Effects of Biogas Substrate Recirculation on Methane Yield and Efficiency of a Liquid-Manure-Based Biogas Plant
Abstract
:1. Introduction
2. Material and Methods
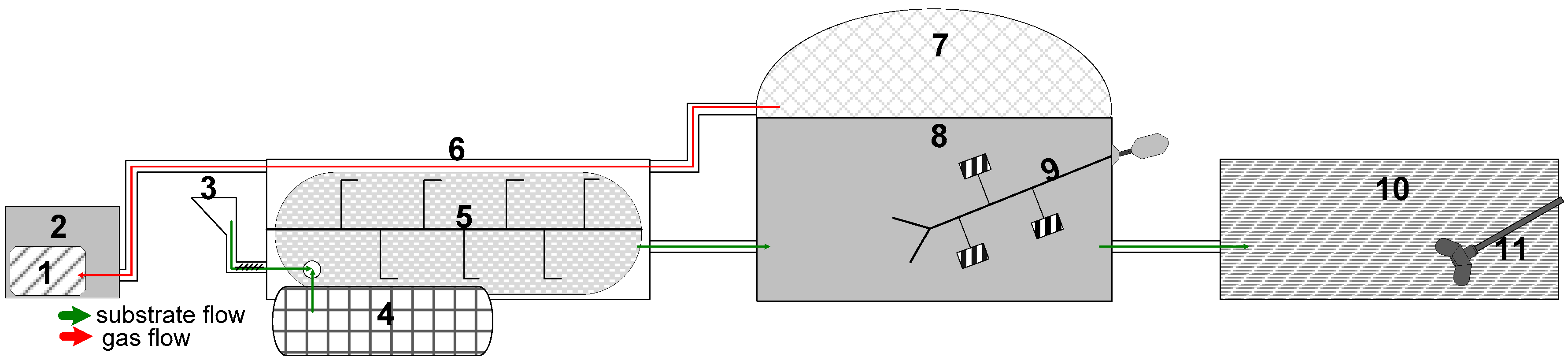
2.1. Recirculation of the Biogas Substrate
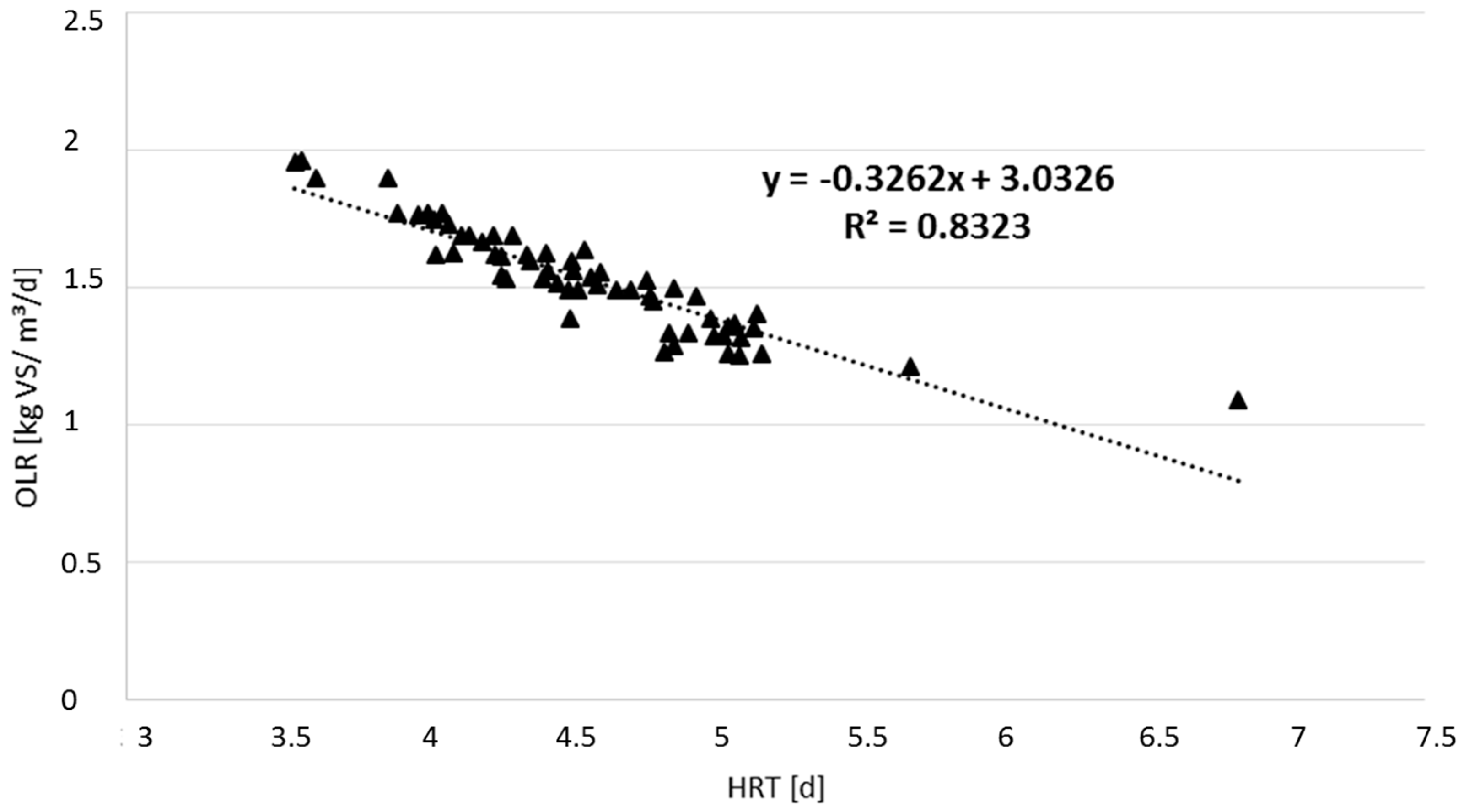
2.2. Calculation of Organic Loading Rate And Hydraulic Retention Time
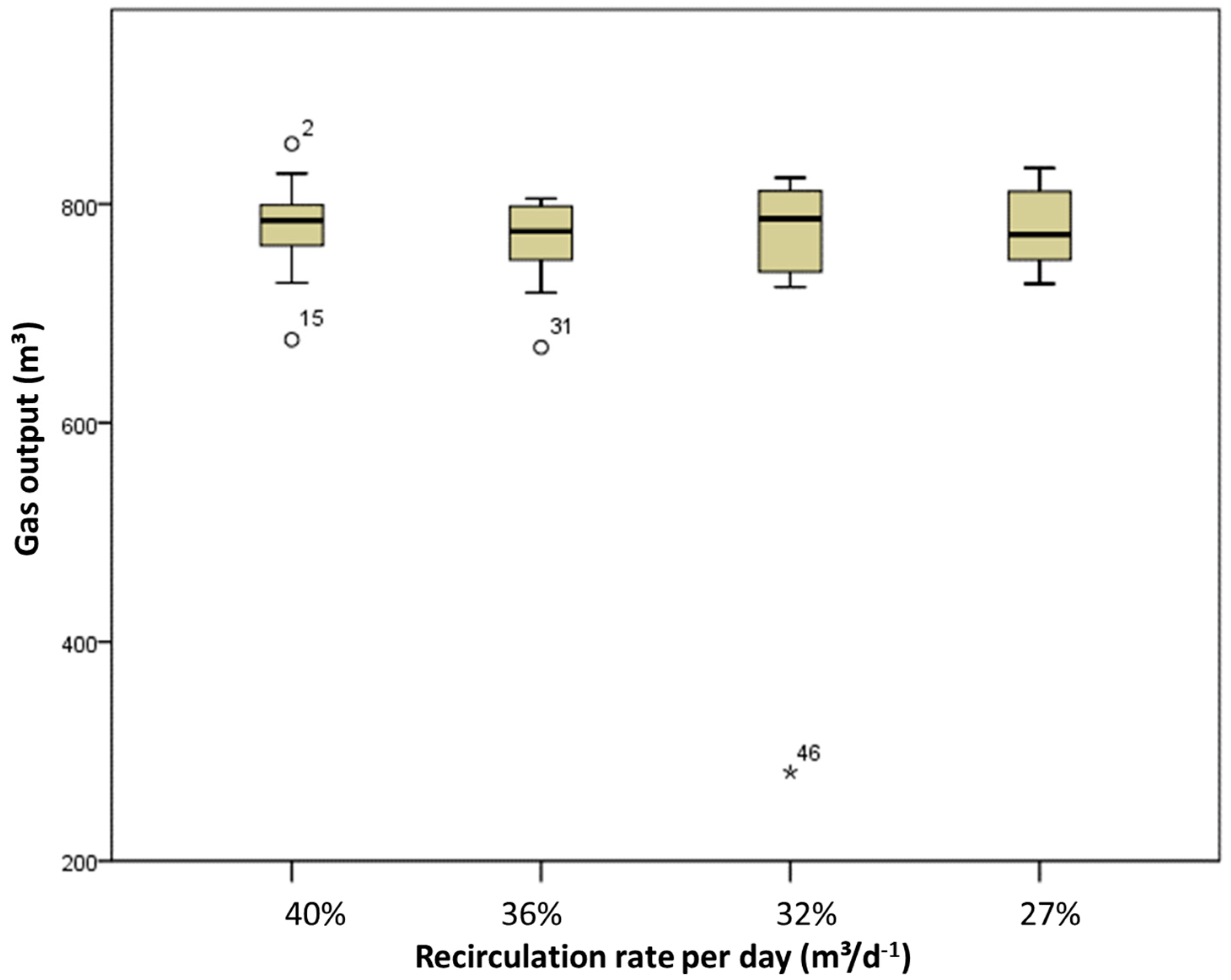
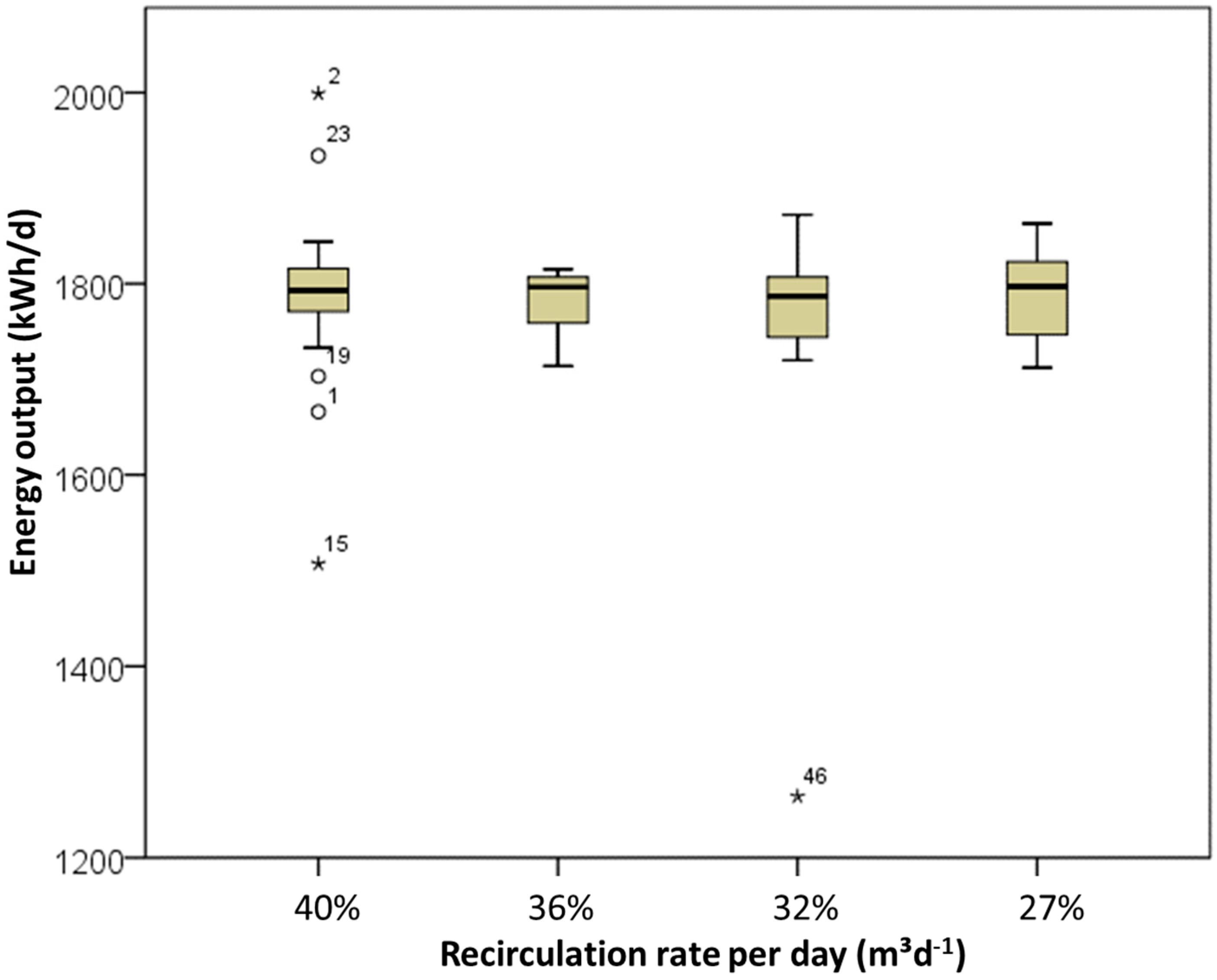
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| c | concentration of organic matter (volatile solids) (% VS) |
| HRT | hydraulic retention time |
| LU | livestock unit (500 kg body weight) |
| substrate added per unit of time (kg/d) | |
| oDM | organic dry matter |
| OLR | organic loading rate |
| RA | recirculation amount |
| volume of substrate added daily | |
| VR | reactor volume m3 |
| VS | volatile solids or organic dry matter |
References
- Asam, Z.; Poulsen, T.G.; Nizami, A.; Rafique, R.; Kiely, G.; Murphy, J.D. How can we improve biomethane production per unit of feedstock in biogas plants? Appl. Energy 2011, 88, 2013–2018. [Google Scholar] [CrossRef]
- Chynoweth, D.P.; Owens, J.M.; Legrand, R. Renewable methane from anaerobic digestion of biomass. Renew. Energy 2001, 22, 1–8. [Google Scholar] [CrossRef]
- Esposito, G.; Frunzo, L.; Panico, A.; Pirozzi, F. Enhanced bio-methane production from co-digestion of different organic wastes. Environ. Technol. 2012, 33, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Buhr, H.O.; Andrews, J.F. The thermophilic anaerobic digestion process. Water Res. 1977, 11, 129–143. [Google Scholar] [CrossRef]
