Investigation of the Importance of Climatic Factors in COVID-19 Worldwide Intensity
Abstract
:1. Introduction
2. Data Collection
3. Data Analysis
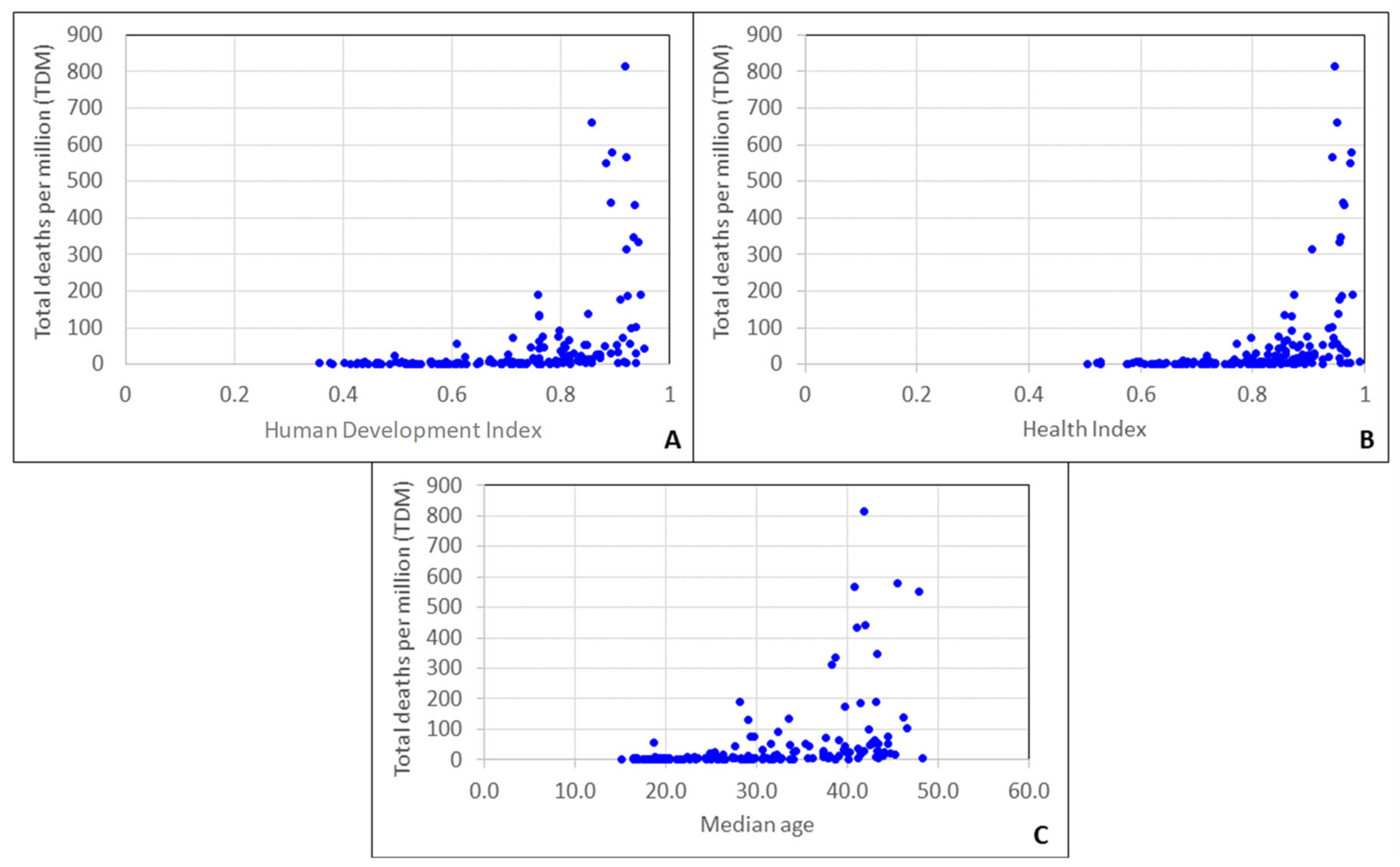
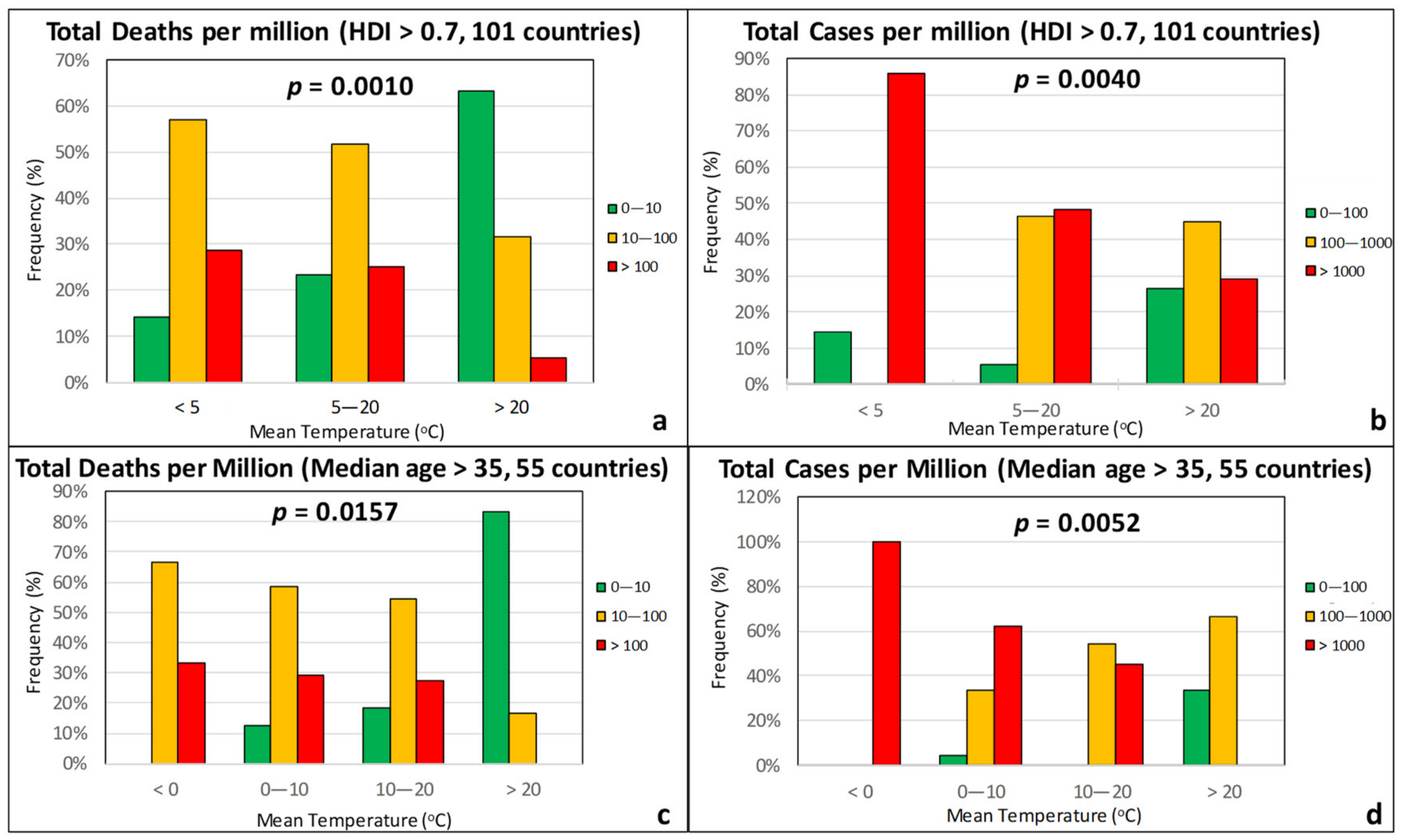
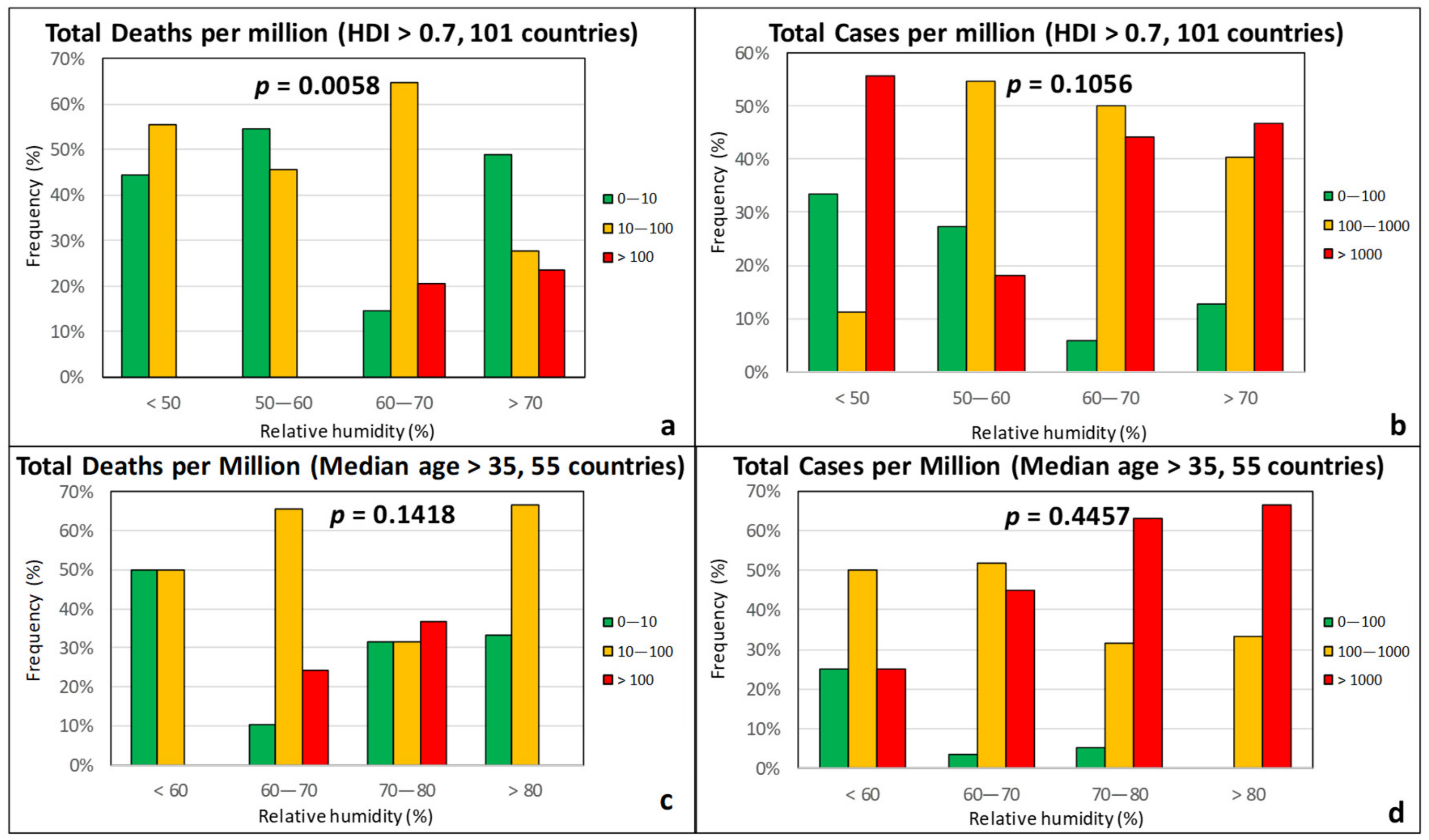
3.1. Climatic Factors
3.2. Sociodemographic Factors
3.3. Government Response Measures
4. Multilinear Regression
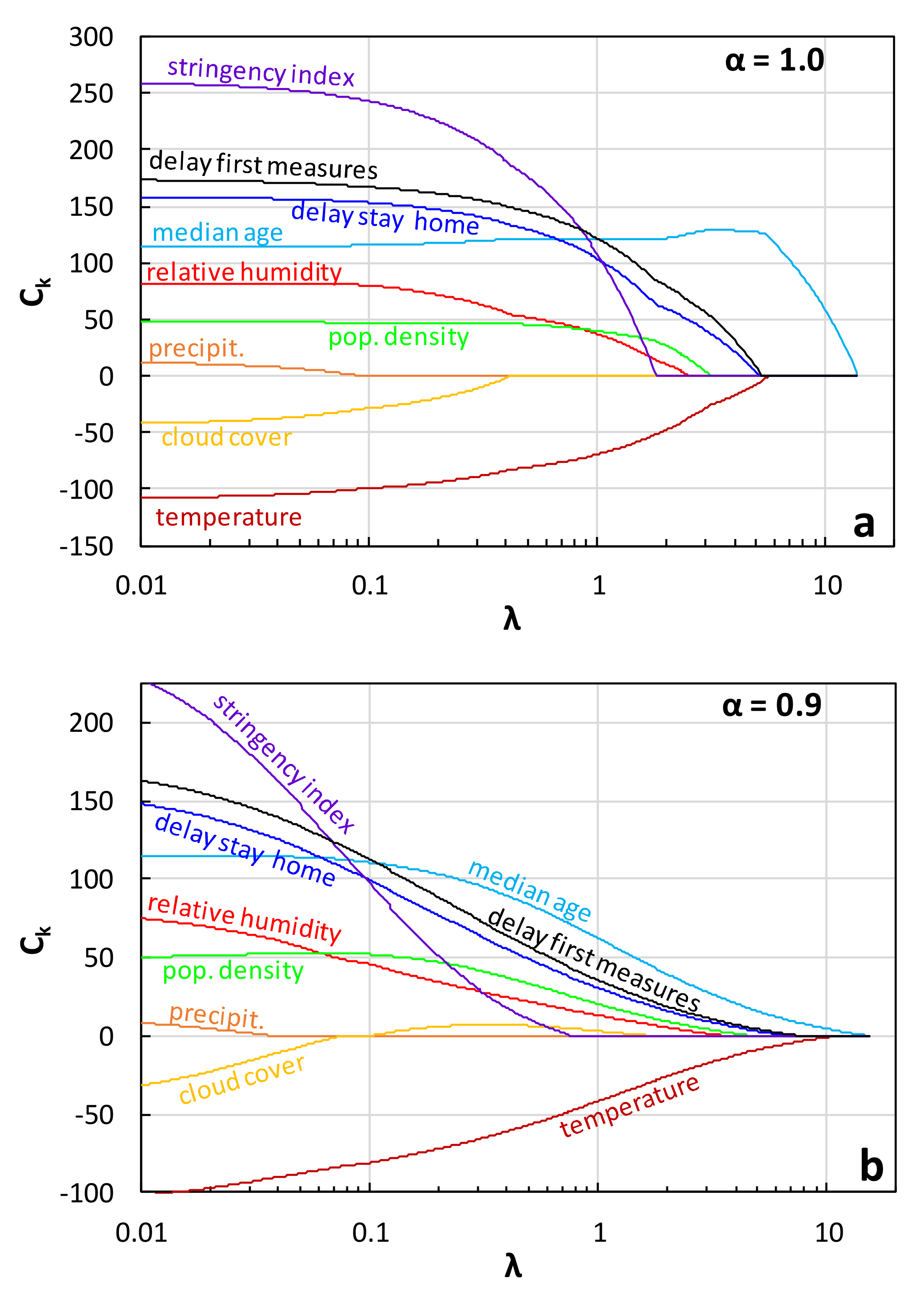
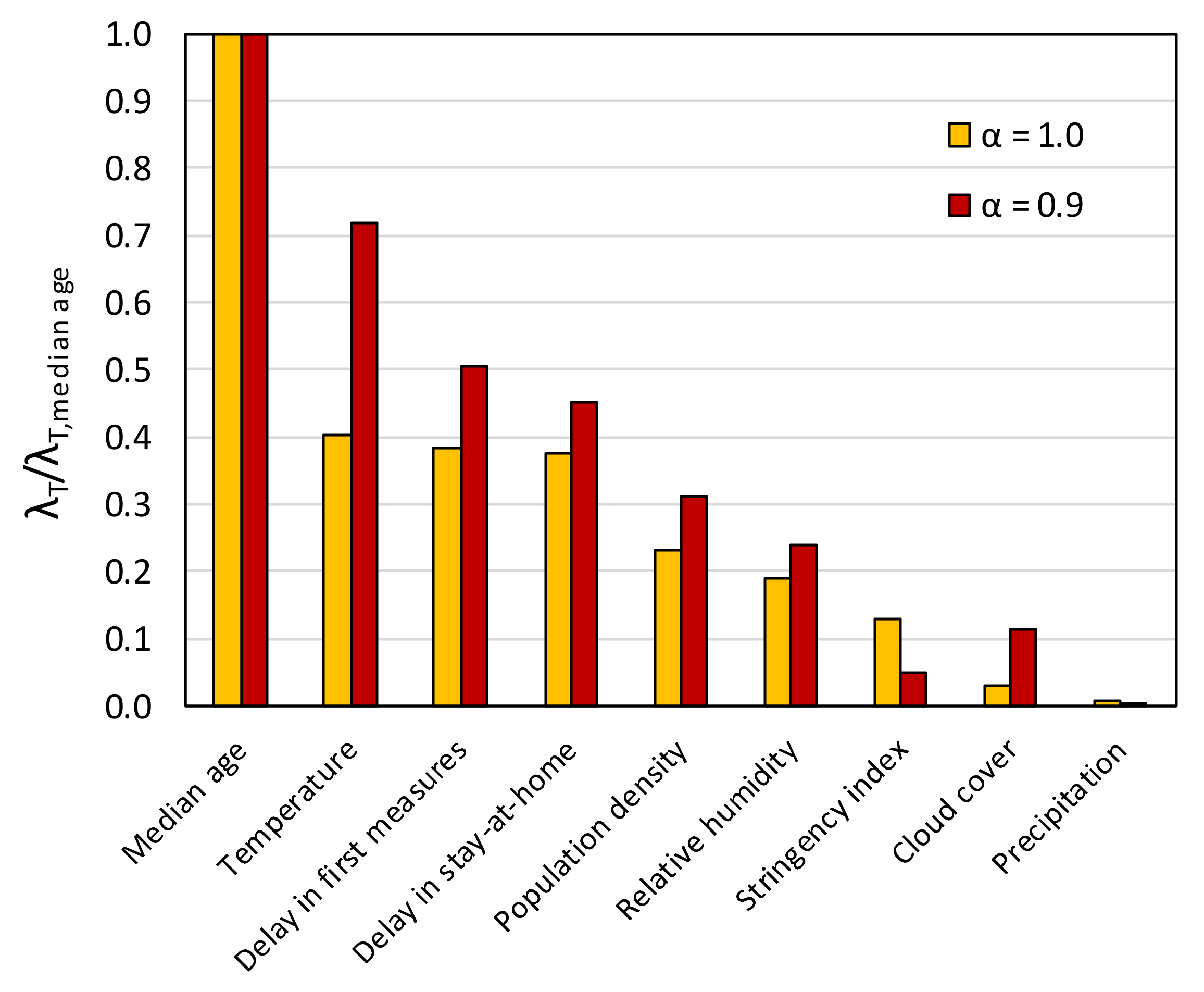
4.1. Methodology
4.2. Forward Stepwise Regression
4.3. Lasso and Elastic Net Regression
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, M.; Jiang, A.; Gong, L.; Luo, L.; Guo, W.; Li, C.; Zheng, J.; Li, C.; Yang, B.; Zeng, J.; et al. Temperature significant change COVID-19 Transmission in 429 cities. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- WHO—World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19-11 March 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 12 March 2020).
- WHO—World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report 132. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200531-covid-19-sitrep-132.pdf?sfvrsn=d9c2eaef_2 (accessed on 2 June 2020).
