Organosolv Fractionation of Softwood Biomass for Biofuel and Biorefinery Applications
Abstract
:1. Introduction
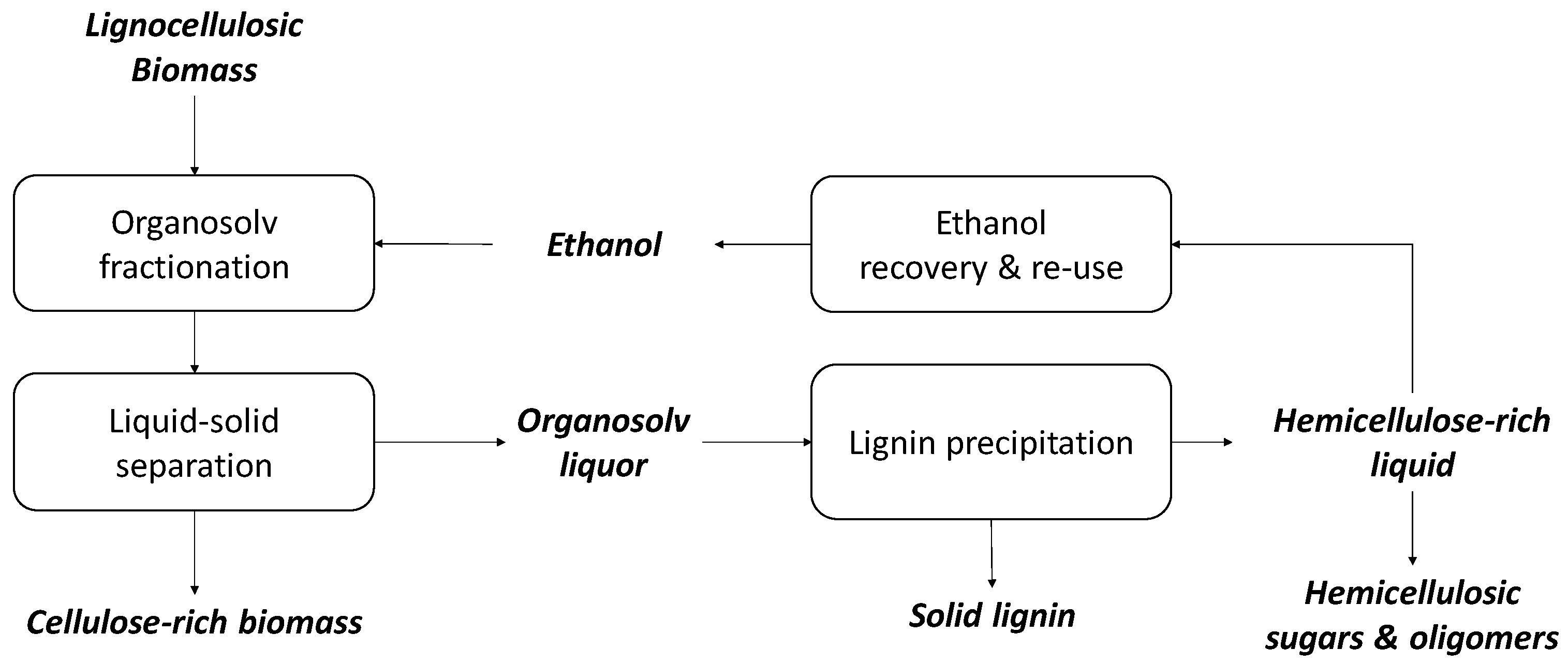
2. Organosolv Pulping
3. Organosolv as a Pretreatment and Fractionation Method
3.1. Uncatalyzed Organosolv Pretreatment
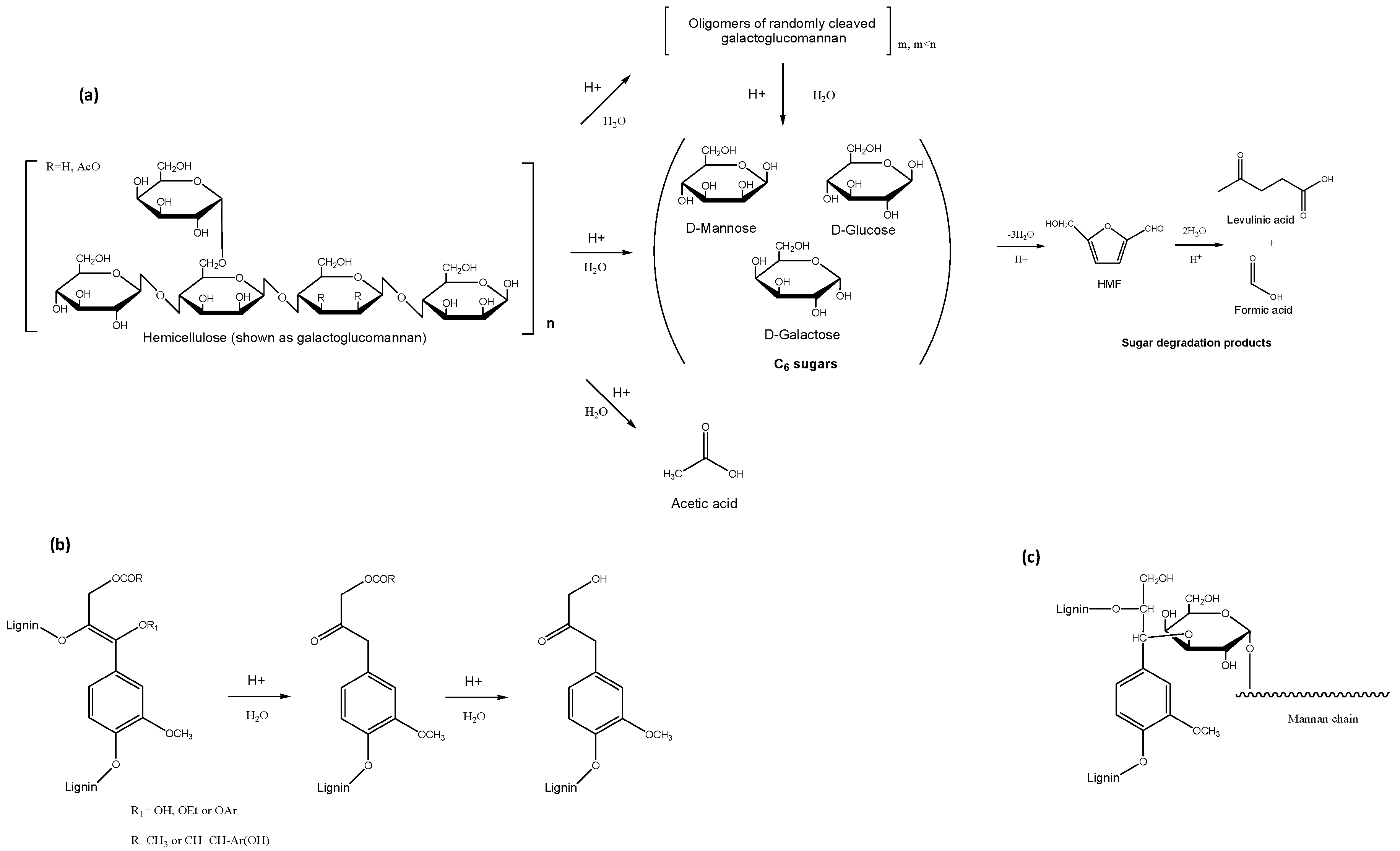
3.2. Acid Catalysed Organosolv Pretreatment
3.2.1. Organic Acids
3.2.2. Mineral Acids
4. The Role of Residual Lignin in the Enzymatic Hydrolysis of Organosolv Pretreated Biomass
5. Valorization of Organosolv Lignins into Chemicals and Materials
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Claassen, P.A.M.; van Lier, J.B.; Lopez Contreras, A.M.; van Niel, E.W.J.; Sijtsma, L.; Stams, A.J.M.; de Vries, S.S.; Weusthuis, R.A. Utilisation of biomass for the supply of energy carriers. Appl. Microbiol. Biotechnol. 1999, 52, 741–755. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. A review of the production of ethanol from softwood. Appl. Microbiol. Biotechnol. 2002, 59, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.O.; Moorebulls, M.R.; Barrier, J.W. An Evaluation of 2 Acid-Hydrolysis Processes for the Conversion of Cellulosic Feedstocks to Ethanol and Other Chemicals. Appl. Biochem. Biotechnol. 1990, 24–25, 773–783. [Google Scholar] [CrossRef]
- Heinonen, J.; Tamminen, A.; Uusitalo, J.; Sainio, T. Ethanol production from wood via concentrated acid hydrolysis, chromatographic separation, and fermentation. J. Chem. Technol Biotechnol. 2012, 87, 689–696. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial Cellulose Utilization: Fundamentals and Biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.D.; Steele-King, C.G.; McQueen-Mason, S.J. Sustainable liquid biofuels from biomass: The writing’s on the walls. New Phytol. 2008, 178, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Picataggio, S.K. Our Challenge is to Acquire Deeper Understanding of Biomass Recalcitrance and Conversion. In Biomass Recalcitrance; Blackwell Publishing Ltd.: Oxford, UK, 2009; p. 1. [Google Scholar]
- Johnson, D.K.; Elander, R.T. Pretreatments for Enhanced Digestibility of Feedstocks. In Biomass Recalcitrance; Blackwell Publishing Ltd.: Oxford, UK, 2009; p. 436. [Google Scholar]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Hodge, D.B.; Andersson, C.; Berglund, K.A.; Rova, U. Detoxification requirements for bioconversion of softwood dilute acid hydrolyzates to succinic acid. Enzym. Microb. Technol. 2009, 44, 309–316. [Google Scholar] [CrossRef]
- Conner, A.H.; Wood, B.F.; Hill, C.G.; Harris, J.F. Kinetic-Model for the Dilute Sulfuric-Acid Saccharification of Lignocellulose. J. Wood Chem. Technol. 1985, 5, 461–489. [Google Scholar] [CrossRef]
- Yoon, S.H.; Cullinan, H.T.; Krishnagopalan, G.A. Reductive modification of alkaline pulping of Southern Pine, integrated with hydrothermal pre-extraction of hemicelluloses. Ind. Eng. Chem. Res. 2010, 49, 5969–5976. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Choli-Papadopoulou, T.; Matis, K.A.; Triantafyllidis, K.S. Optimization of Hydrothermal Pretreatment of Hardwood and Softwood Lignocellulosic Residues for Selective Hemicellulose Recovery and Improved Cellulose Enzymatic Hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4529–4544. [Google Scholar] [CrossRef]