- Ahring, B.K. Methanogenesis in thermophilic biogas reactors. Antonie Van Leeuwenhoek 1995, 67, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Pap, B.; Györkei, Á.; Boboescu, I.Z.; Nagy, I.K.; Bíró, T.; Kondorosi, É.; Maróti, G. Temperature-dependent transformation of biogas-producing microbial communities points to the increased importance of hydrogenotrophic methanogenesis under thermophilic operation. Bioresour. Technol. 2015, 177, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Ahring, B.K. Strategies for the anaerobic digestion of the organic fraction of municipal solid waste: An overview. Water Sci. Technol. 2006, 53, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.; D’Antonio, G.; Esposito, G.; Frunzo, L.; Iodice, P.; Pirozzi, F. The effect of substrate-bulk interaction on hydrolysis modelling in anaerobic digestion process. Sustainability 2014, 6, 8348–8363. [Google Scholar] [CrossRef]
- Yadvika, S.; Santosh, T.R.; Sreekrishnan, T.R.; Kohli, S.; Rana, V. Enhancement of biogas production from solid substrates using different techniques—A review. Bioresour. Technol. 2004, 95, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.; Zeeman, G.; Gijzen, H.; Lettinga, G. Solids removal in upflow anaerobic reactors, a review. Bioresour. Technol. 2003, 90, 1–9. [Google Scholar] [CrossRef]