- Kunst, A.E.; Looman, C.W.N.; MacKenbach, J.P. Outdoor Air Temperature and Mortality in the Netherlands: A Time-Series Analysis. Am. J. Epidemiol. 1993, 137, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Viboud, C.; Pakdaman, K.; Wilson, M.L.; Myers, M.F.; Valleron, A.-J.; Flahault, A. Association of influenza epidemics with global climate variability. Eur. J. Epidemiol. 2004, 19, 1055–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaman, J.; Pitzer, V.E.; Viboud, C.; Grenfell, B.T.; Lipsitch, M. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol. 2010, 8, e1000316. [Google Scholar] [CrossRef]
- Tang, J.W.; Lai, F.Y.; Nymadawa, P.; Deng, Y.M.; Ratnamohan, M.; Petric, M.; Loh, T.P.; Tee, N.W.; Dwyer, D.E.; Barr, I.G.; et al. Comparison of the incidence of influenza in relation to climate factors during 2000–2007 in five countries. J. Med. Virol. 2010, 82, 1958–1965. [Google Scholar] [CrossRef]
- Baumgartner, E.A.; Dao, C.N.; Nasreen, S.; Bhuiyan, M.U.; Mah-E-Muneer, S.; Al Mamun, A.; Sharker, M.A.Y.; Zaman, R.U.; Cheng, P.-Y.; Klimov, A.I.; et al. Seasonality, Timing, and Climate Drivers of Influenza Activity Worldwide. J. Infect. Dis. 2012, 206, 838–846. [Google Scholar] [CrossRef] [Green Version]
- Tamerius, J.D.; Shaman, J.; Alonso, W.J.; Bloom-Feshbach, K.; Uejio, C.K.; Comrie, A.; Viboud, C. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog. 2013, 9, e1003194. [Google Scholar] [CrossRef]
- Xie, J.; Zhu, Y. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020, 724, 138201. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, Y.; Liu, J.; He, X.; Wang, B.; Fu, S.; Yan, J.; Niu, J.; Zhou, J.; Luo, B. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020, 724, 138226. [Google Scholar] [CrossRef]
- Oliveiros, B.; Caramelo, L.; Ferreira, N.C.; Caramelo, F. Role of temperature and humidity in the modulation of the doubling time of COVID-19 cases. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Tosepu, R.; Gunawan, J.; Effendy, D.S.; Ahmad, L.O.A.I.; Lestari, H.; Bahar, H.; Asfian, P. Correlation between weather and Covid-19 pandemic in Jakarta, Indonesia. Sci. Total Environ. 2020, 725, 138436. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Sharma, A.; Kaur, P.; Gugamsetty, B.; Kumar, A. Statistical interpretation of environmental influencing parameters on COVID-19 during the lockdown in Delhi, India. Environ. Dev. Sustain. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Pradhan, B.; Maulud, K.N.A. Estimating the Impact of Daily Weather on the Temporal Pattern of COVID-19 Outbreak in India. Earth Syst. Environ. 2020, 4, 523–534. [Google Scholar] [CrossRef]
- Ahmadi, M.; Sharifi, A.; Dorosti, S.; Ghoushchi, S.J.; Ghanbari, N. Investigation of effective climatology parameters on COVID-19 outbreak in Iran. Sci. Total Environ. 2020, 729, 138705. [Google Scholar] [CrossRef]
- Auler, A.; Cássaro, F.; Da Silva, V.; Pires, L. Evidence that high temperatures and intermediate relative humidity might favor the spread of COVID-19 in tropical climate: A case study for the most affected Brazilian cities. Sci. Total Environ. 2020, 729, 139090. [Google Scholar] [CrossRef]
- Bukhari, Q.; Jameel, Y. Will Coronavirus Pandemic Diminish by Summer? SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Lasisi, T.T.; Eluwole, K.K. Is the weather-induced COVID-19 spread hypothesis a myth or reality? Evidence from the Russian Federation. Environ. Sci. Pollut. Res. 2020, 1–5. [Google Scholar] [CrossRef]
- Araújo, M.B.; Naimi, B. Spread of SARS-CoV-2 Coronavirus likely to be constrained by climate. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Briz-Redón, Á.; Serrano-Aroca, Á. The effect of climate on the spread of the COVID-19 pandemic: A review of findings, and statistical and modelling techniques. Prog. Phys. Geogr. Earth Environ. 2020, 44, 591–604. [Google Scholar] [CrossRef]
- Carleton, T.; Cornetet, J.; Huybers, P.; Meng, K.; Proctor, J. Ultraviolet Radiation Decreases COVID-19 Growth Rates: Global Causal Estimates and Seasonal Implications. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Caspi, G.; Shalit, U.; Kristensen, S.L.; Aronson, D.; Caspi, L.; Rossenberg, O.; Shina, A.; Caspi, O. Climate effect on COVID-19 spread rate: An online surveillance tool. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Liang, H.; Yuan, X.; Hu, Y.; Xu, M.; Zhao, Y.; Zhang, B.; Tian, F.; Zhu, X. Roles of meteorological conditions in COVID-19 transmission on a worldwide scale. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Coro, G. A global-scale ecological niche model to predict SARS-CoV-2 coronavirus infection rate. Ecol. Model. 2020, 143, 109187. [Google Scholar] [CrossRef] [PubMed]
- Ficetola, G.F.; Rubolini, D. Climate affects global patterns of COVID-19 early outbreak dynamics. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Zhou, Q.; Zhang, S.; Yung, K.K.L.; Guo, Y. Non-Linear Modulation of COVID-19 Transmission by Climate Conditions. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Gunthe, S.S.; Swain, B.; Patra, S.S.; Amte, A. On the global trends and spread of the COVID-19 outbreak: Preliminary assessment of the potential relation between location-specific temperature and UV index. J. Public Health 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Hozhabri, H.; Sparascio, F.P.; Sohrabi, H.; Mousavifar, L.; Roy, R.; Scribano, D.; De Luca, A.; Ambrosi, C.; Sarshar, M. The Global Emergency of Novel Coronavirus (SARS-CoV-2): An Update of the Current Status and Forecasting. Int. J. Environ. Res. Public Health 2020, 17, 5648. [Google Scholar] [CrossRef] [PubMed]
- Jamil, T.; Alam, I.; Gojobori, T.; Duarte, C.M. No Evidence for Temperature-Dependence of the COVID-19 Epidemic. Front. Public Health 2020, 8, 436. [Google Scholar] [CrossRef]
- Leung, N.Y.; Bulterys, M.A.; Bulterys, P.L. Predictors of COVID-19 incidence, mortality, and epidemic growth rate at the country level. medRxiv 2020. [Google Scholar] [CrossRef]
- Merow, C.; Urban, M.C. Seasonality and uncertainty in global COVID-19 growth rates. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Notari, A. Temperature dependence of COVID-19 transmission. arXiv 2020, arXiv:2003.12417. Available online: https://arxiv.org/abs/2003.12417 (accessed on 12 September 2020).
- Qi, H.; Xiao, S.; Shi, R.; Ward, M.P.; Chen, Y.; Tu, W.; Su, Q.; Wang, W.; Wang, X.; Zhang, Z. COVID-19 transmission in Mainland China is associated with temperature and humidity: A time-series analysis. Sci. Total Environ. 2020, 728, 138778. [Google Scholar] [CrossRef]
- Rahman, A.; Hossain, G.; Singha, A.C.; Islam, S.; Islam, A. A Retrospective Analysis of Influence of Environmental/Air Temperature and Relative Humidity on Sars-CoV-2 Outbreak. J. Pure Appl. Microbiol. 2020, 14, 1705–1714. [Google Scholar] [CrossRef]
- Sajadi, M.M.; Habibzadeh, P.; Vintzileos, A.; Shokouhi, S.; Miralles-Wilhelm, F.; Amoroso, A. Temperature, Humidity, and Latitude Analysis to Estimate Potential Spread and Seasonality of Coronavirus Disease 2019 (COVID-19). JAMA Netw. Open 2020, 3, e2011834. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.F.F.; Duarte, G.B.; Sobral, A.I.G.D.P.; Marinho, M.L.M.; Melo, A.D.S. Association between climate variables and global transmission oF SARS-CoV-2. Sci. Total Environ. 2020, 729, 138997. [Google Scholar] [CrossRef]
- Sunalini, K.K.; Pavani, A. Effects of temperature and relative humidity n COVID-19. J. Crit. Rev. 2020, 7, 3177–3182. [Google Scholar]
- Wan, X.; Cheng, C.; Zhang, Z. Early transmission of COVID-19 has an optimal temperature but late transmission decreases in warm climate. medRxiv 2020. [Google Scholar] [CrossRef]
- Wang, J.; Tang, K.; Feng, K.; Lv, W. High Temperature and High Humidity Reduce the Transmission of COVID-19. SSRN Electron. J. 2020. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Jing, W.; Liu, J.; Ma, Q.; Yuan, J.; Wang, Y.; Du, M.; Liu, M. Effects of temperature and humidity on the daily new cases and new deaths of COVID-19 in 166 countries. Sci. Total Environ. 2020, 729, 139051. [Google Scholar] [CrossRef]
- Smit, A.J.; Fitchett, J.M.; Engelbrecht, F.A.; Scholes, R.J.; Dzhivhuho, G.; Sweijd, N.A. Winter Is Coming: A Southern Hemisphere Perspective of the Environmental Drivers of SARS-CoV-2 and the Potential Seasonality of COVID-19. Int. J. Environ. Res. Public Health 2020, 17, 5634. [Google Scholar] [CrossRef]
- EC-European Commission. Covid Data for Europe Regions. Available online: https://covid-statistics.jrc.ec.europa.eu/Home/Dashboard (accessed on 5 July 2020).
- Beltekian, D.; Gavrilov, D.; Giattino, C.; Hasell, J.; Macdonald, B.; Mathieu, E.; Ortiz-Ospina, E.; Ritchie, H.; Roser, M. Published Online at OurWorldInData.org. Available online: https://covid.ourworldindata.org/data/owid-covid-data.csv (accessed on 7 July 2020).
- Hale, T.; Webster, S.; Petherick, A.; Phillips, T.; Kira, B. Oxford COVID-19 Government Response Tracker, Blavatnik School of Government. Available online: https://www.bsg.ox.ac.uk/research/research-projects/coronavirus-government-response-tracker#data (accessed on 10 August 2020).
- GDL-Global Data Lab. Subnational Human Development Index (4.0). Available online: https://globaldatalab.org/shdi/2018/indices/?levels=1&interpolation=0&extrapolation=0&nearest_real=0 (accessed on 10 June 2020).
- Copernicus. Essential Climate Variables for Assessment of Climate Variability from 1979 to Present. Available online: https://cds.climate.copernicus.eu/cdsapp#!/dataset/ecv-for-climate-change?tab=doc (accessed on 15 July 2020).
- Copernicus. Copernicus Program of “ERA5 Monthly Averaged Data on Pressure Levels from 1979 to Present”. Available online: https://cds.climate.copernicus.eu/cdsapp#!/dataset/10.24381/cds.6860a573?tab=form (accessed on 15 July 2020).
