Optimization of Ammonia Stripping of Piggery Biogas Slurry by Response Surface Methodology
Abstract
:1. Introduction
2. Test Material, Apparatus and Method
2.1. Test Material
2.2. Test Apparatus
2.3. Test Method
3. Results and Discussion
3.1. Influence of Stripping Time on the Effect of Ammonia Stripping
3.2. Influence of pH Value on the Effect of Ammonia Stripping
3.3. Influence of Gas Flow Rate on the Effect of Ammonia Stripping
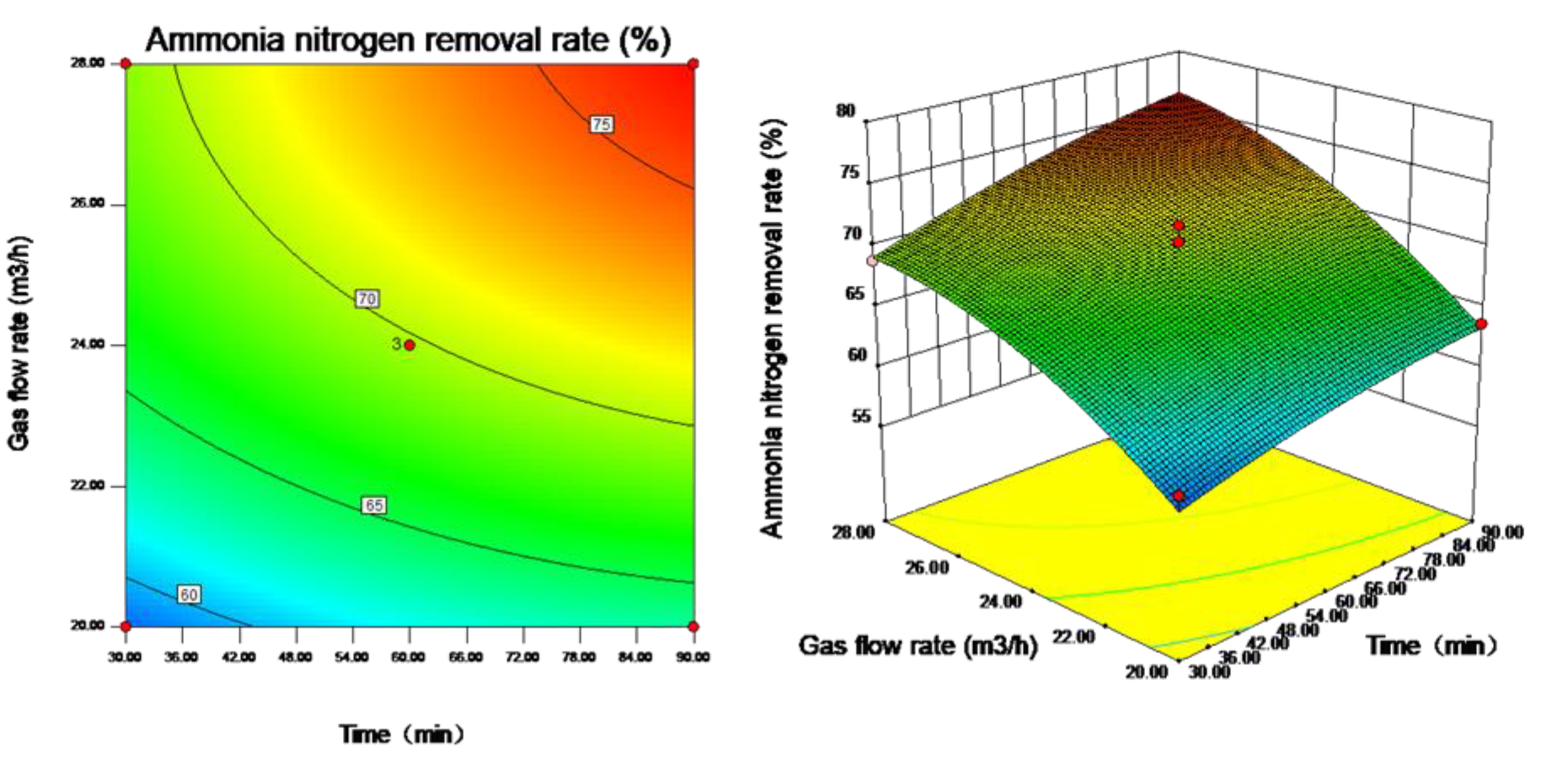
3.4. Response Surface Analysis Results
3.5. Parameter Optimization and Experimental Verification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yan, Y.Y.; Li, Z.F.; Cheng, S.K. A review of anaerobic digested effluent treatment on livestock farm. China Biogas. 2013, 31, 48–52. [Google Scholar]
- Wu, H.Z. A review on the key factors of high ammonia nitrogen wastewater treatment by stripping. J. Green Sci. Technol. 2013, 2, 144–146. [Google Scholar]
- Gustin, S.; Marinsek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Proc. Saf. Environ. Protec. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- Tuerdi, A.; Yang, L.; An, D.; Wang, T. Progresses in air stripping for treatment of ammonia wastewater. Petrochem. Technol. 2014, 43, 1348–1353. [Google Scholar]
- Ferraz, F.M.; Povinelli, J.; Vieira, E.M. Ammonia removal from landfill leachate by air stripping and absorption. Environ. Technol. 2013, 34, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.X. Pilot Study on Treatment of Landfill Leachate by Ammonia Stripping-Biological Contact Oxidation Process. Guangxi University of Technology: Liuzhou, China, 2013. [Google Scholar]
- Park, S.; Kim, M. Innovative ammonia stripping with an electrolyzed water system as pretreatment of thermally hydrolyzed wasted sludge for anaerobic digestion. Water Res. 2015, 68, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.H.; Chen, A.; Lo, K.V. Removal of nitrogen from swine manure wastewaters by ammonia stripping. Bioresour. Technol. 1995, 54, 17–20. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, Y.W.; Jahng, D. Ammonia stripping for enhanced biomethanization of piggery wastewater. J. Hazard. Mater. 2012, 199, 36–42. [Google Scholar] [CrossRef]
- Bonmati, A.; Flotats, X. Air stripping of ammonia from pig slurry: Characterisation and feasibility as a pre-or post-treatment to mesophilic anaerobic digestion. Waste Manag. 2003, 23, 261–272. [Google Scholar] [CrossRef]
- Jin, Y.Y. Experimental Study on the Treatment of Anaerobic Effluent of Dairy Wastewater by Ammonia Stripping and Coagulation. Anhui University of Technology: Ma’anshan, China, 2015. [Google Scholar]
- Lei, X.; Sugiura, N.; Feng, C.; Maekawa, T. Pretreatment of anaerobic digestion effluent with ammonia stripping and biogas purification. J. Hazard. Mater. 2007, 145, 391–397. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; He, M.Z. Experimental Design and Application of Design-Expert and SPSS; Science Press: Beijing, China, 2010. [Google Scholar]