- Garrote, G.; Dominguez, H.; Parajo, J.C. Hydrothermal processing of lignocellulosic materials. Holz als Roh- und Werkstoff 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Brownell, H.H.; Yu, E.K.C.; Saddler, J.N. Steam-explosion pretreatment of wood: Effect of chip size, acid, moisture content and pressure drop. Biotechnol. Bioeng. 1986, 28, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Sassner, P.; Galbe, M.; Zacchi, G. Steam pretreatment of Salix with and without SO2 impregnation for production of bioethanol. Appl. Biochem. Biotechnol. 2005, 124, 1101–1117. [Google Scholar] [CrossRef]
- Sassner, P.; Galbe, M.; Zacchi, G. Techno-economic evaluation of bioethanol production from three different lignocellulosic materials. Biomass Bioenergy 2008, 32, 422–430. [Google Scholar] [CrossRef]
- Tengborg, C.; Stenberg, K.; Galbe, M.; Zacchi, G.; Larsson, S.; Palmqvist, E.; Hahn-Hagerdal, B. Comparison of SO2 and H2SO4 impregnation of softwood prior to steam pretreatment on ethanol production. Appl. Biochem. Biotechnol. 1998, 70–72, 3–15. [Google Scholar] [CrossRef]
- Ramos, L.P. The chemistry involved in the steam treatment of lignocellulosic materials. Quim. Nova 2003, 26, 863–871. [Google Scholar] [CrossRef]
- Ojeda, K.; Sanchez, E.; El-Halwagi, M.; Kafarov, V. Exergy analysis and process integration of bioethanol production from acid pre-treated biomass: Comparison of SHF, SSF and SSCF pathways. Chem. Eng. J. 2011, 176–177, 195–201. [Google Scholar] [CrossRef]
- Eriksson, G.; Kjellstrom, B.; Lundqvist, B.; Paulrud, S. Combustion of wood hydrolysis residue in a 150 kW powder burner. Fuel 2004, 83, 1635–1641. [Google Scholar] [CrossRef]
- Bozell, J.J. An evolution from pretreatment to fractionation will enable successful development of the integrated biorefinery. BioResources 2010, 5, 1326–1327. [Google Scholar]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Paszner, L.; Behera, N.C. Beating Behaviour and Sheet Strength Development of Coniferous Organosolv Fibers. Holzforschung 1985, 39, 51–61. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Ragauskas, A.J. Fundamentals of Biomass Pretreatment by Fractionation. In Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; John Wiley & Sons, Ltd.: Chichester, UK, 2013; p. 201. [Google Scholar]
- Farías-Sánchez, J.C.; López-Miranda, J.; Castro-Montoya, A.J.; Saucedo-Luna, J.; Carrillo-Parra, A.; López-Albarrán, P.; Pineda-Pimentel, M.G.; Rutiaga-Quiñones, J.G. Comparison of five pretreatments for the production of fermentable sugars obtained from Pinus pseudostrobusl L. Wood. EXCLI J. 2015, 14, 430–438. [Google Scholar] [PubMed]
- Muñoz, C.; Mendonça, R.; Baeza, J.; Berlin, A.; Saddler, J.; Freer, J. Bioethanol production from bio- organosolv pulps of Pinus radiata and Acacia dealbata. J. Chem. Technol. Biotechnol. 2007, 82, 767–774. [Google Scholar] [CrossRef]
