Multiscale Modeling of a Packed Bed Chemical Looping Reforming (PBCLR) Reactor †
Abstract
:1. Introduction
2. Methodology
2.1. PR-DNS Setup
2.2. 1D Packed Bed Model
3. Results
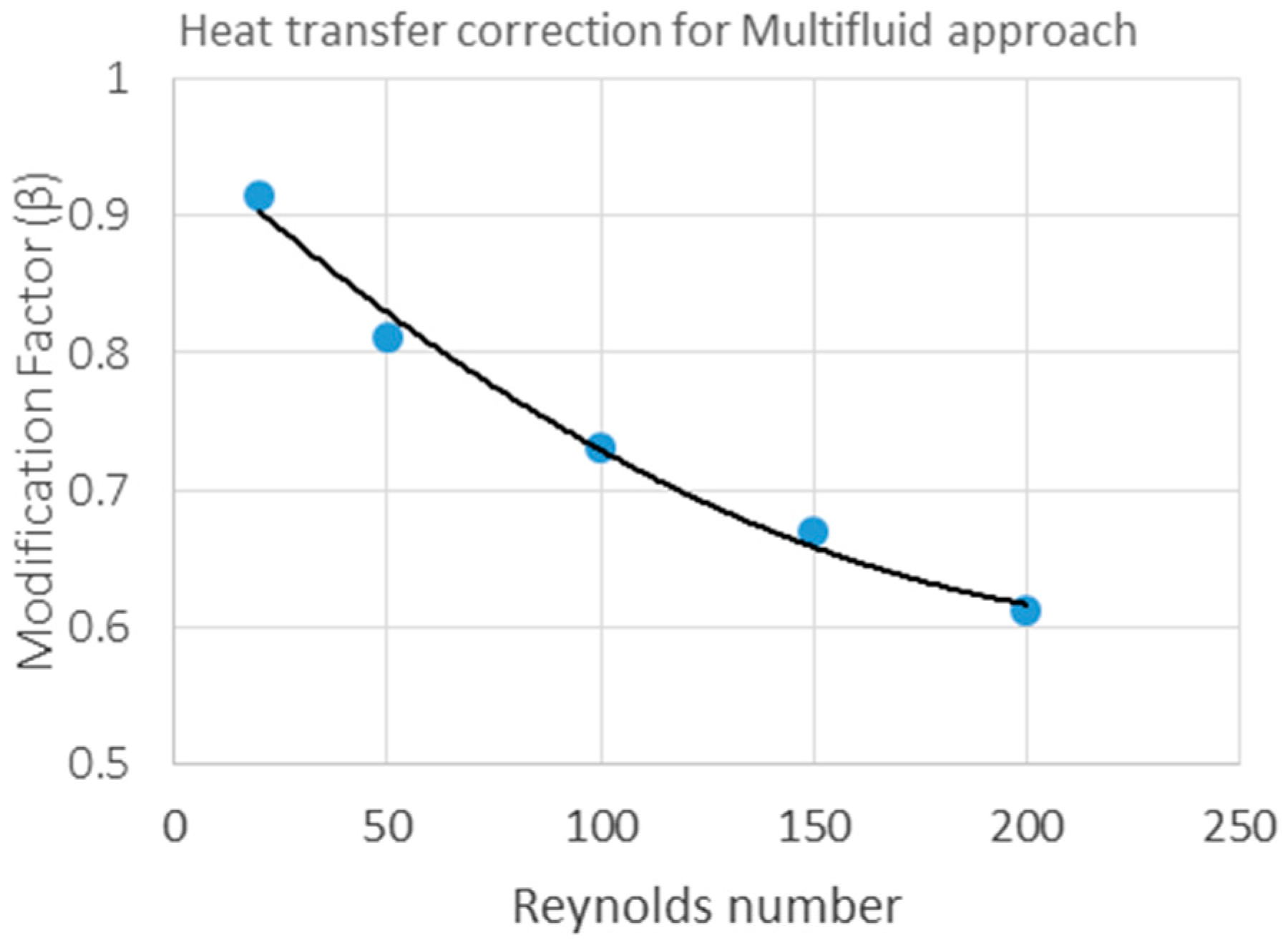
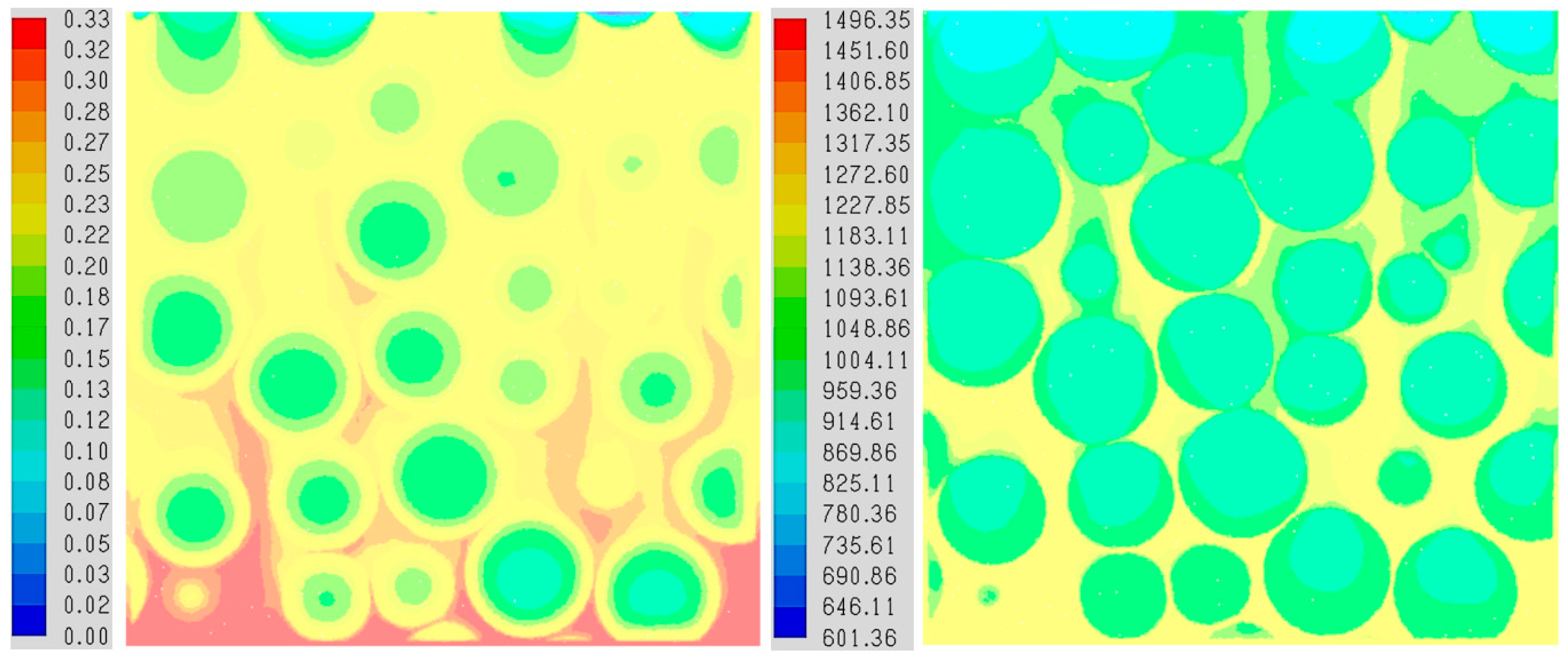
3.1. PR-DNS Results
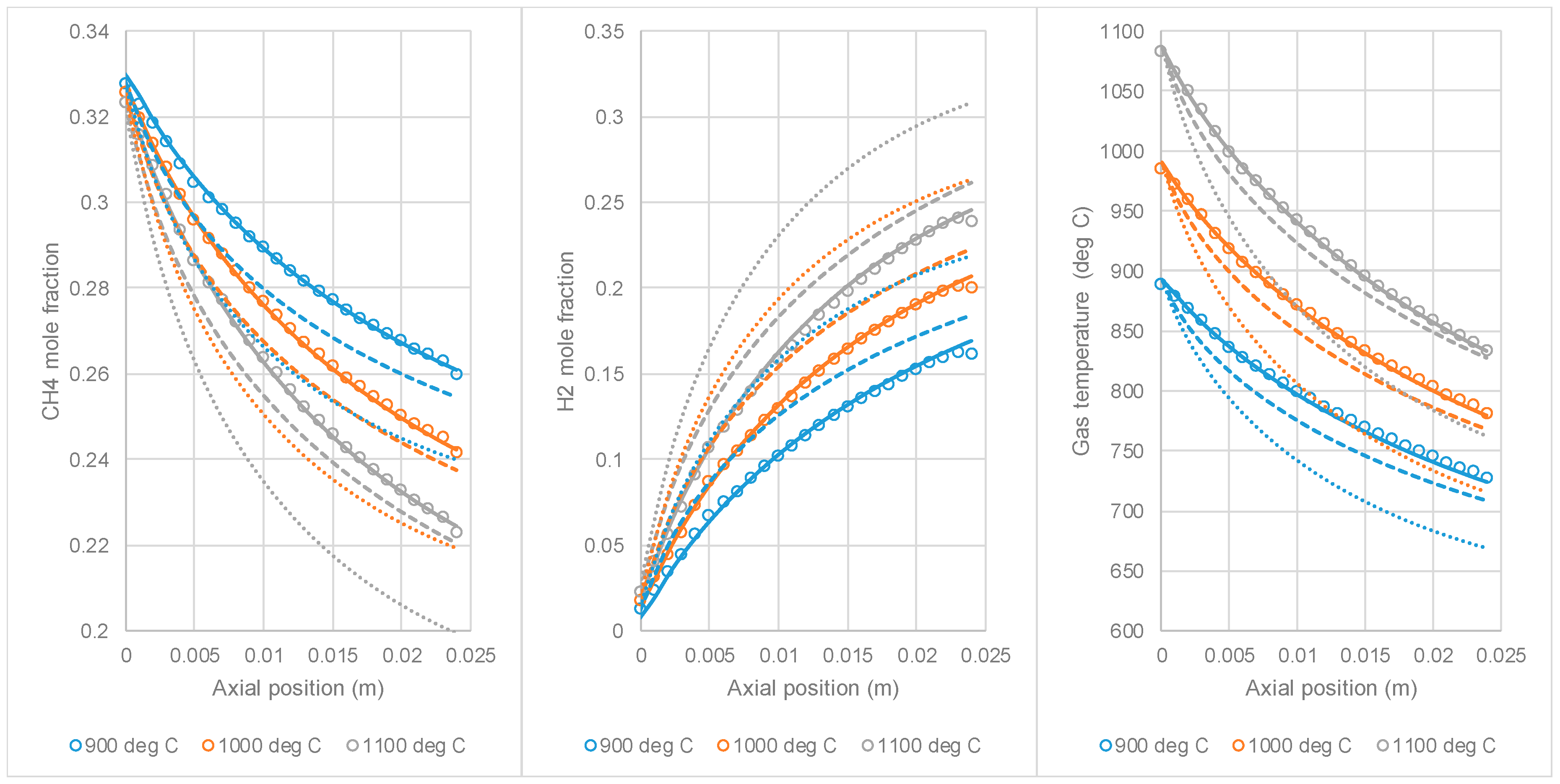
3.2. Comparison of 1D Model to PR-DNS Results
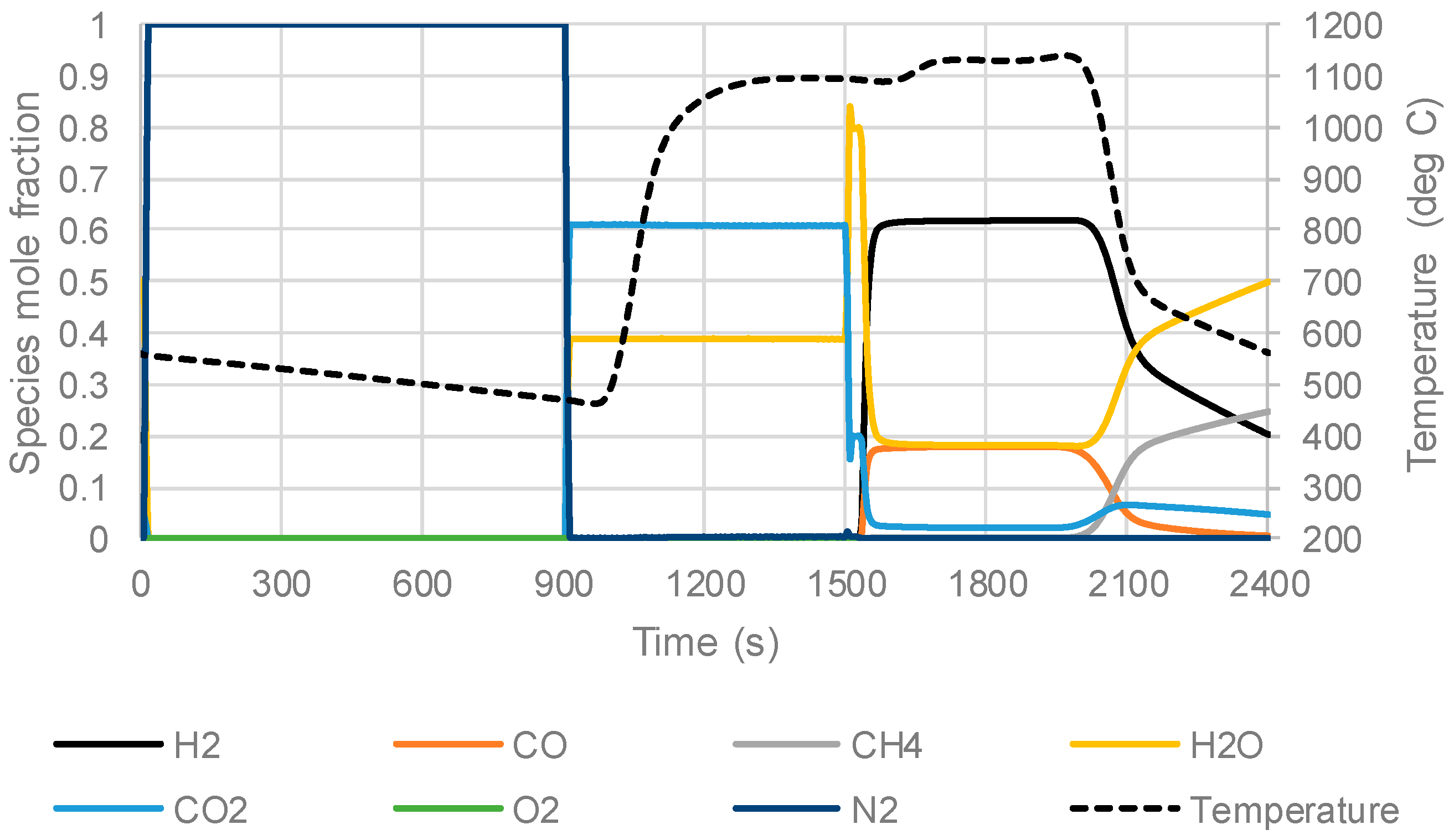
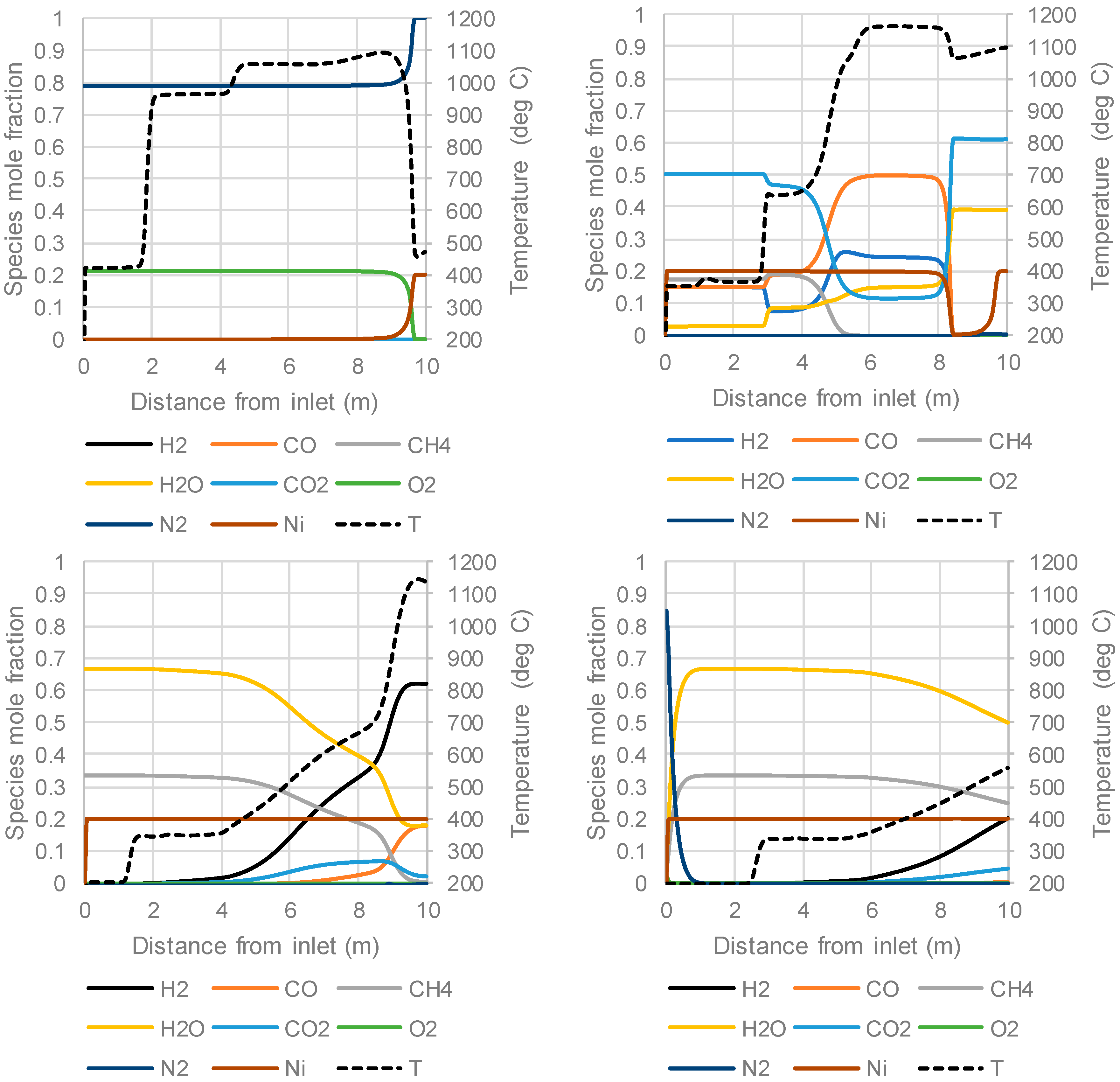
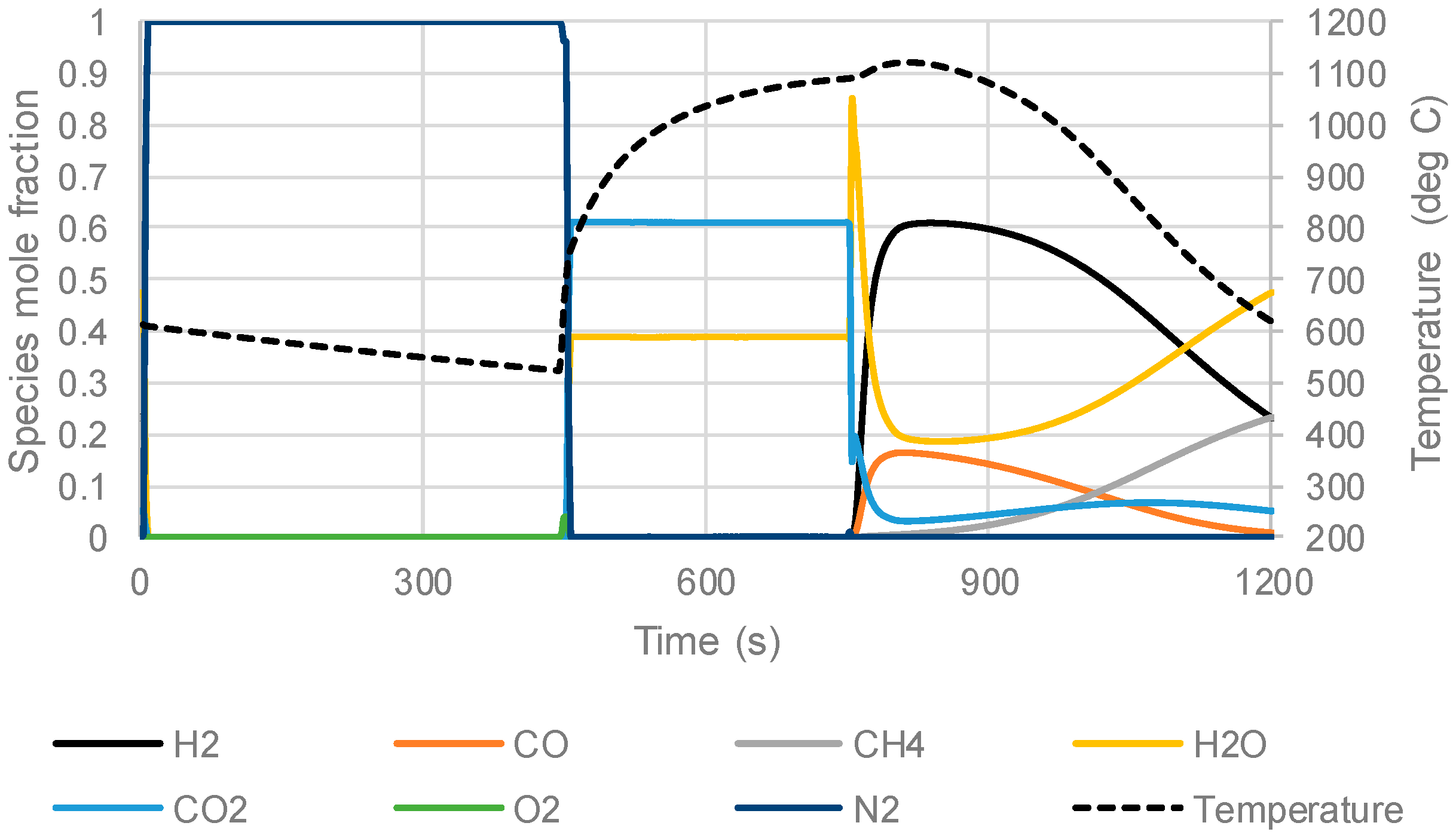
3.3. The Packed Bed Chemical Looping Reforming Process
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| Greek Symbols | |
| α | Volume fraction |
| ε | Void fraction |
| φ | Thiele modulus (Th) |
| Effectiveness factor | |
| ρ | Density (kg/m3) |
| Latin Symbols | |
| a | Characteristic length of spherical particle (rp/3) |
| Cp | Specific heat capacity of gas [J/kg·K] |
| Ea | Activation energy [J/mol] |
| he | effective heat transfer coefficient [W/m2·K] |
| k0 | Arrhenius constant [1/s] |
| kg | Thermal conductivity of gas [W/m·K] |