- Eccles, R. An Explanation for the Seasonality of Acute Upper Respiratory Tract Viral Infections. Acta Oto-Laryngol. 2002, 122, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Mourtzoukou, E.G.; Falagas, M. Exposure to cold and respiratory tract infections. Int. J. Tuberc. Lung Dis. 2007, 11, 938–943. [Google Scholar] [PubMed]
- Foxman, E.F.; Storer, J.A.; Fitzgerald, M.E.; Wasik, B.R.; Hou, L.; Zhao, H.; Turner, P.E.; Pyle, A.M.; Iwasaki, A. Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proc. Natl. Acad. Sci. USA 2015, 112, 827–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagripanti, J.-L.; Lytle, C.D. Inactivation of Influenza Virus by Solar Radiation. Photochem. Photobiol. 2007, 83, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.P.; Stilianakis, N.I. Inactivation of influenza A viruses in the environment and modes of transmission: A critical review. J. Infect. 2008, 57, 361–373. [Google Scholar] [CrossRef]
- Lowen, A.C.; Steel, J. Roles of Humidity and Temperature in Shaping Influenza Seasonality. J. Virol. 2014, 88, 7692–7695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marr, L.C.; Tang, J.W.; Van Mullekom, J.; Lakdawala, S.S. Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J. R. Soc. Interface 2019, 16, 20180298. [Google Scholar] [CrossRef]