- Hideno, A.; Kawashima, A.; Fukuoka, M.; Endo, T.; Honda, K.; Morita, M. Effect of alcohol-based organosolv treatment combined with short-time ball milling on the enzymatic hydrolysis of Japanese cypress (Chamaecyparis obtusa). Wood Sci. Technol. 2013, 47, 381–393. [Google Scholar] [CrossRef]
- Agnihotri, S.; Johnsen, I.A.; Bøe, M.S.; Øyaas, K.; Moe, S. Ethanol organosolv pretreatment of softwood (Picea abies) and sugarcane bagasse for biofuel and biorefinery applications. Wood Sci. Technol. 2015, 49, 881–896. [Google Scholar] [CrossRef]
- Mabee, W.E.; Gregg, D.J.; Arato, C.; Berlin, A.; Bura, R.; Gilkes, N.; Pan, X.; Pye, E.K.; Saddler, J.N. Updates on softwood-to-ethanol process development. Appl. Biochem. Biotechnol. 2006, 129, 55–70. [Google Scholar] [CrossRef]
- Pan, X.; Xie, D.; Yu, R.W.; Lam, D.; Saddler, J.N. Pretreatment of lodgepole pine killed by mountain pine beetle using the ethanol organosolv process: Fractionation and process optimization. Ind. Eng. Chem. Res. 2007, 46, 2609–2617. [Google Scholar] [CrossRef]
- Pan, X.; Xie, D.; Yu, R.W.; Saddler, J.N. The bioconversion of mountain pine beetle-killed lodgepole pine to fuel ethanol using the organosolv process. Biotechnol. Bioeng. 2008, 101, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, P.; Miller, S.J.; Ragauskas, A.J. Effects of organosolv pretreatment and enzymatic hydrolysis on cellulose structure and crystallinity in Loblolly pine. Carbohydr. Res. 2010, 345, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Aaltonen, O.; Ylinen, P. Organosolv pulping—Methods and pulp properties. Biomass 1987, 13, 45–65. [Google Scholar] [CrossRef]
- Worster, H.E. The present and future of alkali pulping. Pulp Paper Can 1974, 75, 345. [Google Scholar]
- Treiber, E. Zukunftsentwicklung der Zellstoffherstellung und damit zusammenhängende Forschungsaufgaben. Lenztnger Ber. 1976, 42, 26. [Google Scholar]
- Kleinert, T.N. Short Note. Ethanol-Water Deglinification of Sizeable Pieces of Wood. Disintegration into Stringlike Fiber Bundles. Holzforschung 1975. [Google Scholar] [CrossRef]
- McGee, J.K.; April, G.C. Chemicals from renewable resources: Hemicellulose behavior during organosolv delignification of southern yellow pine. Chem. Eng. Commun. 1982, 19, 49–56. [Google Scholar] [CrossRef]
- Lange, W.; Schweers, W.; Beinhoff, O. Über Eigenschaften und Abbaubarkeit von mit Alkohol-Wasser-Gemischen isolierten Ligninen—5. Mitteilung: Orientierende Untersuchungen über Ausbeuten und Eigenschaften von unter verschiedenen Aufschlußbedingungen mit und ohne Katalysatorzusatz isolierten Ligninen. Holzforschung 1981, 35, 119–124. [Google Scholar]
- Nguyen, Q.; Tucker, M.; Keller, F.; Beaty, D.; Connors, K.; Eddy, F. Dilute acid hydrolysis of softwoods. Appl. Biochem. Biotechnol. 1999, 77, 133–142. [Google Scholar] [CrossRef]
- Uraki, Y.; Sano, Y. Polyhydric Alcohol Pulping at Atmospheric Pressure: An Effective Method for Organosolv Pulping of Softwoods. Holzforschung 1999, 53. [Google Scholar] [CrossRef]