- Michele, P.; Giuliana, D.; Carlo, M.; Sergio, S.; Fabrizio, A. Optimization of solid state anaerobic digestion of the OFMSW by digestate recirculation: A new approach. Waste Manag. 2015, 35, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, Å.; Jarvis, A.; Stenberg, B.; Mathisen, B.; Svensson, B.H. Anaerobic digestion of alfalfa silage with recirculation of process liquid. Bioresour. Technol. 2007, 98, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Bories, A.; Raynal, J.; Bazile, F. Anaerobic digestion of high-strength distillery wastewater (cane molasses stillage) in a fixed-film reactor. Biol. Wastes 1988, 23, 251–267. [Google Scholar] [CrossRef]
- Rincón, B.; Borja, R.; González, J.M.; Portillo, M.C.; Sáiz-Jiménez, C. Influence of organic loading rate and hydraulic retention time on the performance, stability and microbial communities of one-stage anaerobic digestion of two-phase olive mill solid residue. Biochem. Eng. J. 2008, 40, 253–261. [Google Scholar] [CrossRef]
- Munk, B.; Bauer, C.; Gronauer, A.; Lebuhn, M. Population dynamics of methanogens during acidification of biogas fermenters fed with maize silage. Eng. Life Sci. 2010, 10, 496–508. [Google Scholar] [CrossRef]
- Estevez, M.M.; Sapci, Z.; Linjordet, R.; Schnürer, A.; Morken, J. Semi-continuous anaerobic co-digestion of cow manure and steam-exploded Salix with recirculation of liquid digestate. J. Environ. Manag. 