Institutes of Health. In Preterm Birth: Causes, Consequences, and Prevention; Behrman, R.E., Butler, A.S., Eds.; National Academies Press, National Academy of Sciences: Washington, DC, USA, 2007. [Google Scholar]
- Voegtline, K.M.; Costigan, K.A.; Kivlighan, K.T.; Laudenslager, M.L.; Henderson, J.L.; DiPietro, J.A. Concurrent levels of maternal salivary cortisol are unrelated to self-reported psychological measures in low-risk pregnant women. Arch. Women’s Ment. Health 2013, 16, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.L.; Kitzman, H.; Veazie, P. The effects of stress on birth weight in low-income, unmarried black women. Women’s Health Issues 2009, 19, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Reagan, P.B.; Salsberry, P.J. Cross race comparisons between SES health gradients among African-American and white women at mid-life. Soc. Sci. Med. 2014, 108, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Borrell, L.N.; Rodriguez-Alvarez, E.; Savitz, D.A.; Baquero, M.C. Parental Race/Ethnicity and Adverse Birth Outcomes in New York City: 2000-2010. Am. J. Public Health 2016, 106, 1491–1497. [Google Scholar] [CrossRef]
- Loggins Clay, S.; Griffin, M.; Averhart, W. Black/White disparities in pregnant women in the United States: An examination of risk factors associated with Black/White racial identity. Health Soc. Care Community 2018. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.W.; David, R.J.; Handler, A.; Wall, S.; Andes, S. Very low birthweight in African American infants: The role of maternal exposure to interpersonal racial discrimination. Am. J. Public Health 2004, 94, 2132–2138. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, L.; Lobel, M. Explaining racial disparities in adverse birth outcomes: Unique sources of stress for Black American women. Soc. Sci. Med. 2011, 72, 977–983. [Google Scholar] [CrossRef]
- Rippe, R.C.; Noppe, G.; Windhorst, D.A.; Tiemeier, H.; van Rossum, E.F.; Jaddoe, V.W.; Verhulst, F.C.; Bakermans-Kranenburg, M.J.; van IJzendoorn, M.H.; van den Akker, E.L. Splitting hair for cortisol? Associations of socio-economic status, ethnicity, hair color, gender and other child characteristics with hair cortisol and cortisone. Psychoneuroendocrinology 2016, 66, 56–64. [Google Scholar] [CrossRef]
- DeFur, P.L.; Evans, G.W.; Cohen Hubal, E.A.; Kyle, A.D.; Morello-Frosch, R.A.; Williams, D.R. Vulnerability as a function of individual and group resources in cumulative risk assessment. Environ. Health Perspect. 2007, 115, 817–824. [Google Scholar] [CrossRef]
- Sexton, K. Sociodemographic aspects of human susceptibility to toxic chemicals: Do class and race matter for realistic risk assessment? Environ. Toxicol. Pharmacol. 1997, 4, 261–269. [Google Scholar] [CrossRef]
- Chakraborty, J.; Maantay, J.A.; Brender, J.D. Disproportionate proximity to environmental health hazards: Methods, models, and measurement. Am. J. Public Health 2011, 101 (Suppl. 1), S27–S36. [Google Scholar] [CrossRef]
- Brown, P. Race, class, and environmental health: A review and systematization of the literature. Environ. Res. 1995, 69, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.; Tilghman, J.; Rosenbaum, A.; Payne-Sturges, D.C. U.S. EPA authority to use cumulative risk assessments in environmental decision-making. Int. J. Environ. Res. Public Health 2012, 9, 1997–2019. [Google Scholar] [CrossRef] [PubMed]