- Abramovitch Rudolph, A.; Iyanar, K. Organosolv Pulping Using a Microwave Oven. Holzforschung 1994, 48, 349–354. [Google Scholar] [CrossRef]
- Green, J.; Sanyer, N. Alkaline pulping in aqueous alcohols and amines. Tappi 1982, 65, 133–136. [Google Scholar]
- El Hage, R.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Effects of process severity on the chemical structure of Miscanthus ethanol organosolv lignin. Polym. Degrad. Stab. 2010, 95, 997–1003. [Google Scholar] [CrossRef]
- Paszner, L. Topochemistry of Softwood Delignification by Alkali Earth Metal Salt Catalysed Organosolv Pulping. Holzforschung 1989, 43, 159–168. [Google Scholar] [CrossRef]
- Mittal, A.; Chatterjee, S.G.; Scott, G.M.; Amidon, T.E. Modeling xylan solubilization during autohydrolysis of sugar maple wood meal: Reaction kinetics. Holzforschung 2009, 63, 307. [Google Scholar] [CrossRef]
- Garrote, G.; Dominguez, H.; Parajo, J.C. Kinetic modelling of corncob autohydrolysis. Process Biochem. 2001, 36, 571–578. [Google Scholar] [CrossRef]
- Conner, A.H. Kinetic Modeling of Hardwood Prehydrolysis I. Xylan Removal by Water Prehydrolysis. Wood Fiber Sci. 1984, 16, 268. [Google Scholar]
- Conner, A.H.; Lorenz, L.F. Kinetic modeling of hardwood prehydrolysis. Part III. Water and dilute acetic acid prehydrolysis of southern red oak. Wood Fiber Sci. 1986, 18, 248. [Google Scholar]
- Mašura, M. Prehydrolysis of beechwood. Wood Sci. Technol. 1987, 21, 89–100. [Google Scholar]
- Lesar, B.; Humar, M.; Hora, G.; Hachmeister, P.; Schmiedl, D.; Pindel, E.; Siika-aho, M.; Liitia, T. Utilization of recycled wood in biorefineries: Preliminary results of steam explosion and ethanol/water organosolv pulping without a catalyst. Eur. J. Wood Wood Prod. 2016, 74, 711–723. [Google Scholar] [CrossRef]
- Xu, C.; Liao, B.; Shi, W. Organosolv Pretreatment of Pine Sawdust for Bio-ethanol Production. In Green Energy and Technology; Springer: Berlin/Heidelberg, Germany, 2013; p. 435. [Google Scholar]
- Fissore, A.; Carrasco, L.; Reyes, P.; Rodríguez, J.; Freer, J.; Mendonça, R.T. Evaluation of a combined brown rot decay-chemical delignification process as a pretreatment for bioethanol production from Pinus radiata wood chips. J. Ind. Microbiol. Biotechnol. 2010, 37, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Arato, C.; Gilkes, N.; Gregg, D.; Mabee, W.; Pye, K.; Xiao, Z.; Zhang, X.; Saddler, J. Biorefining of softwoods using ethanol organosolv pulping: Preliminary evaluation of process streams for manufacture of fuel-grade ethanol and co-products. Biotechnol. Bioeng. 2005, 90, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Berlin, A.; Muñoz, C.; Gilkes, N.; Alamouti, S.M.; Chung, P.; Kang, K.-Y.Y.; Maximenko, V.; Baeza, J.; Freer, J.; Mendonça, R.; et al. An evaluation of British Columbian beetle-killed hybrid spruce for bioethanol production. Appl. Biochem. Biotechnol. 2007, 137–140, 267–280. [Google Scholar] [PubMed]