2014, 136, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Wu, S.; Zhang, W.; Dong, R. Performance of two-stage vegetable waste anaerobic digestion depending on varying recirculation rates. Bioresour. Technol. 2014, 162, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.; Nordberg, Å.; Mathisen, B.; Svensson, B.H. Stimulation of conversion rates and bacterial activity in a silage-fed two-phase biogas process by initiating liquid recirculation. Antonie Van Leeuwenhoek 1995, 68, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Baader, W.; Braunschweig, F.R. Performance of completely filled vertical through-flow anaerobic digester. In Alternative Sources of Energy for Agriculture; FFTC Book Series 28; FFTC: Taipei, Taiwan, 1985; pp. 51–65. [Google Scholar]
- Zuo, Z.; Wu, S.; Zhang, W.; Dong, R. Effects of organic loading rate and effluent recirculation on the performance of two-stage anaerobic digestion of vegetable waste. Bioresour. Technol. 2013, 146, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Aslanzadeh, S.; Rajendran, K.; Jeihanipour, A.; Taherzadeh, M. The effect of effluent recirculation in a semi-continuous two-stage anaerobic digestion system. Energies 2013, 6, 2966–2981. [Google Scholar] [CrossRef]
- British Standards Institution. EN 12176:1998: Characterization of Sludge. Determination of pH Value; 13.030.20; BSI: London, UK, 1998. [Google Scholar]
- British Standards Institution. EN 12880:2000: Characterization of Sludges. Determination of Dry Residue and Water Content; 13.030.20; BSI: London, UK, 2000. [Google Scholar]