- Cui, J.Q.; Tang, J.; Wang, Y.; Zhang, S. Optimization on microwave extraction process of mignonette from peanut shells by response surface methodology. Chem. Bioeng. 2017, 34, 42–46. [Google Scholar]
- D’Archivio, A.A.; Maggi, M.A.; Ruggieri, F. Investigation by response surface methodology of extraction of caffeine, gallic acid and selected catechins from tea using water-ethanol mixtures. Food Anal. Methods. 2016, 9, 2773–2779. [Google Scholar] [CrossRef]
- Vijayakumar, T.P.; Boopathy, P. Optimization of ingredients for noodle preparation using response surface methodology. J. Food Sci. Technol. 2014, 51, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.F.; Li, P.; Tu, R.R.; Zhao, X.; Yuan, G.; Wu, Y. Optimizing the process conditions for preparing dialdehyde starch with high aldehyde content by acidolysis oxidation based on response surface methodology. Mater. Rep. 2019, 33, 335–341. [Google Scholar]
- Adlan, M.N.; Palaniandy, P.; Aziz, H.A. Optimization of coagulation and dissolved air flotation (DAF) treatment of semi-aerobic landfill leachate using response surface methodology (RSM). Desalination. 2011, 277, 74–82. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Optimized waste tea activated carbon for adsorption of methylene blue and acid blue 29 dyes using response surface methodology. Chem. Eng. J. 2011, 175, 233–243. [Google Scholar] [CrossRef]
- Bashir, M.J.K.; Aziz, H.A.; Yusoff, M.S.; Nordin Adlan, M. Application of response surface methodology (RSM) for optimization of ammoniacal nitrogen removal from semi-aerobic landfill leachate using ion exchange resin. Desalination. 2010, 254, 154–161. [Google Scholar] [CrossRef]
- Box, G.; Behnken, D. Some new three level designs for the study of quantitative variables. Technometrics. 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Sui, Q.W. Combined of Ammonia Stripping and Membrane Bioreactor Processes for Anaerobically Digested Swine Wastewater Treatment. Chinese Academy of Agricultural Sciences: Beijing, China, 2014. [Google Scholar]
- Du, X. A Study on Optimal Design and Robust Design for Complex Multiple Response Problems; Tianjin University: Tianjin, China, 2012. [Google Scholar]
- Harrington, E.C. The desirability function. Ind. Qual. Control. 1965, 21, 494–498. [Google Scholar]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Hossini, H.; Rezaee, A.; Ayati, B.; Hossein Mahvi, A. Optimizing ammonia volatilization by air stripping from aquatic solutions using response surface methodology (RSM). Desalinat. Water Treat. 2016, 57, 11765–11772. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, YQ.; Zhang, L. Study on optimal stripping of ammonia nitrogen wastewater by response surface method. J. Yangtze Univ. 2018, 15, 31–34. [Google Scholar]