- Mirmohamadsadeghi, S.; Karimi, K.; Zamani, A.; Amiri, H.; Horváth, I.S. Enhanced solid-state biogas production from lignocellulosic biomass by organosolv pretreatment. BioMed Res. Int. 2014, 2014, 350414. [Google Scholar] [CrossRef] [PubMed]
- Nitsos, C.; Stoklosa, R.; Karnaouri, A.; Vörös, D.; Lange, H.; Hodge, D.; Crestin, C.; Rova, U.; Christakopoulos, P. Isolation and Characterization of Organosolv and Alkaline Lignins from Hardwood and Softwood Biomass. ACS Sustain. Chem. Eng. 2016, 4, 5181–5193. [Google Scholar] [CrossRef]
- Matsakas, L.; Nitsos, C.; Vörös, D.; Rova, U.; Christakopoulos, P. High-Titer Methane from Organosolv-Pretreated Spruce and Birch. Energies 2017, 10, 263. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K. Improvement of acetone, butanol, and ethanol production from woody biomass using organosolv pretreatment. Bioprocess Biosyst. Eng. 2015, 38, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Nitsos, C.K.; Matis, K.A.; Triantafyllidis, K.S. Optimization of Hydrothermal Pretreatment of Lignocellulosic Biomass in the Bioethanol Production Process. ChemSusChem 2013, 6, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, Y.; Lee, S.H.; Endo, T. Cost reduction and feedstock diversity for sulfuric acid-free ethanol cooking of lignocellulosic biomass as a pretreatment to enzymatic saccharification. Bioresour. Technol. 2009, 100, 4783–4789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arato, C.; Pye, E.K.; Gjennestad, G. The Lignol Approach to Biorefining of Woody Biomass to Produce Ethanol and Chemicals. In Twenty-Sixth Symposium on Biotechnology for Fuels and Chemicals; Davison, B.H., Evans, B.R., Finkelstein, M., McMillan, J.D., Eds.; Humana Press: Totowa, NJ, USA, 2005; p. 871. [Google Scholar]
- Guragain, Y.N.; Bastola, K.P.; Madl, R.L.; Vadlani, P.V. Novel Biomass Pretreatment Using Alkaline Organic Solvents: A Green Approach for Biomass Fractionation and 2, 3-Butanediol Production. Bioenergy Res. 2016, 9, 643–655. [Google Scholar] [CrossRef]
- Gregg, D.J.; Boussaid, A.; Saddler, J.N. Techno-economic evaluations of a generic wood-to-ethanol process: Effect of increased cellulose yields and enzyme recycle. Bioresour. Technol. 1998, 63, 7–12. [Google Scholar] [CrossRef]
- Wingren, A.; Galbe, M.; Zacchi, G. Techno-Economic Evaluation of Producing Ethanol from Softwood: Comparison of SSF and SHF and Identification of Bottlenecks. Biotechnol. Prog. 2003, 19, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-J.; Sano, Y. Atmospheric Acetic Acid Pulping of Rice Straw IV: Physico-Chemical Characterization of Acetic Acid Lignins from Rice Straw and Woods. Part 2. Chemical Structures. Holzforschung 1999. [Google Scholar] [CrossRef]