- Raffington, L.; Prindle, J.; Keresztes, A.; Binder, J.; Heim, C.; Shing, Y.L. Blunted cortisol stress reactivity in low-income children relates to lower memory function. Psychoneuroendocrinology 2018, 90, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Hackman, D.A.; Farah, M.J.; Meaney, M.J. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2010, 11, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Morello-Frosch, R.; Zuk, M.; Jerrett, M.; Shamasunder, B.; Kyle, A.D. Understanding The Cumulative Impacts Of Inequalities In Environmental Health: Implications For Policy. Health Aff. 2011, 30, 879–887. [Google Scholar] [CrossRef]
- Carrico, C.; Gennings, C.; Wheeler, D.C.; Factor-Litvak, P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J. Agric. Biol. Environ. Stat. 2014, 20, 100–120. [Google Scholar] [CrossRef] [PubMed]
- Yorita Christensen, K.L.; Carrico, C.K.; Sanyal, A.J.; Gennings, C. Multiple classes of environmental chemicals are associated with liver disease: NHANES 2003-2004. Int. J. Hyg. Environ. Health 2013, 216, 703–709. [Google Scholar] [CrossRef]
- Grewal, J.; Grantz, K.L.; Zhang, C.; Sciscione, A.; Wing, D.A.; Grobman, W.A.; Newman, R.B.; Wapner, R.; D’Alton, M.E.; Skupski, D.; et al. Cohort Profile: NICHD Fetal Growth Studies-Singletons and Twins. Int. J. Epidemiol. 2018, 47, 25–25l. [Google Scholar] [CrossRef]
- Cohen, S. Perceived Stress in a Probability Sample of the United States; Sage: Newbury Park, CA, USA, 1988; pp. 31–67. [Google Scholar]
- Hewitt, P.L.; Flett, G.L.; Mosher, S.W. The Perceived Stress Scale: Factor structure and relation to depression symptoms in a psychiatric sample. J. Psychopathol. Behav. Assess. 1992, 14, 247–257. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Eberhard-Gran, M.; Eskild, A.; Tambs, K.; Opjordsmoen, S.; Samuelsen, S.O. Review of validation studies of the Edinburgh Postnatal Depression Scale. Acta Psychiatr. Scand. 2001, 104, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.R.; Himes, J.H.; Kaufman, R.B.; Mor, J.; Kogan, M. A United States national reference for fetal growth. Obstet. Gynecol. 1996, 87, 163–168. [Google Scholar] [CrossRef]
- Institute of Medicine. Weight Gain during Pregnancy: Reexamining the Guidelines; Institute of Medicine: Washington, DC, USA, 2009. [Google Scholar]
- Pollack, A.Z.; Louis, G.M.; Chen, Z.; Peterson, C.M.; Sundaram, R.; Croughan, M.S.; Sun, L.; Hediger, M.L.; Stanford, J.B.; Varner, M.W.; et al. Trace elements and endometriosis: The ENDO study. Reprod. Toxicol. 2013, 42, 41–48. [Google Scholar] [CrossRef]
- McKelvey, W.; Gwynn, R.C.; Jeffery, N.; Kass, D.; Thorpe, L.E.; Garg, R.K.; Palmer, C.D.; Parsons, P.J. A biomonitoring study of lead, cadmium, and mercury in the blood of New York City adults. Environ. Health Perspect. 2007, 115, 1435–1441. [Google Scholar] [CrossRef]
- Birdsall, R.E.; Kiley, M.P.; Segu, Z.M.; Palmer, C.D.; Madera, M.; Gump, B.B.; MacKenzie, J.A.; Parsons, P.J.; Mechref, Y.; Novotny, M.V.; et al. Effects of lead and mercury on the blood proteome of children. J. Proteome Res. 2010, 9, 4443–4453. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Harel, O.; Little, R.J. How well quantified is the limit of quantification? Epidemiology 2010, 21 (Suppl. 4), S10–S16. [Google Scholar] [CrossRef]