| Nu | Nusselt number (h dp/kg) |
| P | Pressure [Pa], 1 bar = 101,325 Pa |
| Pr | Prandtl number (μ Cp/kg) |
| R | Gas constant [8.314 J/mol/K] |
| r | Radius [m] |
| Re | Reynolds number (ρ us dp/μ) |
| Sc | Schmidt number (μ/ρ D) |
| T | Temperature [K] |
| us | Superficial velocity of the gas [m/s] |
| xi | Mass fraction of species i |
| yi | Mole fraction of species i |
| Sub/superscripts | |
| g | Gas |
| p | Particle |
References
- Ramachandran, P.A.; Doraiswamy, L.K. Modeling of noncatalytic gas-solid reactions. AIChE J. 1982, 28, 881–900. [Google Scholar] [CrossRef]
- Karthik, G.M.; Buwa, V.V. Effect of particle shape on fluid flow and heat transfer for methane steam reforming reactions in a packed bed. AIChE J. 2017, 63, 366–377. [Google Scholar] [CrossRef]
- Augier, F.; Idoux, F.; Delenne, J.Y. Numerical simulations of transfer and transport properties inside packed beds of spherical particles. Chem. Eng. Sci. 2010, 65, 1055–1064. [Google Scholar] [CrossRef]
- Dixon, A.G.; Nijemeisland, M.; Stitt, H. CFD simulation of reaction and heat transfer near the wall of a fixed bed. Int. J. Chem. React. Eng. 2003. [Google Scholar] [CrossRef]
- Magnico, P. Pore-scale simulations of unsteady flow and heat transfer in tubular fixed beds. AIChE J. 2009, 55, 849–867. [Google Scholar] [CrossRef]
- Nijemeisland, M.; Dixon, A.G.; Hugh Stitt, E. Catalyst design by cfd for heat transfer and reaction in steam reforming. Chem. Eng. Sci. 2004, 59, 5185–5191. [Google Scholar] [CrossRef]
- Dixon, A.G. Local transport and reaction rates in a fixed bed reactor tube: Endothermic steam methane reforming. Chem. Eng. Sci. 2017, 168, 156–177. [Google Scholar] [CrossRef]
- Singhal, A.; Cloete, S.; Radl, S.; Quinta-Ferreira, R.; Amini, S. Heat transfer to a gas from densely packed beds of monodisperse spherical particles. Chem. Eng. J. 2017, 314, 27–37. [Google Scholar] [CrossRef]
- Singhal, A.; Cloete, S.; Radl, S.; Quinta-Ferreira, R.; Amini, S. Heat transfer to a gas from densely packed beds of cylindrical particles. Chem. Eng. Sci. 2017, 172, 1–12. [Google Scholar] [CrossRef]