- Tu, M.; Zhang, X.; Paice, M.; MacFarlane, P.; Saddler, J.N. The potential of enzyme recycling during the hydrolysis of a mixed softwood feedstock. Bioresour. Technol. 2009, 100, 6407–6415. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, L.F.; Chandra, R.P.; Saddler, J.N. Fibre size does not appear to influence the ease of enzymatic hydrolysis of organosolv-pretreated softwoods. Bioresour. Technol. 2012, 107, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Saddler, J.N. Cellulose accessibility limits the effectiveness of minimum cellulase loading on the efficient hydrolysis of pretreated lignocellulosic substrates. Biotechnol. Biofuels 2011, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, L.F.; Chandra, R.P.; Saddler, J.N. The effect of varying organosolv pretreatment chemicals on the physicochemical properties and cellulolytic hydrolysis of mountain pine beetle-killed lodgepole pine. Appl. Biochem. Biotechnol. 2010, 161, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, T.; Karita, S.; Araki, Y.; Watanabe, S.; Oyadomari, M.; Takada, R.; Tanaka, F.; Abe, K.; Watanabe, T.; Honda, Y.; et al. Analysis of exposed cellulose surfaces in pretreated wood biomass using carbohydrate-binding module (CBM)–cyan fluorescent protein (CFP). Biotechnol. Bioeng. 2010, 105, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Nakagame, S.; Chandra, R.P.; Saddler, J.N. The effect of isolated lignins, obtained from a range of pretreated lignocellulosic substrates, on enzymatic hydrolysis. Biotechnol. Bioeng. 2010, 105, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Nakagame, S.; Chandra, R.P.; Kadla, J.F.; Saddler, J.N. Enhancing the enzymatic hydrolysis of lignocellulosic biomass by increasing the carboxylic acid content of the associated lignin. Biotechnol. Bioeng. 2011, 108, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, L.F.; Chandra, R.P.; Saddler, J.N. The effects of increasing swelling and anionic charges on the enzymatic hydrolysis of organosolv-pretreated softwoods at low enzyme loadings. Biotechnol. Bioeng. 2011, 108, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Tu, M.B.; Shi, Z.Q.; Zheng, K.; Olmos, L.G.; Yu, S.Y. Contrasting effects of hardwood and softwood organosolv lignins on enzymatic hydrolysis of lignocellulose. Bioresour. Technol. 2014, 163, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Pan, X. Correlation between lignin physicochemical properties and inhibition to enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2016, 113, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, J.Y.; Fu, S.Y. Effects of Lignin−Metal Complexation on Enzymatic Hydrolysis of Cellulose. J. Agric. Food Chem. 2010, 58, 7233–7238. [Google Scholar] [CrossRef] [PubMed]
- Kurabi, A.; Berlin, A.; Gilkes, N.; Kilburn, D.; Bura, R.; Robinson, J.; Markov, A.; Skomarovsky, A.; Gusakov, A.; Okunev, O.; et al. Enzymatic hydrolysis of steam-exploded and ethanol organosolv-pretreated douglas-fir by novel and commercial fungal cellulases. Appl. Biochem. Biotechnol. 2005, 121, 219–230. [Google Scholar] [CrossRef]
- Berlin, A.; Gilkes, N.; Kilburn, D.; Bura, R.; Markov, A.; Skomarovsky, A.; Okunev, O.; Maximenko, V.; Gregg, D.; Sinitsyn, A.; et al. Evaluation of novel fungal cellulase preparations for ability to hydrolyze softwood substrates—Evidence for the role of accessory enzymes. Enzym. Microb. Technol. 2005, 37, 175–184. [Google Scholar] [CrossRef]