- Johnstone, E.B.; Louis, G.M.; Parsons, P.J.; Steuerwald, A.J.; Palmer, C.D.; Chen, Z.; Sun, L.; Hammoud, A.O.; Dorais, J.; Peterson, C.M. Increased urinary cobalt and whole blood concentrations of cadmium and lead in women with uterine leiomyomata: Findings from the ENDO Study. Reprod. Toxicol. 2014, 49, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Renzetti, S.; Curtin, P.; Just, A.; Gennings, C. Generalized Weighted Quantile Sum Regression, R Package version 1.0.0; 2016. [Google Scholar]
- Lefmann, T.; Combs-Orme, T.; Orme, J.G. Examining the inter-correlated effects of low income, life stress, and race on birth outcomes: A representative state study. Soc. Work Health Care 2017, 1–20. [Google Scholar] [CrossRef]
- Rider, C.V.; Dourson, M.L.; Hertzberg, R.C.; Mumtaz, M.M.; Price, P.S.; Simmons, J.E. Incorporating nonchemical stressors into cumulative risk assessments. Toxicol. Sci. 2012, 127, 10–17. [Google Scholar] [CrossRef]
- Czarnota, J.; Gennings, C.; Colt, J.S.; De Roos, A.J.; Cerhan, J.R.; Severson, R.K.; Hartge, P.; Ward, M.H.; Wheeler, D.C. Analysis of Environmental Chemical Mixtures and Non-Hodgkin Lymphoma Risk in the NCI-SEER NHL Study. Environ. Health Perspect. 2015, 123, 965–970. [Google Scholar] [CrossRef] [Green Version]
- Horton, M.K.; Blount, B.C.; Valentin-Blasini, L.; Wapner, R.; Whyatt, R.; Gennings, C.; Factor-Litvak, P. CO-occurring exposure to perchlorate, nitrate and thiocyanate alters thyroid function in healthy pregnant women. Environ. Res. 2015, 143, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Artacho-Cordón, F.; León, J.; Sáenz, J.M.; Fernández, M.F.; Martin-Olmedo, P.; Olea, N.; Arrebola, J.P. Contribution of Persistent Organic Pollutant Exposure to the Adipose Tissue Oxidative Microenvironment in an Adult Cohort: A Multipollutant Approach. Environ. Sci. Technol. 2016, 50, 13529–13538. [Google Scholar] [CrossRef]
- Sanders, A.P.; Gennings, C.; Svensson, K.; Motta, V.; Mercado-Garcia, A.; Solano, M.; Baccarelli, A.A.; Tellez-Rojo, M.M.; Wright, R.O.; Burris, H.H. Bacterial and cytokine mixtures predict the length of gestation and are associated with miRNA expression in the cervix. Epigenomics 2017, 9, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Gennings, C.; Carrico, C.; Factor-Litvak, P.; Krigbaum, N.; Cirillo, P.M.; Cohn, B.A. A cohort study evaluation of maternal PCB exposure related to time to pregnancy in daughters. Environ. Health 2013, 12, 66. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Czarnota, J.; Jones, R.M. Estimating an area-level socioeconomic status index and its association with colonoscopy screening adherence. PLoS ONE 2017, 12, e0179272. [Google Scholar] [CrossRef]
- Nieves, J.W.; Gennings, C.; Factor-Litvak, P.; Hupf, J.; Singleton, J.; Sharf, V.; Oskarsson, B.; Fernandes Filho, J.A.; Sorenson, E.J.; D’Amico, E.; et al. Association Between Dietary Intake and Function in Amyotrophic Lateral Sclerosis. JAMA Neurol. 2016, 73, 1425–1432. [Google Scholar] [CrossRef] [Green Version]
- Howe, C.G.; Li, Z.; Zens, M.S.; Palys, T.; Chen, Y.; Channon, J.Y.; Karagas, M.R.; Farzan, S.F. Dietary B Vitamin Intake Is Associated with Lower Urinary Monomethyl Arsenic and Oxidative Stress Marker 15-F. J. Nutr. 2017, 147, 2289–2296. [Google Scholar] [CrossRef]