- British Standards Institution. EN 12879:2000: Characterization of Sludges. Determination of the Loss of Ignition of Dry Mass; 13.030.20; BSI: London, UK, 2000. [Google Scholar]
- Al Seadi, T.; Rutz, D.; Prassl, H.; Köttner, M.; Finsterwalder, T.; Volk, S.; Janssen, R. Biogas Handbook; University of Southern Denmark: Esbjerg, Denmark, 2008. [Google Scholar]
- Hu, Y.; Shen, F.; Yuan, H.; Zou, D.; Pang, Y.; Liu, Y.; Zhu, B.; Chufo, W.A.; Jaffar, M.; Li, X. Influence of recirculation of liquid fraction of the digestate (LFD) on maize stover anaerobic digestion. Biosyst. Eng. 2014, 127, 189–196. [Google Scholar] [CrossRef]
- Chen, Y.; Rößler, B.; Zielonka, S.; Wonneberger, A.; Lemmer, A. Effects of organic loading rate on the performance of a pressurized anaerobic filter in two-phase anaerobic digestion. Energies 2014, 7, 736–750. [Google Scholar] [CrossRef]
- Babaee, A.; Shayegan, J. Effect of organic loading rates (OLR) on production of methane from anaerobic digestion of vegetables waste. In Proceedings of the World Renewable Energy Congress, Linköping, Sweden, 8–13 May 2011.
- Estevez, M.M.; Linjordet, R.; Morken, J. Organic loading rate effect on anaerobic digestion: Case study on co digestion of lignocellulosic pre-treated material with cow manure. In Proceedings of the International Conference of Agricultural Engineering, Valencia, Spain, 8–12 July 2012.