- Glasser, W.G.; Davé, V.; Frazier, C.E. Molecular Weight Distribution of (Semi-) Commercial Lignin Derivatives. J. Wood Chem. Technol. 1993, 13, 545–559. [Google Scholar] [CrossRef]
- Gordobil, O.; Moriana, R.; Zhang, L.; Labidi, J.; Sevastyanova, O. Assesment of technical lignins for uses in biofuels and biomaterials: Structure-related properties, proximate analysis and chemical modification. Ind. Crops Prod. 2016, 83, 155–165. [Google Scholar] [CrossRef]
- Yang, X.; Li, N.; Lin, X.; Pan, X.; Zhou, Y. Selective Cleavage of the Aryl Ether Bonds in Lignin for Depolymerization by Acidic Lithium Bromide Molten Salt Hydrate under Mild Conditions. J. Agric. Food Chem. 2016, 64, 8379–8387. [Google Scholar] [CrossRef] [PubMed]
- Ropponen, J.; Räsänen, L.; Rovio, S.; Ohra-aho, T.; Liitiä, T.; Mikkonen, H.; van de Pas, D.; Tamminen, T. Solvent extraction as a means of preparing homogeneous lignin fractions. Holzforschung 2011, 543. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Fiţigău, F.I.; Gosselink, R.J.A.; Frissen, A.E.; Stoutjesdijk, J.; Peter, F. Fractionation of five technical lignins by selective extraction in green solvents and characterisation of isolated fractions. Ind. Crops Prod. 2014, 62, 481–490. [Google Scholar] [CrossRef]
- Prinsen, P.; Narani, A.; Hartog, A.F.; Wever, R.; Rothenberg, G. Dissolving Lignin in Water through Enzymatic Sulfation with Aryl Sulfotransferase. ChemSusChem 2017, 10, 2267–2273. [Google Scholar] [CrossRef] [PubMed]
- Oasmaa, A.; Alén, R.; Meier, D. Catalytic hydrotreatment of some technical lignins. Bioresour. Technol. 1993, 45, 189–194. [Google Scholar] [CrossRef]
- Hu, J.; Shen, D.; Wu, S.; Zhang, H.; Xiao, R. Composition Analysis of Organosolv Lignin and Its Catalytic Solvolysis in Supercritical Alcohol. Energy Fuels 2014, 28, 4260–4266. [Google Scholar] [CrossRef]
- Løhre, C.; Kleinert, M.; Barth, T. Organosolv extraction of softwood combined with lignin-to-liquid-solvolysis as a semi-continuous percolation reactor. Biomass Bioenergy 2017, 99, 147–155. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, B.; Qi, Z.; Li, C.; Ji, J.; Dai, T.; Wang, P.A.; Zhang, P.T. Valorization of Lignin to Simple Phenolic Compounds over Tungsten Carbide: Impact of Lignin Structure. ChemSusChem 2017, 10, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.R.; Schuchardt, U. Oxidation of organosolv lignins in acetic acid. Appl. Biochem. Biotechnol. 1999, 77, 127–132. [Google Scholar] [CrossRef]
- Harmita, H.; Karthikeyan, K.G.; Pan, X. Copper and cadmium sorption onto kraft and organosolv lignins. Bioresour. Technol. 2009, 100, 6183–6191. [Google Scholar] [CrossRef] [PubMed]
- Podschun, J.; Stücker, A.; Saake, B.; Lehnen, R. Structure–Function Relationships in the Phenolation of Lignins from Different Sources. ACS Sustain. Chem. Eng. 2015, 3, 2526–2532. [Google Scholar] [CrossRef]
- Gordobil, O.; Egüés, I.; Labidi, J. Modification of Eucalyptus and Spruce organosolv lignins with fatty acids to use as filler in PLA. React. Funct. Polym. 2016, 104, 45–52. [Google Scholar] [CrossRef]