| Parameter | Control | ||
|---|---|---|---|
| Time (min) | pH | Gas Flow Rate (m3/h) | |
| Time (min) | 15, 30, 45, 60, 75, 90, 105, 120 | 10.5 | 20 |
| pH | 120 | 11, 11.5, 12 | 20 |
| Gas flow rate (m3/h) | 120 | 10.5 | 20, 24, 28 |
| Level | Factor A Time (min) | Factor B pH | Factor C Gas Flow Rate (m3/h) |
|---|---|---|---|
| −1 | 30 | 11 | 20 |
| 0 | 60 | 11.5 | 24 |
| 1 | 90 | 12 | 28 |
| Number | Factor A | Factor B | Factor C | Response Y |
|---|---|---|---|---|
| Time (min) | pH | Gas Flow Rate (m3/h) | Ammonia Nitrogen Removal Rate (%) | |
| 1 | 30(−1) | 11(−1) | 24(0) | 64.40 |
| 2 | 90(1) | 11(−1) | 24(0) | 71.99 |
| 3 | 30(−1) | 12(1) | 24(0) | 69.65 |
| 4 | 90(1) | 12(1) | 24(0) | 76.06 |
| 5 | 30(−1) | 11.5(0) | 20(−1) | 59.52 |
| 6 | 90(1) | 11.5(0) | 20(−1) | 63.64 |
| 7 | 30(−1) | 11.5(0) | 28(1) | 68.85 |
| 8 | 90(1) | 11.5(0) | 28(1) | 75.21 |
| 9 | 60(0) | 11(−1) | 20(−1) | 56.16 |
| 10 | 60(0) | 12(1) | 20(−1) | 68.65 |
| 11 | 60(0) | 11(−1) | 28(1) | 75.16 |
| 12 | 60(0) | 12(1) | 28(1) | 76.48 |
| 13 | 60(0) | 11.5(0) | 24(0) | 71.71 |
| 14 | 60(0) | 11.5(0) | 24(0) | 70.39 |
| 15 | 60(0) | 11.5(0) | 24(0) | 67.11 |
| SS | df | MS | F | P | Significance | |
|---|---|---|---|---|---|---|
| Model | 489.60 | 9 | 54.40 | 13.82 | 0.0050 | ** |
| A | 74.91 | 1 | 74.91 | 19.03 | 0.0073 | ** |
| B | 66.87 | 1 | 66.87 | 16.99 | 0.0092 | ** |
| C | 284.77 | 1 | 284.77 | 72.33 | 0.0004 | ** |
| AB | 0.35 | 1 | 0.35 | 0.088 | 0.7782 | |
| AC | 1.25 | 1 | 1.25 | 0.32 | 0.5968 | |
| BC | 31.19 | 1 | 31.19 | 7.92 | 0.0373 | * |
| A2 | 2.13 | 1 | 2.13 | 0.54 | 0.4950 | |
| B2 | 8.85 | 1 | 8.85 | 2.25 | 0.1941 | |
| C2 | 17.42 | 1 | 17.42 | 4.42 | 0.0894 | |
| Residual | 19.68 | 5 | 3.94 | |||
| Lack of Fit | 8.46 | 3 | 2.82 | 0.50 | 0.7180 | |
| Pure Error | 11.22 | 2 | 5.61 | |||
| Cor Total | 509.29 | 14 |
| Number | Time (min) | pH | Gas Flow Rate (m3/h) | Ammonia Nitrogen Removal Rate (%) |
|---|---|---|---|---|
| 1 | 90 | 11 | 28 | 73.74 |
| 2 | 90 | 11 | 28 | 75.17 |
| 3 | 90 | 11 | 28 | 72.88 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, M.; Dong, H.; Zhu, Z.; Zhan, Y. Optimization of Ammonia Stripping of Piggery Biogas Slurry by Response Surface Methodology. Int. J. Environ. Res. Public Health 2019, 16, 3819. https://doi.org/10.3390/ijerph16203819
Zou M, Dong H, Zhu Z, Zhan Y. Optimization of Ammonia Stripping of Piggery Biogas Slurry by Response Surface Methodology. International Journal of Environmental Research and Public Health. 2019; 16(20):3819. https://doi.org/10.3390/ijerph16203819
Chicago/Turabian StyleZou, Mengyuan, Hongmin Dong, Zhiping Zhu, and Yuanhang Zhan. 2019. "Optimization of Ammonia Stripping of Piggery Biogas Slurry by Response Surface Methodology" International Journal of Environmental Research and Public Health 16, no. 20: 3819. https://doi.org/10.3390/ijerph16203819