- Singhal, A.; Cloete, S.; Radl, S.; Quinta-Ferreira, R.; Amini, S. Comparison of particle-resolved direct numerical simulation and 1d modelling of catalytic reactions in a packed bed. In Progress in Applied CFD—CFD2017, Proceedings of the 12th International Conference on CFD in Oil & Gas, Metallurgical and Process Industries, Trondheim, Norway, 30 May–1 June 2017; SINTEF Academic Press: Oslo, Norway, 2017; pp. 667–674. [Google Scholar]
- Singhal, A.; Cloete, S.; Quinta-Ferreira, R.; Amini, S. Multiscale modelling of packed bed chemical looping reforming. Energy Procedia 2017, 136, 349–355. [Google Scholar] [CrossRef]
- Cloete, S.; Gallucci, F.; van Sint Annaland, M.; Amini, S. Gas switching as a practical alternative for scaleup of chemical looping combustion. Energy Technol. 2016, 4, 1286–1298. [Google Scholar] [CrossRef]
- Singhal, A.; Cloete, S.; Quinta-Ferreira, R.; Amini, S. Comparison of particle-resolved direct numerical simulation and 1d modelling of catalytic reactions in a cylindrical particle bed. In Proceedings of the V International Conference on Particle-Based Methods, Fundamentals and Applications, Hannover, Germany, 26–28 September 2017; International Center for Numerical Methods in Engineering (CIMNE): Barcelona, Spain, 2017; pp. 802–812. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Yang, W.; Cloete, S.; Morud, J.; Amini, S. An effective reaction rate model for gas-solid reactions with high intra-particle diffusion resistance. Int. J. Chem. React. Eng. 2016, 14, 331–342. [Google Scholar] [CrossRef]
- Francisco Morgado, J.; Cloete, S.; Morud, J.; Gurker, T.; Amini, S. Modelling study of two chemical looping reforming reactor configurations: Looping vs. Switching. Powder Technol. 2016, 316, 599–613. [Google Scholar] [CrossRef]
- Wassie, S.A.; Gallucci, F.; Zaabout, A.; Cloete, S.; Amini, S.; van Sint Annaland, M. Hydrogen production with integrated CO2 capture in a novel gas switching reforming reactor: Proof-of-concept. Int. J. Hydrogen Energy 2017, 42, 14367–14379. [Google Scholar] [CrossRef]
- Szekely, J.; Evans, J.W.; Sohn, H.Y. Gas-Solid Reactions; Academic Press: New York, NY, USA, 1976; p. 612. [Google Scholar]