- Martin, V.; Pfeiffer, D.U.; Zhou, X.; Xiao, X.; Prosser, D.J.; Guo, F.; Gilbert, M. Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in China. PLoS Pathog. 2011, 7, e1001308. [Google Scholar] [CrossRef]
- Adly, H.M.; Aljahdali, I.A.; Garout, M.A.; Khafagy, A.A.; Saati, A.A.; Saleh, S.A.K. Correlation of COVID-19 Pandemic with Healthcare System Response and Prevention Measures in Saudi Arabia. Int. J. Environ. Res. Public Health 2020, 17, 6666. [Google Scholar] [CrossRef]
- Kheirallah, K.A.; Alsinglawi, B.; Alzoubi, A.; Saidan, M.; Mubin, O.; Alorjani, M.S.; Mzayek, F. The Effect of Strict State Measures on the Epidemiologic Curve of COVID-19 Infection in the Context of a Developing Country: A Simulation from Jordan. Int. J. Environ. Res. Public Health 2020, 17, 6530. [Google Scholar] [CrossRef]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza Virus Transmission Is Dependent on Relative Humidity and Temperature. PLoS Pathog. 2007, 3, e151. [Google Scholar] [CrossRef] [PubMed]
- Hanley, B.P.; Borup, B. Aerosol influenza transmission risk contours: A study of humid tropics versus winter temperate zone. Virol. J. 2010, 7, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamidi, S.; Sabouri, S.; Ewing, R. Does Density Aggravate the COVID-19 Pandemic? J. Am. Plan. Assoc. 2020, 86, 495–509. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report—89. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200418-sitrep-89-covid-19.pdf (accessed on 1 May 2020).