- Mitro, S.D.; Johnson, T.; Zota, A.R. Cumulative Chemical Exposures during Pregnancy and Early Development. Curr. Environ. Health Rep. 2015, 2, 367–378. [Google Scholar] [CrossRef]
- Al-Gubory, K.H. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reprod. Biomed. Online 2014, 29, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Chung, E.K.; Mathew, L.; Elo, I.T.; Coyne, J.C.; Culhane, J.F. Depressive symptoms in disadvantaged women receiving prenatal care: The influence of adverse and positive childhood experiences. Ambul. Pediatr. 2008, 8, 109–116. [Google Scholar] [CrossRef]
- Dionisio, K.L.; Baxter, L.K.; Chang, H.H. An empirical assessment of exposure measurement error and effect attenuation in bipollutant epidemiologic models. Environ. Health Perspect. 2014, 122, 1216–1224. [Google Scholar] [CrossRef]

| Participant Characteristic | n | % | |
|---|---|---|---|
| Race | |||
| Non-Hispanic White | 557 | 27.33 | |
| Non-Hispanic Black | 532 | 26.10 | |
| Hispanic | 559 | 27.43 | |
| Asian & Pacific Islander | 390 | 19.14 | |
| Income | |||
| Less than $30,000 | 489 | 27.78 | |
| $30,000–$39,999 | 155 | 8.81 | |
| $40,000–$49,999 | 138 | 7.84 | |
| $50,000–$74,999 | 216 | 12.27 | |
| $75,000–$99,999 | 241 | 13.69 | |
| $100,000 or more | 521 | 29.60 | |
| Education | |||
| Less than high school | 208 | 10.21 | |
| High school diploma or GED or equivalent | 350 | 17.17 | |
| Some college or Associate degree | 593 | 29.10 | |
| Bachelor’s degree | 509 | 24.98 | |
| Master’s degree or Advanced degree | 378 | 18.55 | |
| Perceived Stress | |||
| No (<75tth percentile) | 1433 | 78.82 | |
| Yes (≥75th Percentile) | 385 | 21.18 | |
| Depression | |||
| No | 1980 | 97.15 | |
| Yes | 58 | 2.85 | |
| SGA | |||
| No | 1875 | 92.59 | |
| Yes | 150 | 7.41 | |
| Preterm Birth | |||
| No | 1902 | 93.74 | |
| Yes | 127 | 6.26 | |
| Low Birthweight | |||
| No | 1921 | 94.86 | |
| Yes | 104 | 5.14 | |
| Weight gain | |||
| Adequate | 566 | 30.64 | |
| Under | 317 | 17.16 | |
| Over | 964 | 52.19 | |
| Parity | |||
| 0 | 1006 | 49.36 | |
| 1 | 696 | 34.15 | |
| 2+ | 336 | 16.49 | |
| Marital Status | |||
| Not married or cohabitating | 490 | 24.07 | |
| Married or cohabitating | 1546 | 75.93 | |
| Age | |||
| ≤24 | 583 | 28.61 | |
| 25–35 | 1171 | 57.46 | |
| >35 | 284 | 13.94 | |
| Variables Measured Continuously | |||
| Mean (SD) | Median | 25th and 75th percentiles | |
| Perceived Stress | 28.51 (9.09) | 28.00 | 26.00, 30.00 |
| Depression | 4.67 (3.38) | 4.00 | 2.00, 7.00 |
| Weight gain (kg) | 15.31(5.99) | 14.97 | 11.79, 18.60 |
| Age | 28.21 (4.7) | 29 | 24.00, 32.00 |
| Pb (µg/dL) 1,2 | 0.51 (5.22) | 0.11 | 0.06, 0.22 |
| Cd (µg/L) 1,3 | 0.03 (0.40) | 0.01 | 0.01, 0.02 |
| Hg (µg/L) 1,4 | 0.32 (0.37) | 0.22 | 0.11, 0.43 |
| Participant Characteristic | OR | 95% CI | p-Value |
|---|---|---|---|
| Race | 0.0003 | ||
| Not Black | Ref | ||
| Non-Hispanic Black | 2.46 | 1.59, 3.81 | |
| Income | |||
| Less than <$30,000 | Ref | 0.01 | |
| $30,000–$39,999 | 1.38 | 0.82, 2.34 | |
| $40,000–$49,999 | 1.06 | 0.69, 1.88 | |
| $50,000–$74,999 | 0.94 | 0.56, 1.59 | |
| ≥$75,000 | 0.57 | 0.37, 0.86 | |
| Education | |||
| HS or Less | Ref | ||
| Some college or Associate degree | 0.81 | 0.57, 1.17 | 0.002 |
| At least college degree | 0.53 | 0.36, 0.76 | |
| Log Pb | 1.18 | 1.04, 1.35 | 0.01 |
| Log Cd | 1.22 | 1.03, 1.35 | 0.01 |
| Log Hg | 0.91 | 0.78, 1.45 | |