- Kuratorium für Technik und Bauwesen in der Landwirtschaft (KTBL). Faustzahlen Biogas: 3. Ausgabe, Tabellenwerk, Kuratorium für Technik und Bauwesen in der Landwirtschaft; Kuratorium für Technik und Bauwesen in der Landwirtschaft: Darmstadt, Germany, 2013. [Google Scholar]
- Gourdon, R.; Vermande, P. Effects of propionic acid concentration on anaerobic digestion of pig manure. Biomass 1987, 13, 1–12. [Google Scholar] [CrossRef]
- Zhang, T.; Mao, C.; Zhai, N.; Wang, X.; Yang, G. Influence of initial pH on thermophilic anaerobic co-digestion of swine manure and maize stalk. Waste Manag. 2015, 35, 119–126. [Google Scholar] [CrossRef] [PubMed]
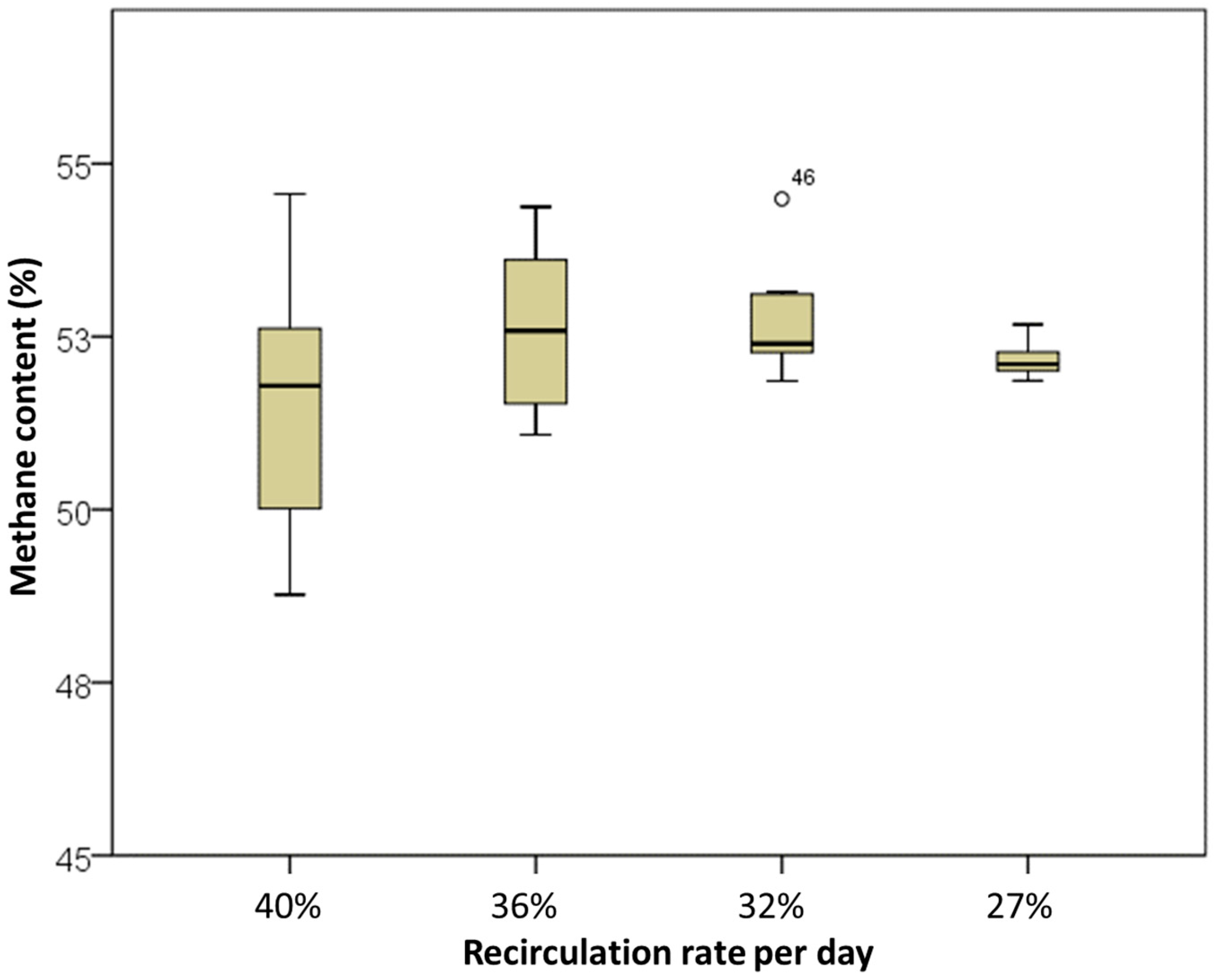
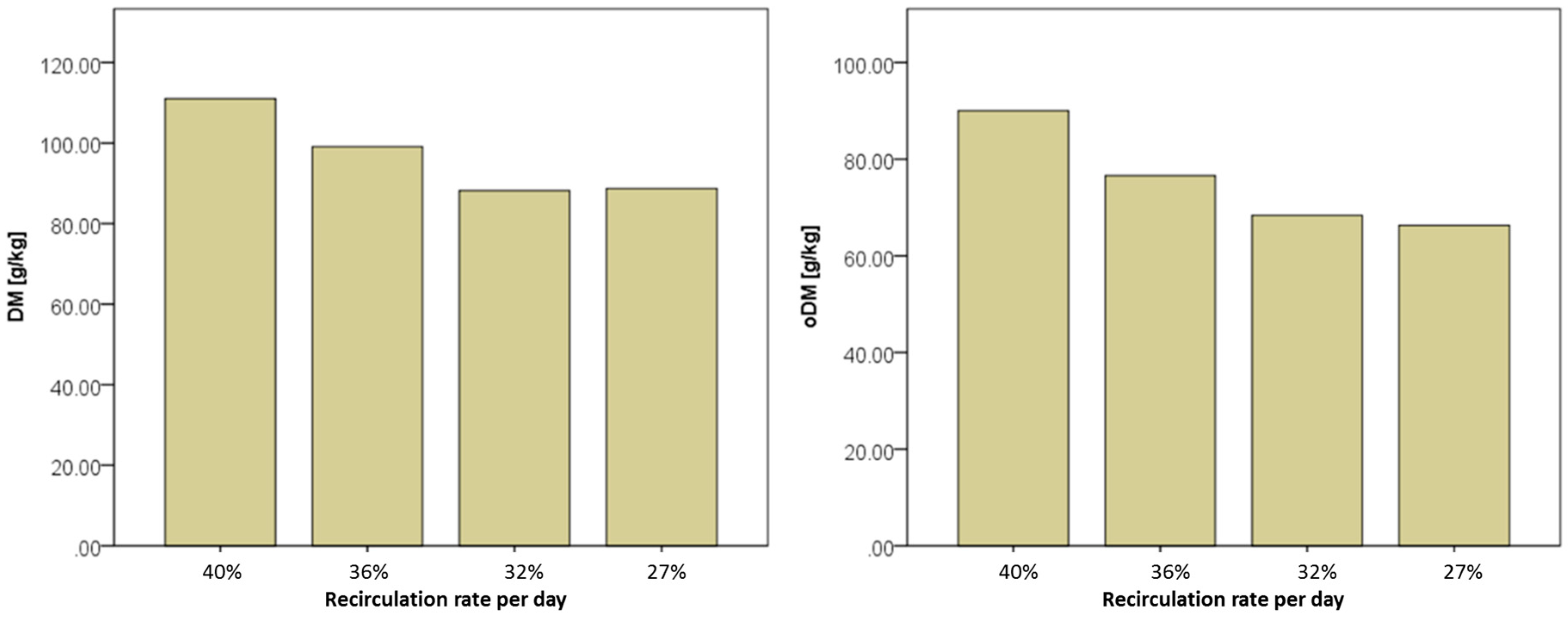
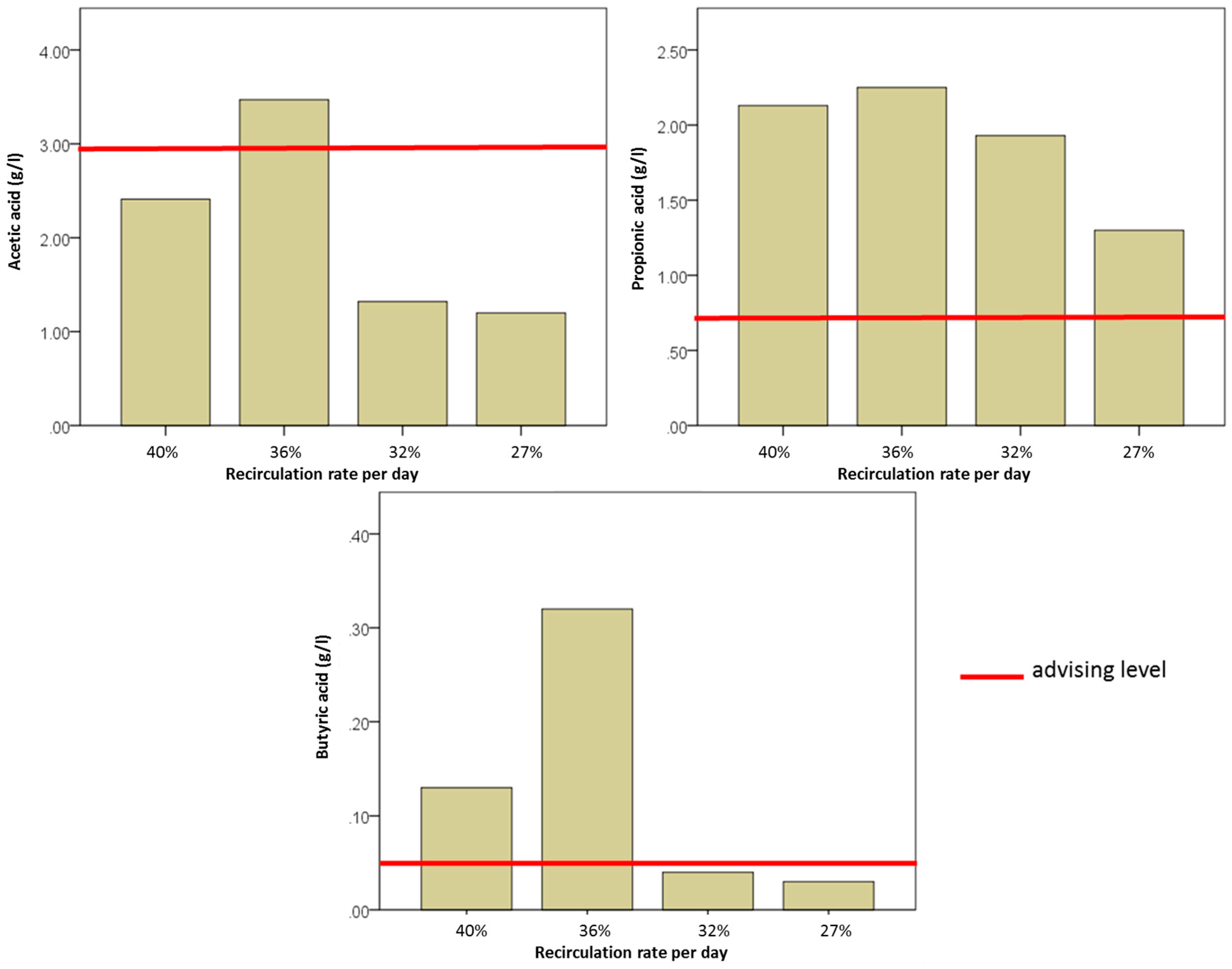

| RA (SR) m3/d | Percentage of Fermenter Volume (120 m3) | Fresh Substrate (SF) m3/d | Total Substrate Input (ST) m3/d | RR Ratio of SR to ST | |
|---|---|---|---|---|---|
| Theoretical | In Practice | ||||
| 10.0 | 8% | 15.0 | 25.0 | 24.8 | 40% |
| 8.5 | 7% | 15.0 | 23.5 | 23.7 | 36% |
| 7.0 | 6% | 15.0 | 22.0 | 22.3 | 32% |
| 5.5 | 5% | 15.0 | 20.5 | 20.6 | 27% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, F.P.C.; Maack, G.-C.; Buescher, W. Effects of Biogas Substrate Recirculation on Methane Yield and Efficiency of a Liquid-Manure-Based Biogas Plant. Energies 2017, 10, 325. https://doi.org/10.3390/en10030325
Müller FPC, Maack G-C, Buescher W. Effects of Biogas Substrate Recirculation on Methane Yield and Efficiency of a Liquid-Manure-Based Biogas Plant. Energies. 2017; 10(3):325. https://doi.org/10.3390/en10030325
Chicago/Turabian StyleMüller, Frauke P. C., Gerd-Christian Maack, and Wolfgang Buescher. 2017. "Effects of Biogas Substrate Recirculation on Methane Yield and Efficiency of a Liquid-Manure-Based Biogas Plant" Energies 10, no. 3: 325. https://doi.org/10.3390/en10030325