- Ryan, T.P. Modern Engineering Statistics; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Zhou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2005, 67, 301–320. [Google Scholar] [CrossRef] [Green Version]
- Hoerl, A.E.; Kennard, R.W. Ridge Regression: Biased Estimation for Nonorthogonal Problems. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]


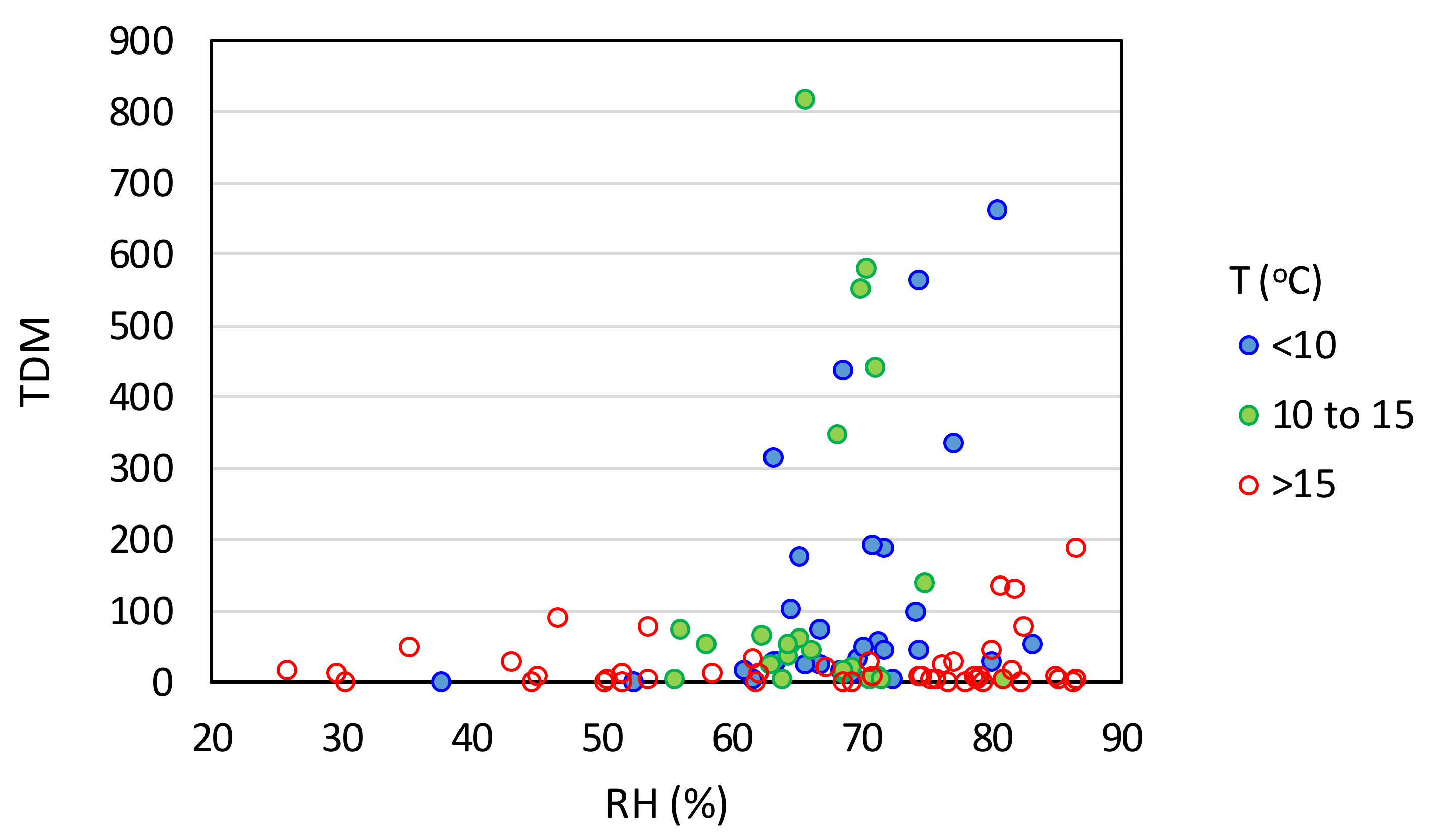
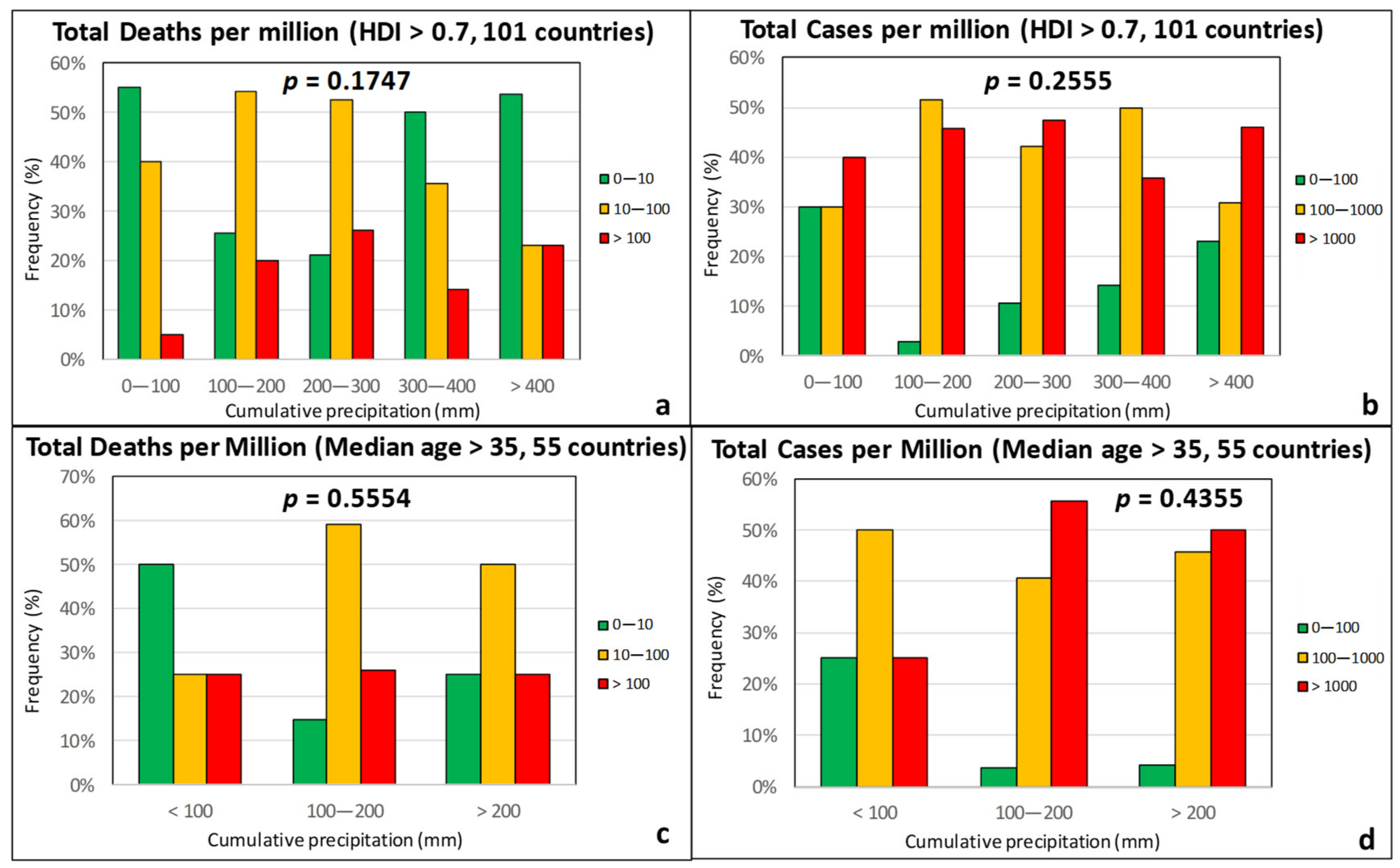
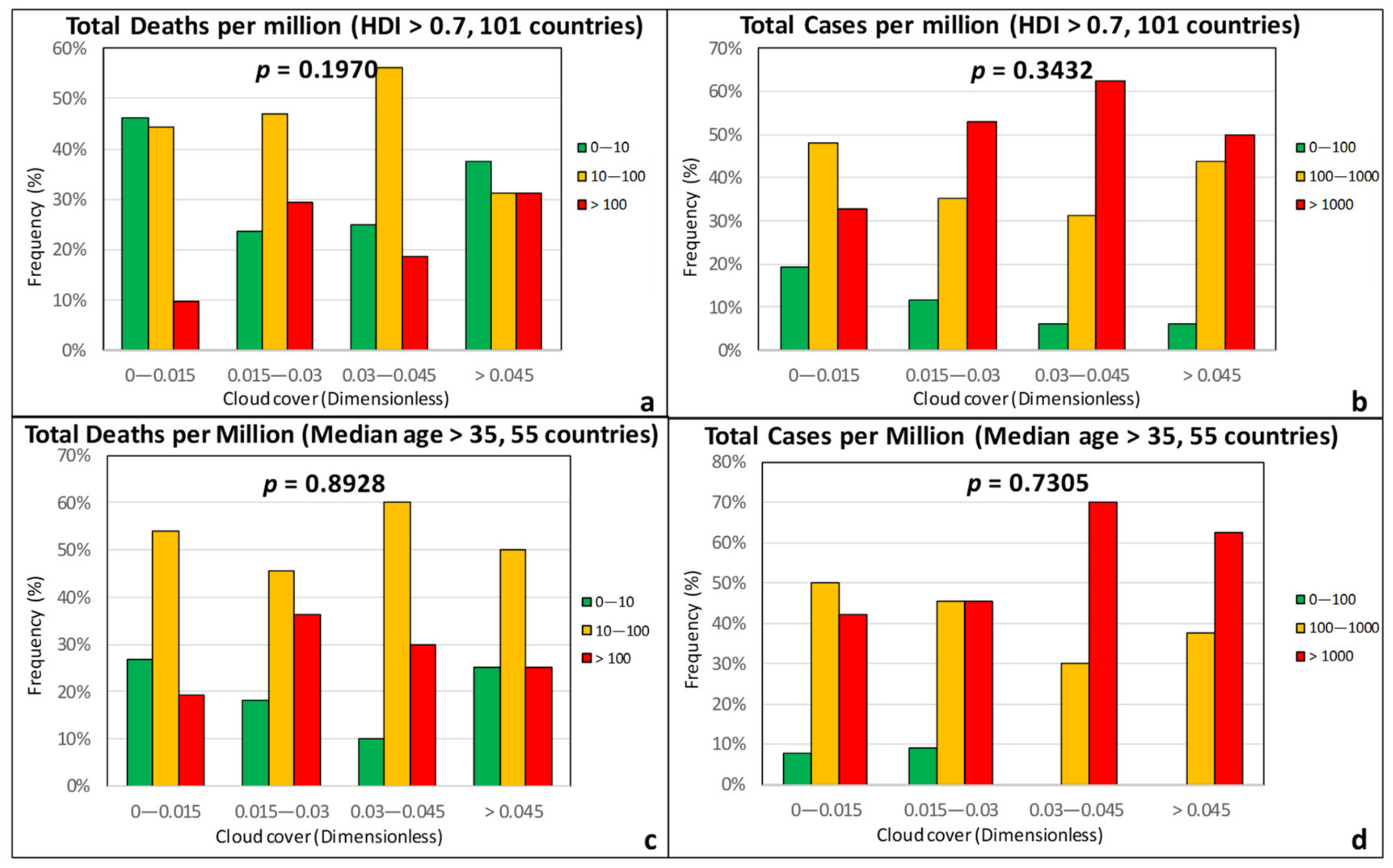
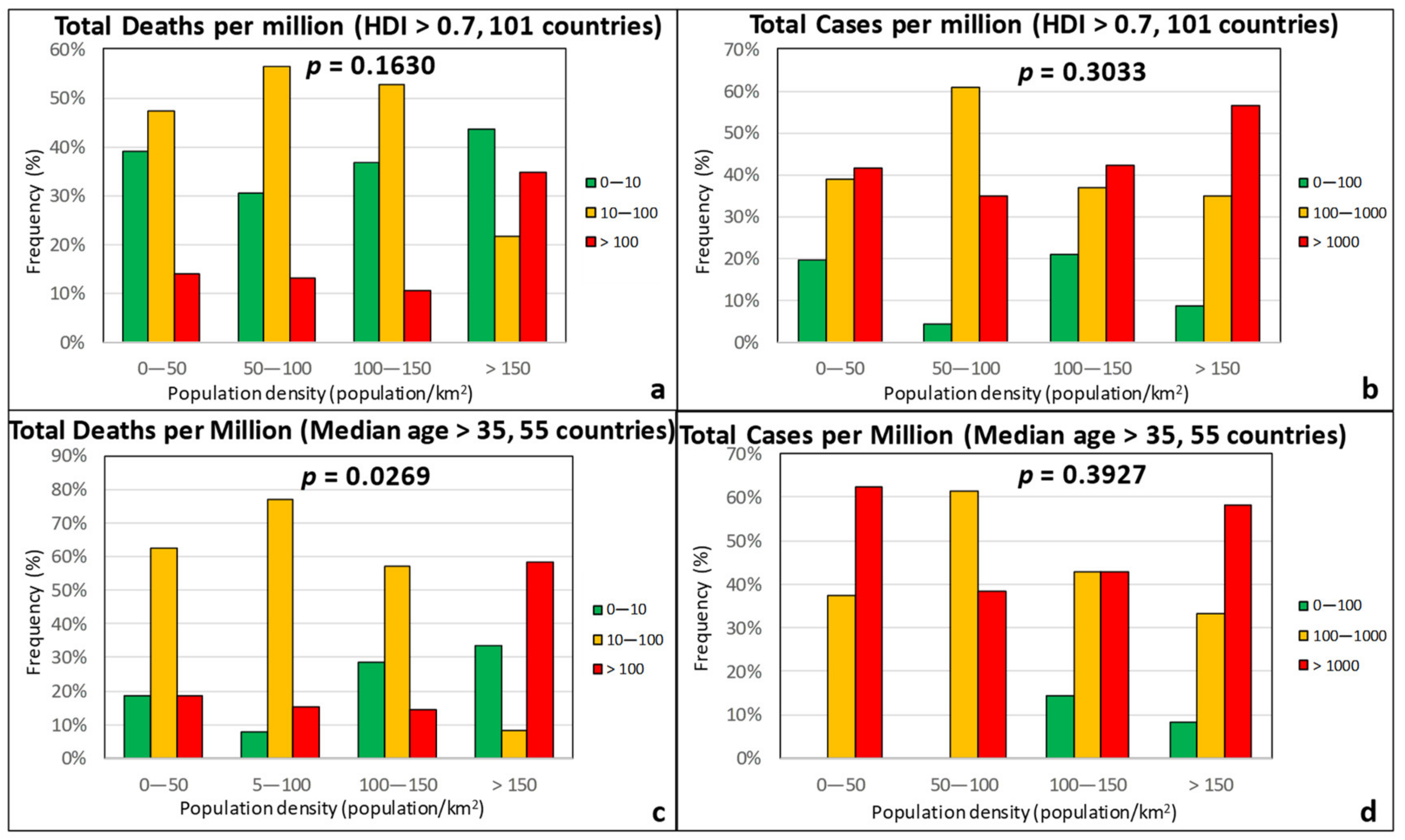
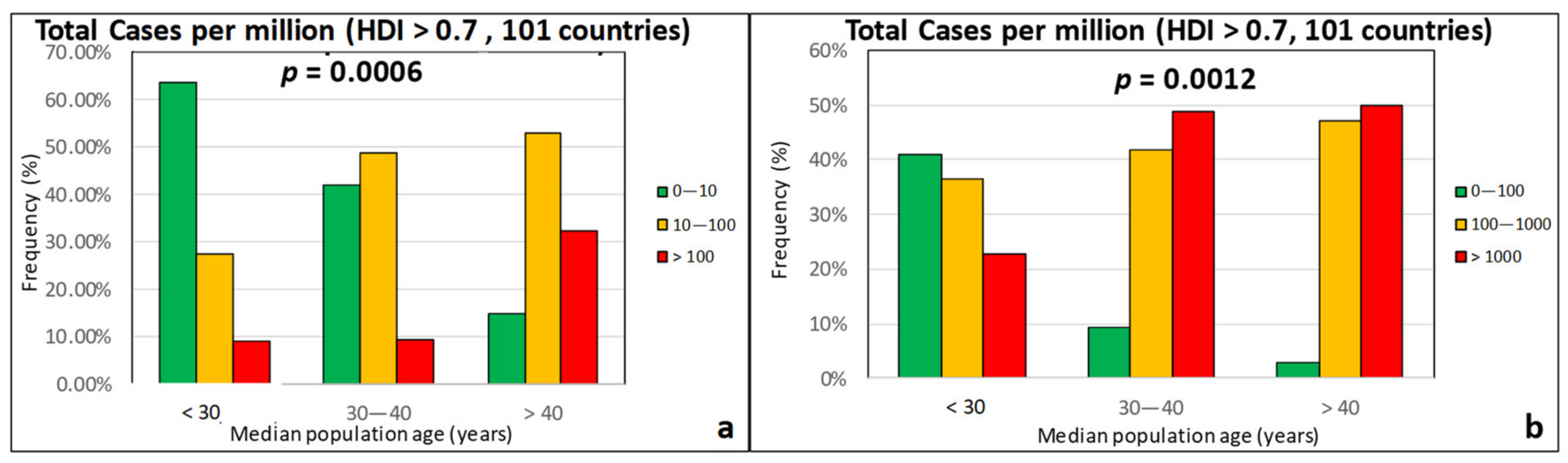

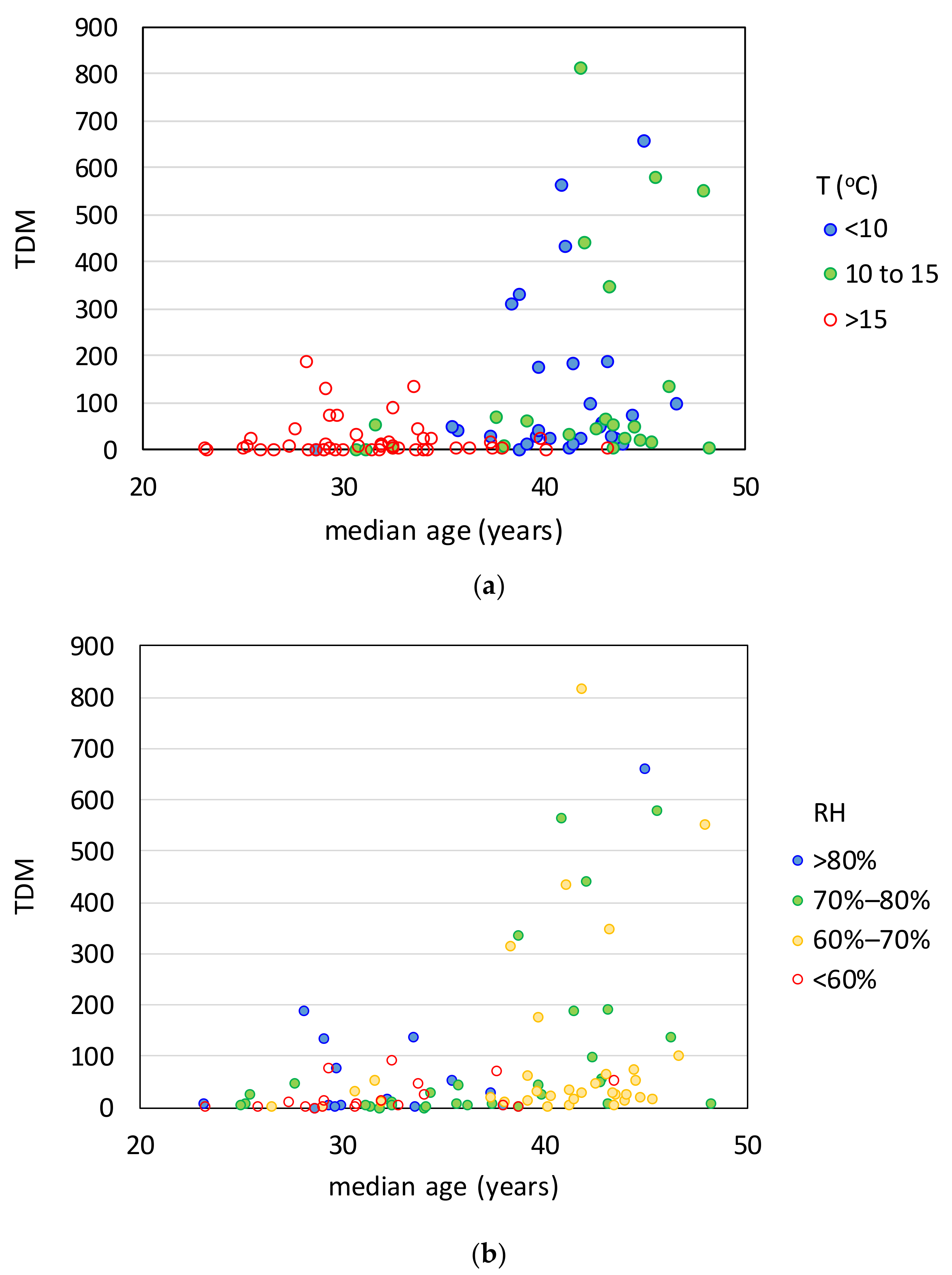
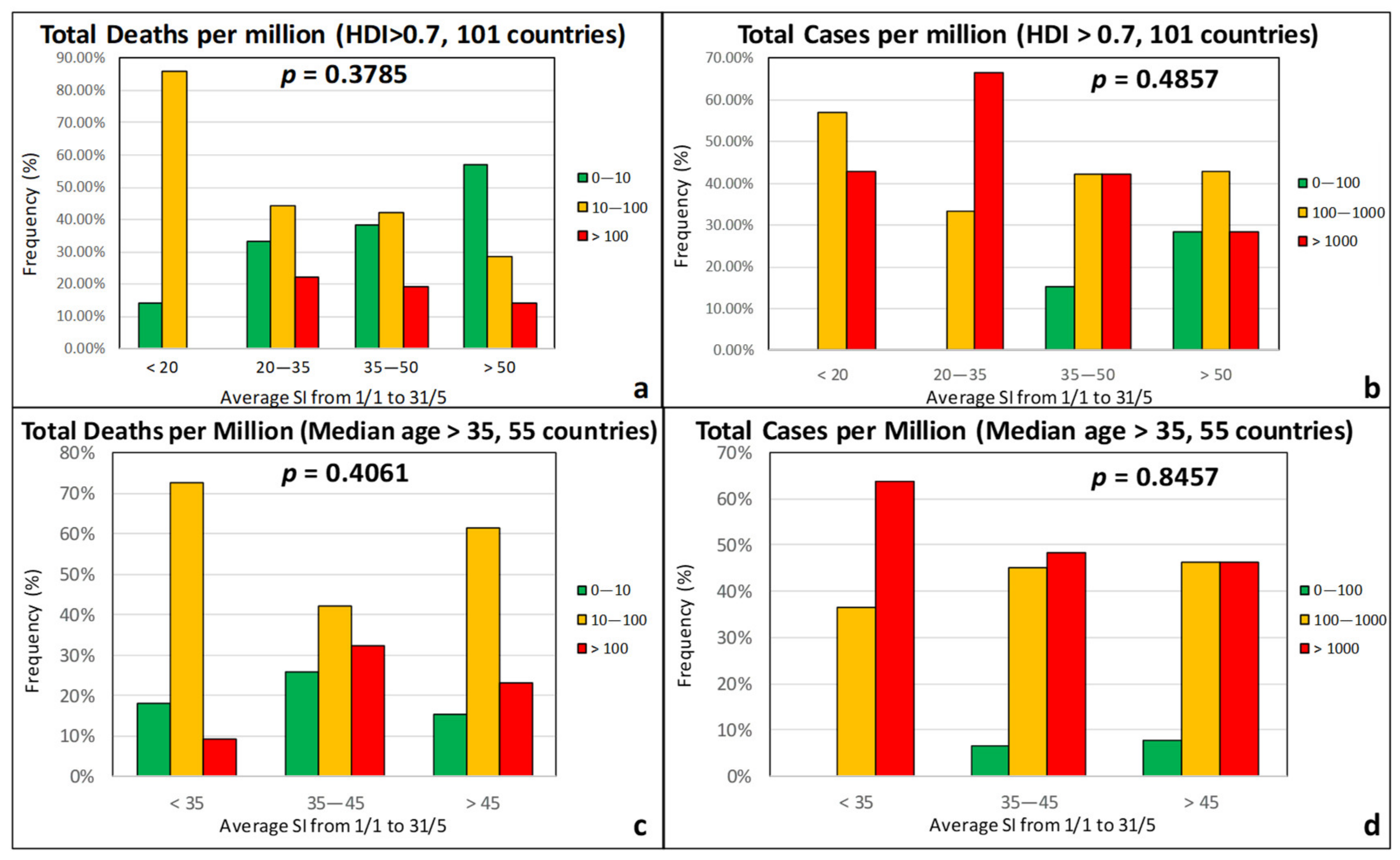
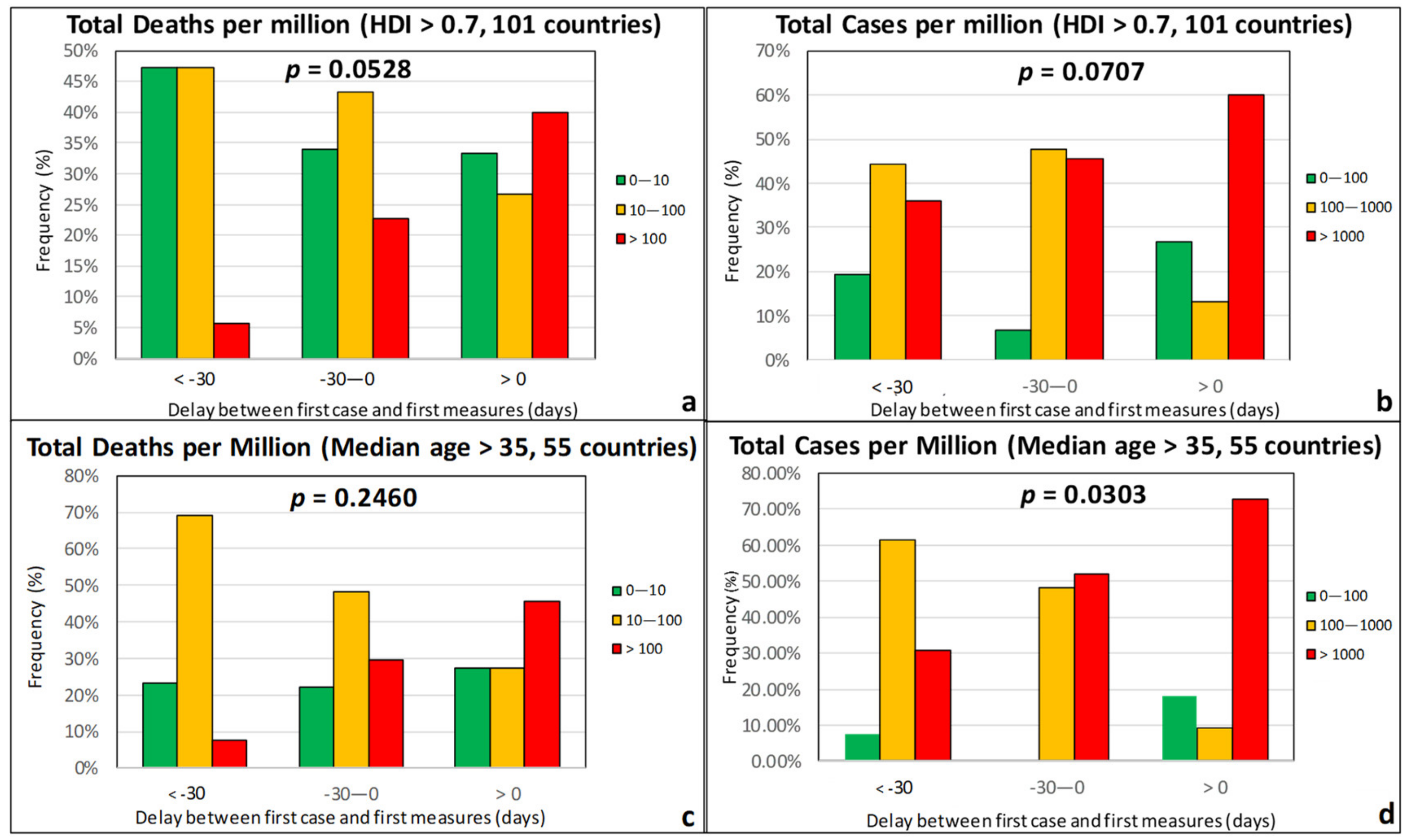
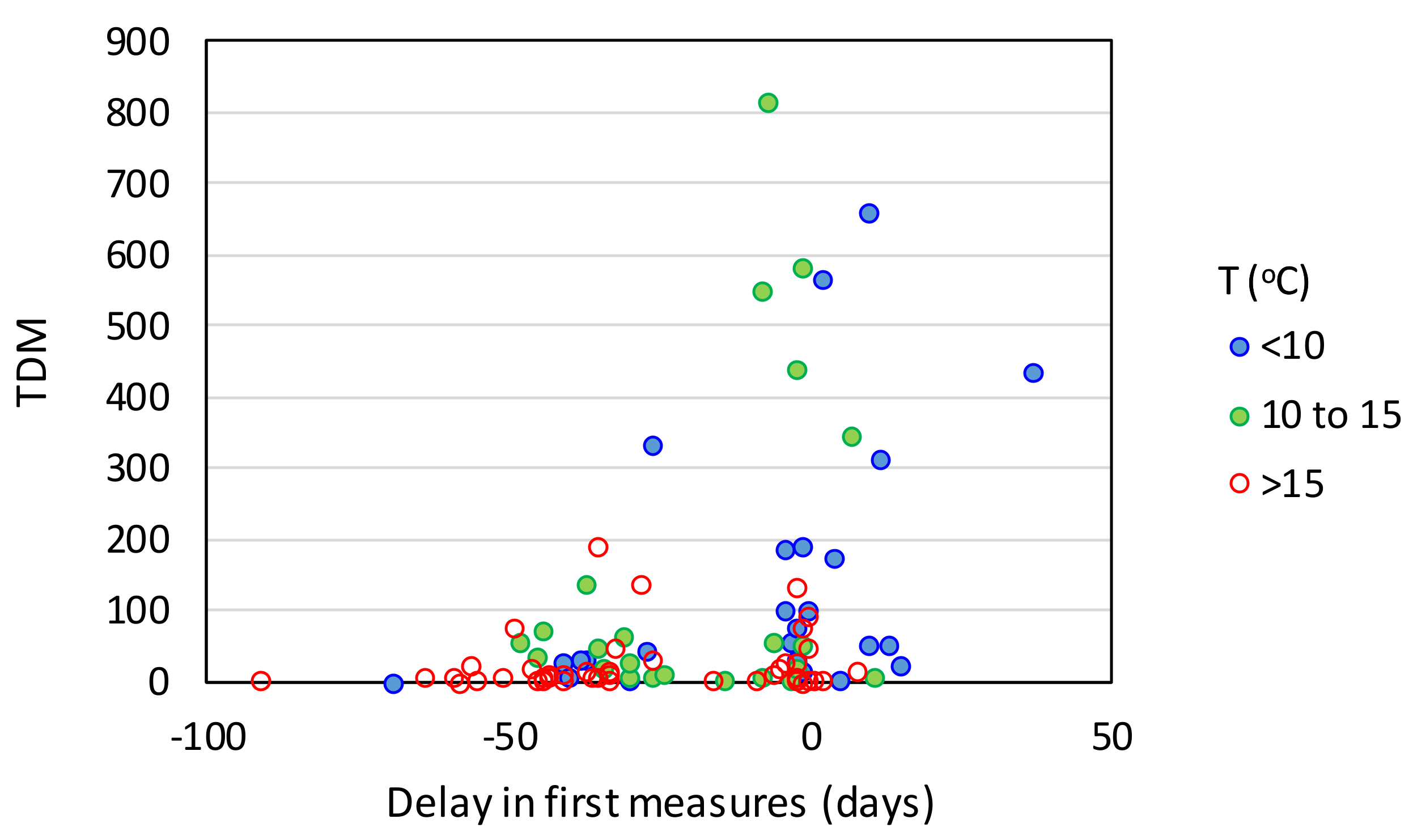
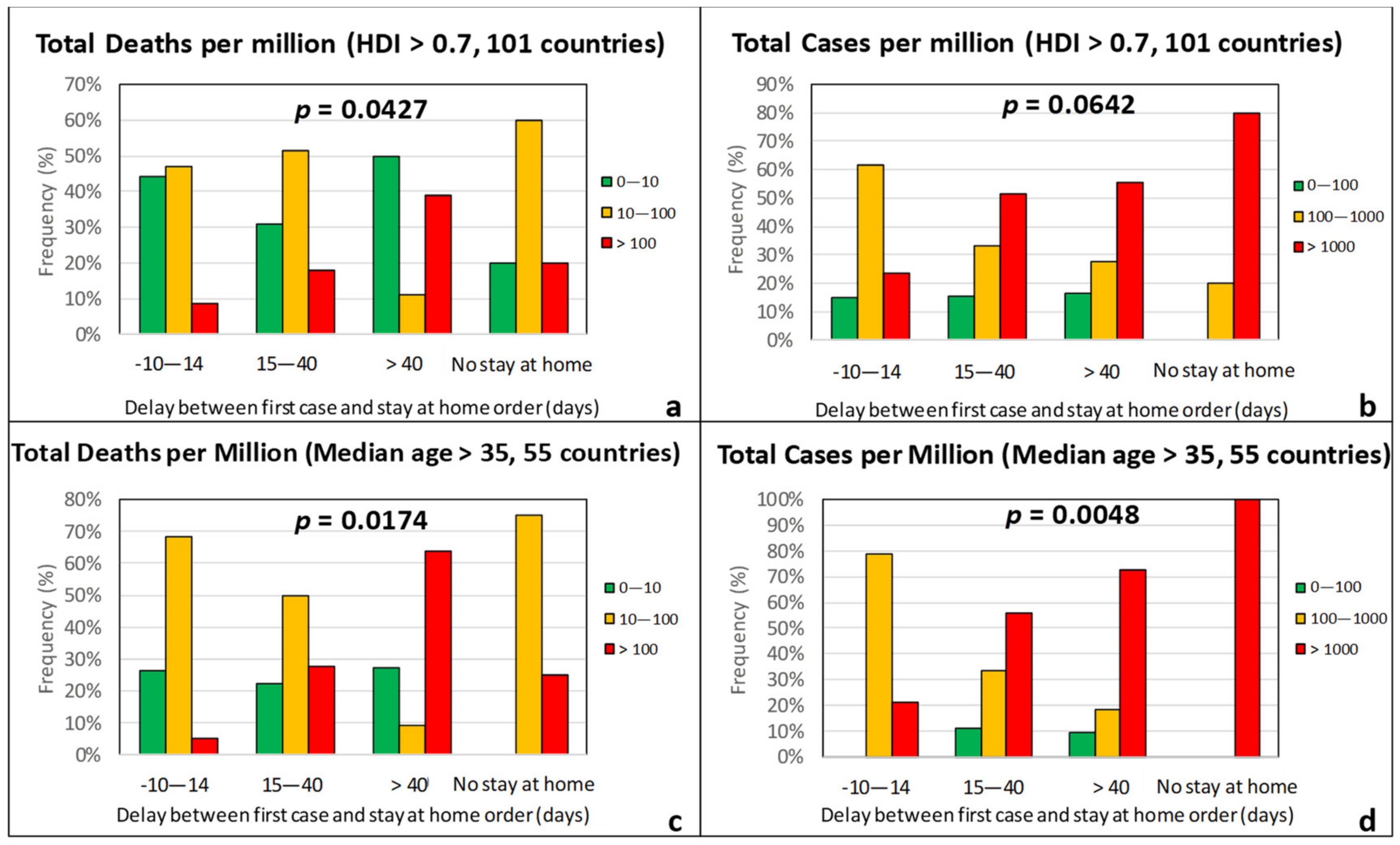

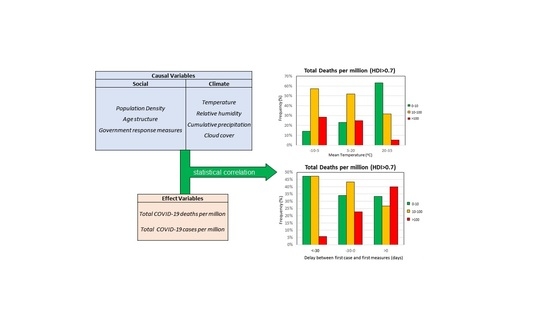
| Effect Variables | Causal Variables | Filtering Variables |
|---|---|---|
| Total Deaths per Million (TDM) Total Cases per Million (TCM) | Climatic Temperature (°C) Relative humidity (%) Cumulative precipitation (mm) Cloud cover Sociodemographic Population density (persons/km2) Median age (years) Percentage of Population over 65 (%) Government response Stringency IndexDelay between first case and first measures (days) Delay between first case and “stay at home” order (days) | Human Development Index (HDI) Median Age (years) |