- Xu, J.; Froment, G.F. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Oliveira, E.L.G.; Grande, C.A.; Rodrigues, A.E. Methane steam reforming in large pore catalyst. Chem. Eng. Sci. 2010, 65, 1539–1550. [Google Scholar] [CrossRef]
- Noorman, S.; van Sint Annaland, M.; Kuipers, J.A.M. Packed bed reactor technology for chemical-looping combustion. Ind. Eng. Chem. Res. 2007, 46, 4212–4220. [Google Scholar] [CrossRef]
- Abad, A.; Adánez, J.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Celaya, J. Mapping of the range of operational conditions for Cu-, Fe-, and Ni-based oxygen carriers in chemical-looping combustion. Chem. Eng. Sci. 2007, 62, 533–549. [Google Scholar] [CrossRef] [Green Version]
- Ergun, S. Fluid flow through packed columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Tsotsas, E.; Martin, H. Thermal conductivity of packed beds: A review. Chem. Eng. Process. Process Intensif. 1987, 22, 19–37. [Google Scholar] [CrossRef]
- Robie, R.A.; Hemingway, B.S. Thermodynamic Properties of Minerals and Related Substances at 298.15 k and 1 Bar (105 Pascals) Pressure and at Higher Temperatures; Series Number 2131; U.S. Geological Survey: Washington, DC, USA, 1995.
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Particle diameter (dp) (m) | 0.005 | Inlet mole fraction ratio (CH4:H2O) | 1:2 |
| Packed bed voidage | 0.355 | Specific heat capacity (Cp) (solid) (J/kg/k) | 1200 |
| Particle void fraction (internal) | 0.3 | Thermal conductivity (solid) (W/m K) | 1.0 |
| Density (solid) (kg/m3) | 2500 | Operating pressure (bar) | 20 |
| Gas velocity (m/s) | 0.5 | Inlet temperature (°C) | 1100, 1000, 900 |
| Stream | Stage Time (s) | Inlet Velocity (m/s) | Inlet Temperature (°C) | Composition (Mole Fraction) | |
|---|---|---|---|---|---|
| Compressed air to oxidation stage | 900 | 0.4 | 420 | O2 | 0.21 |
| N2 | 0.79 | ||||
| PSA off-gas fuel to reduction stage | 600 | 0.2 | 350 | H2 | 0.150 |
| CO | 0.150 | ||||
| CH4 | 0.175 | ||||
| H2O | 0.025 | ||||
| CO2 | 0.500 | ||||