| Perceived Stress (75th percentile) | 1.04 | 0.70, 1.07 | 0.02 |
| Depression | 0.32 | 0.08, 1.33 | 0.10 |
| Weight Gain | |||
| adequate | Ref | <0.0001 | |
| under | 1.56 | 1.05, 2.33 | |
| over | 0.56 | 0.33, 0.81 | |
| Parity | |||
| 0 | Ref | ||
| 1 | 0.61 | 0.43, 0.86 | 0.004 |
| 2+ | 0.59 | 0.38, 0.91 | |
| Married vs. Nonmarried | 0.61 | 0.44, 0.83 | 0.002 |
| Age | |||
| ≤24 | Ref | <0.0001 | |
| 25–35 | 0.52 | 0.38, 0.72 | |
| >35 | 0.46 | 0.28, 0.78 | |
| Variables | Pb | Cd | Hg | Income 1 | Education 1 | Perceived Stress | Depression |
|---|---|---|---|---|---|---|---|
| Pb | 0.22 | 0.01 | 0.08 | 0.05 | 0.00 | 0.02 | |
| p-value | <0.0001 | 0.53 | 0.01 | 0.02 | 0.93 | 0.29 | |
| Cd | 0.09 | 0.02 | 0.00 | −0.02 | 0.05 | ||
| p-value | <0.0001 | 0.24 | 0.82 | 0.42 | 0.03 | ||
| Hg | −0.13 | −0.12 | −0.01 | 0.05 | |||
| p-value | <0.0001 | <0.0001 | 0.63 | 0.03 | |||
| Income | 0.68 | −0.02 | 0.16 | ||||
| p-value | <0.0001 | 0.42 | <0.0001 | ||||
| Education | −0.07 | 0.10 | |||||
| p-value | 0.002 | <0.0001 | |||||
| Perceived Stress | 0.31 | ||||||
| p-value | <0.0001 | ||||||
| Depression | |||||||
| p-value |
| Domain | Unadjusted Models Using Highest Tertile as Exposed | Unadjusted Individual Domains, without Race/Ethnicity Included | Adjusted Models with All Individual Domains as Exposures | |||
|---|---|---|---|---|---|---|
| OR | CI | OR | CI | OR | CI | |
| Metals | 1.16 | 0.97, 1.38 | 1.18 | 0.95, 1.46 | 1.17 | 0.93, 1.48 |
| Psychosocial | 1.20 | 0.96, 1.52 | 1.16 | 0.90, 1.50 | 1.24 | 0.95, 1.62 |
| Demographic | 1.35 | 1.08, 1.68 * | 1.29 | 1.01, 1.65 * | 1.10 | 0.80, 1.50 |
| Total Cumulative | 1.21 | 1.06, 1.37 * | - | - | 1.17 | 1.02, 1.35 * |
| Domain | Variable | Unadjusted | Adjusted | β (SE) | p-Value |
|---|---|---|---|---|---|
| Metals | Pb | 0.49 | 0.48 | 0.30 (0.18) | 0.10 |
| Cd | 0.17 | 0.52 | |||
| Hg | 0.34 | 0.00 | |||
| Psychosocial | Depression | 0.82 | 0.67 | 0.04 (0.19) | 0.85 |
| Perceived Stress | 0.18 | 0.33 | |||
| Sociodemographic | Income | 0.69 | 0.12 | 0.41 (0.20) | 0.04 |
| Education | 0.31 | 0.88 | |||
| Cumulative WQS variable | Pb | 0.28 | 0.03 | 0.37 (0.38) | 0.33 |
| Cd | 0.07 | 0.30 | |||
| Hg | 0.01 | 0.06 | |||
| Depression | 0.15 | 0.14 | |||
| Perceived Stress | 0.14 | 0.15 | |||
| Income | 0.20 | 0.22 | |||
| Education | 0.14 | 0.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zilversmit Pao, L.; Harville, E.W.; Wickliffe, J.K.; Shankar, A.; Buekens, P. The Cumulative Risk of Chemical and Nonchemical Exposures on Birth Outcomes in Healthy Women: The Fetal Growth Study. Int. J. Environ. Res. Public Health 2019, 16, 3700. https://doi.org/10.3390/ijerph16193700
Zilversmit Pao L, Harville EW, Wickliffe JK, Shankar A, Buekens P. The Cumulative Risk of Chemical and Nonchemical Exposures on Birth Outcomes in Healthy Women: The Fetal Growth Study. International Journal of Environmental Research and Public Health. 2019; 16(19):3700. https://doi.org/10.3390/ijerph16193700
Chicago/Turabian StyleZilversmit Pao, Leah, Emily W. Harville, Jeffrey K. Wickliffe, Arti Shankar, and Pierre Buekens. 2019. "The Cumulative Risk of Chemical and Nonchemical Exposures on Birth Outcomes in Healthy Women: The Fetal Growth Study" International Journal of Environmental Research and Public Health 16, no. 19: 3700. https://doi.org/10.3390/ijerph16193700