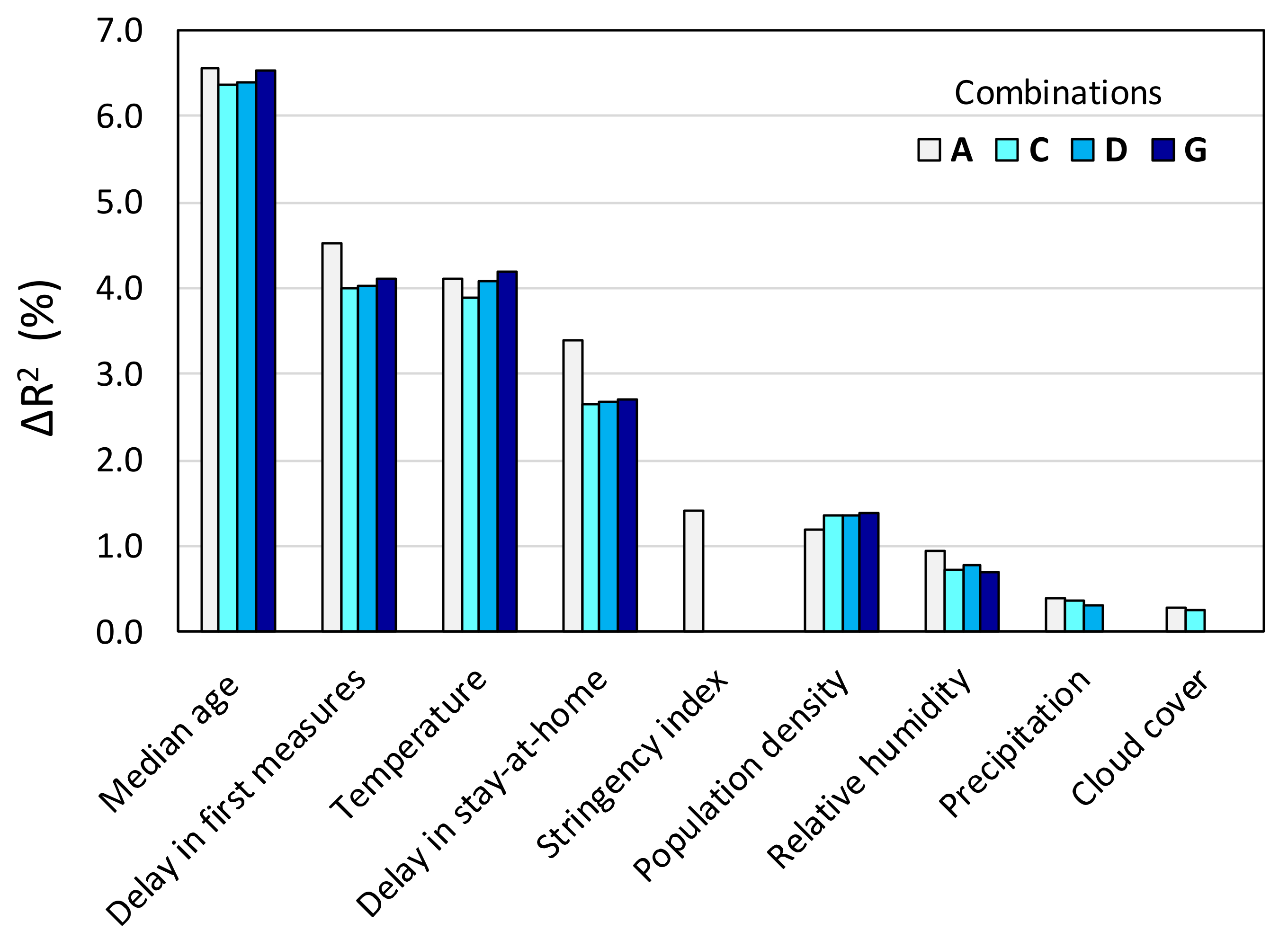
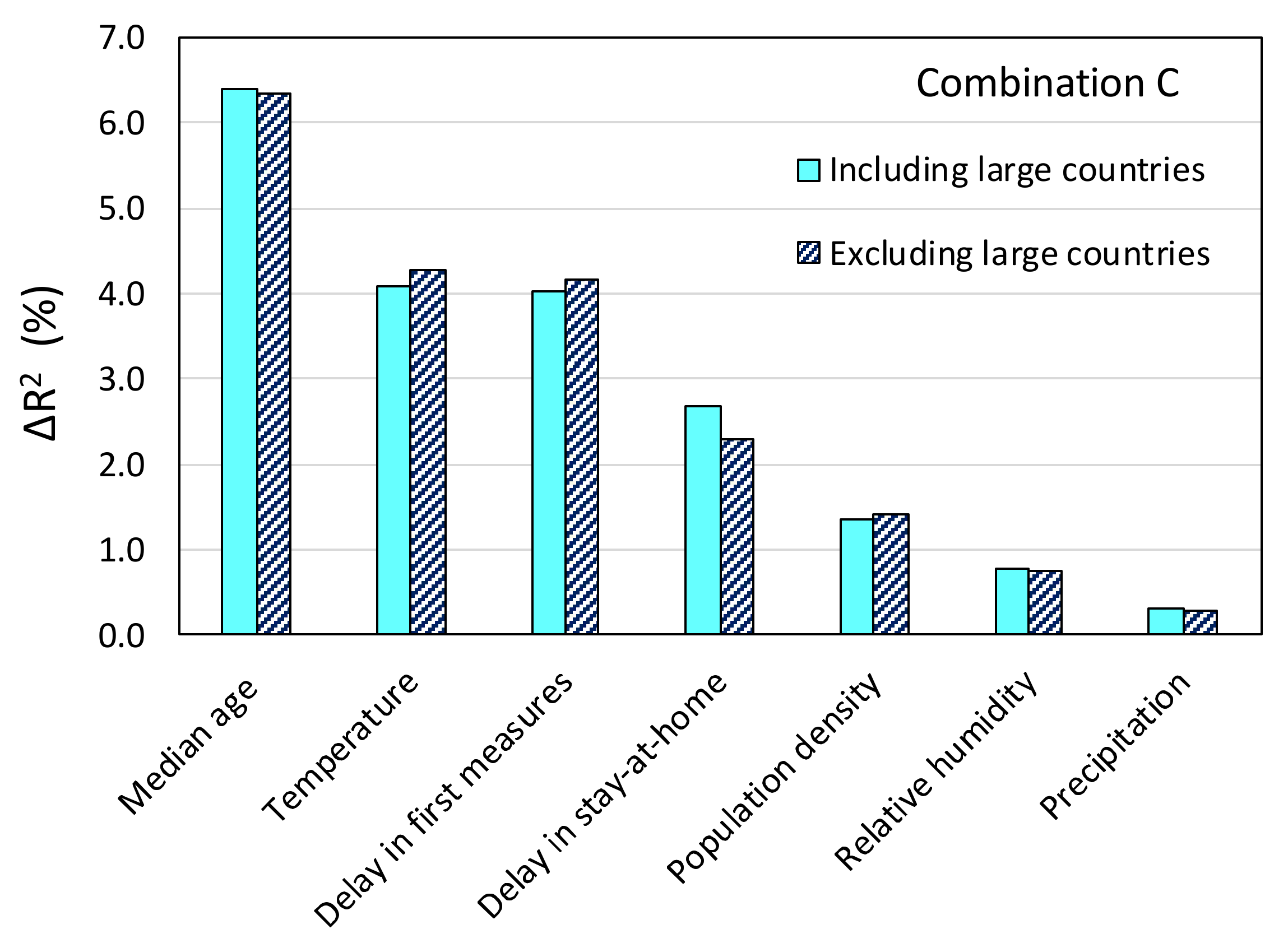
| Variable | Combination | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | |
| Temperature | 0.041 | 0.042 | 0.039 | 0.041 | 0.042 | 0.037 | 0.042 | 0.045 | 0.046 | 0.047 |
| Relative humidity | 0.009 | 0.010 | 0.007 | 0.008 | 0.010 | 0.007 | 0.008 | 0.008 | 0.007 | |
| Precipitation | 0.004 | 0.004 | 0.004 | 0.003 | 0.002 | 0.004 | 0.003 | |||
| Cloud cover | 0.003 | 0.003 | ||||||||
| Population density | 0.012 | 0.012 | 0.014 | 0.013 | 0.015 | 0.014 | 0.013 | 0.013 | 0.016 | |
| Median age | 0.066 | 0.067 | 0.064 | 0.064 | 0.067 | 0.069 | 0.065 | 0.067 | 0.068 | 0.076 |
| Stringency index | 0.014 | 0.014 | ||||||||
| Delay in first measures | 0.045 | 0.045 | 0.040 | 0.040 | 0.041 | 0.042 | 0.041 | 0.047 | 0.048 | |
| Delay in stay-at-home | 0.034 | 0.034 | 0.027 | 0.027 | 0.027 | 0.026 | 0.027 | 0.034 | ||
| Variable | Coefficient | Combination | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | ||
| Temperature | C1 | −108.9 | −88.9 | −86.7 | −89.7 | −93.8 | −60.6 | −86.8 | −103.9 | −99.1 | −90.9 |
| Relative humidity | C2 | 82.2 | 79.1 | 34.8 | 34.9 | 62.7 | 46.1 | 34.7 | 54.2 | 40.6 | |
| Precipitation | C3 | 13.4 | −17.9 | 11.3 | 16.3 | 45.0 | −14.8 | 28.1 | |||