| Methane and steam to the reforming stage | 900 | 0.25 | 200 | CH4 | 0.333 |
| H2O | 0.667 | ||||
| Parameter | Value |
|---|---|
| Bed length | 10 m |
| Active content (fully reduced) | 20% Ni |
| Oxygen carrier density | 2500 kg/m3 |
| Reactor void fraction | 0.4 |
| Particle void fraction | 0.3 |
| Particle tortuosity | 3 |
| Particle diameter | 5 mm |
| Reactor outlet pressure | 18 bar |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singhal, A.; Cloete, S.; Quinta-Ferreira, R.; Amini, S. Multiscale Modeling of a Packed Bed Chemical Looping Reforming (PBCLR) Reactor. Energies 2017, 10, 2056. https://doi.org/10.3390/en10122056
Singhal A, Cloete S, Quinta-Ferreira R, Amini S. Multiscale Modeling of a Packed Bed Chemical Looping Reforming (PBCLR) Reactor. Energies. 2017; 10(12):2056. https://doi.org/10.3390/en10122056
Chicago/Turabian StyleSinghal, Arpit, Schalk Cloete, Rosa Quinta-Ferreira, and Shahriar Amini. 2017. "Multiscale Modeling of a Packed Bed Chemical Looping Reforming (PBCLR) Reactor" Energies 10, no. 12: 2056. https://doi.org/10.3390/en10122056