| Cloud cover | C4 | −43.5 | 6.6 | ||||||||
| Population density | C5 | 48.8 | 47.5 | 72.9 | 73.3 | 80.3 | 70.3 | 70.3 | 64.9 | 78.9 | |
| Median age | C6 | 113.8 | 120.2 | 109.2 | 108.1 | 112.6 | 125.7 | 106.4 | 110.1 | 107.1 | 127.7 |
| Stringency index | C7 | 261.0 | 246.9 | ||||||||
| Delay in first measures | C8 | 174.2 | 167.7 | 128.5 | 129.2 | 130.9 | 133.9 | 127.5 | 156.0 | 153.6 | |
| Delay in stay-at-home | C9 | 158.8 | 157.3 | 94.7 | 94.4 | 94.3 | 91.4 | 95.5 | 122.1 | ||
| Intercept | C0 | −267.7 | −270.4 | −52.7 | −50.4 | −35.3 | −78.2 | −54.1 | −31.5 | −37.5 | −3.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzampoglou, P.; Loukidis, D. Investigation of the Importance of Climatic Factors in COVID-19 Worldwide Intensity. Int. J. Environ. Res. Public Health 2020, 17, 7730. https://doi.org/10.3390/ijerph17217730
Tzampoglou P, Loukidis D. Investigation of the Importance of Climatic Factors in COVID-19 Worldwide Intensity. International Journal of Environmental Research and Public Health. 2020; 17(21):7730. https://doi.org/10.3390/ijerph17217730
Chicago/Turabian StyleTzampoglou, Ploutarchos, and Dimitrios Loukidis. 2020. "Investigation of the Importance of Climatic Factors in COVID-19 Worldwide Intensity" International Journal of Environmental Research and Public Health 17, no. 21: 7730. https://doi.org/10.3390/ijerph17217730