- Kühnel, I.; Saake, B.; Lehnen, R. Comparison of different cyclic organic carbonates in the oxyalkylation of various types of lignin. React. Funct. Polym. 2017, 120, 83–91. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Evtuguin, D.V.; Costa, L.C.; Graca, M.P.F.; Fernandes, A.J.S.; Correia, M.R.P.; Gomes, M.T.S.R.; Oilveria, J.A.B.P. Potentiometric chemical sensors from lignin-poly(propylene oxide) copolymers doped by carbon nanotubes. Analyst 2013, 138, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Evtuguin, D.V.; Andreolety, J.P.; Gandini, A. Polyurethanes based on oxygen-organosolv lignin. Eur. Polym. J. 1998, 34, 1163–1169. [Google Scholar] [CrossRef]
- Poursorkhabi, V.; Mohanty, A.; Misra, M. Characterization of electrospun lignin-based carbon fibers. AIP Conf. Proc. 2015, 1664, 150003. [Google Scholar]
| Type of Softwood | Glucan | Hemicellulose | Galactan | Mannan | Xylan | Arabinan | Lignin | Extractives | Ash | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 42.98 | 23.55 | n.a. | n.a. | n.a. | n.a. | 28.94 | 5.11 | 0.19 | [29] |
| B | 49.5 | 24.1 | 2.9 | 12.30 | 7.60 | 1.30 | 25.60 | 2.96 | 1.7 | [30] |
| C | 44.9 | 14.9 | n.a. | 10.50 | 4.40 | n.a. | 33.00 | n.a. | n.a. | [31] |
| D | 38.8 | n.a. | 17.2 a GGM | n.a. | 5.0 | n.a. | 26.4 | 0.4 | 0.3 | [32] |
| E | 43.00 | 23.00 | 2.00 | 13.00 | 3.00 | 1.00 | 28.00 | n.a. | n.a. | [33] |
| F | 45.42 | 21.37 | 2.00 | 11.78 | 6.34 | 1.25 | 25.08 | 4.66 | 0.26 | [34] |
| G | 45.01 | 23.56 | 2.60 | 12.06 | 7.37 | 1.53 | 28.05 | 4.98 | 0.25 | [35] |
| H | 42.0 | 21.6 | 2.6 | 9.6 | 7.8 | 1.6 | 29.9 | n.a. | n.a. | [36] |
| Biomass | T (°C) | T (min) | Ethanol (%) | Catalyst | Organosolv Solids Content (wt. %) | Hydrolysis Yield %/Time h | Bioethanol Yield %/Time h | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucan | Hemicellulose | Lignin | ||||||||
| Uncatalyzed | ||||||||||
| L | 220 | 180 | 35 | - | - | - | - | 71.4/48 | - | [54] |
| A | 80 | 90 | 60 | - | - | - | - | 20/72 | - | [29] |
| K | 190 | 240 | 50 | - | - | - | - | 24/72 | - | [55] |
| B | 200 | 60 | 60 | - | 92.1 | 5.79 | 6.1 | 100/72 | 65/18 | [30] |
| B | 200 | 60 | 60 | - | 82.4 | 4.7 | 7.0 | 70/24 | 95/96 | [56] |
| Acid-catalyzed | ||||||||||
| E | 200 | 60 | 25 | 1% a | - | - | - | 100/48 | - | |
| H | 170 | 60 | 65 | 1.10% b | 33.3 | 0.5 | 11.6 | 70/80 | - | [28] |
| D | 217.5 | 90 | 63 | 0.05 mol L−1 c | 38.7 | 3.6 d | 11.8 | 100/48 | - | [32] |
| E | 188 | 15 | 50 | pH~2.4 b | 90 | - | - | >90/24 | >90/8 | [33] |
| M | n.a | n.a | n.a | H2SO4 | 89.73 | - | 6.4 | 100/24 | 90/8 | [57] |
| G | 187 | 60 | 65 | 1.10% b | 91.30 | 1.16 | 11.16 | 100/12 | - | [35] |
| F | 187 | 60 | 65 | 1.10% b | 87.86 | - | 16.27 | 100/12 | - | [35] |
| J | 185 | 36 | 50 | 1.2% | 68.15 | 6.42 | 26.43 | >90%/72 | 80/24 | [58] |
| N | 150 | 30 | 75 | 1.0% b | 51.3 | 20.2 | 27.8 | - | 71.4 e | [59] |
| D | 182 | 60 | 60 | 1.0% b | 69.07 | 1.15 | 25.03 | 69.1/48 | 245.5 f | [60,61] |
| N | 180 | 60 | 75 | 1.0% b | 68.1 | 12.12 | 21.1 | 17.3/72 | 87.8 g/72 | [62] |
| Biomass | T (°C) | T (min) | Ethanol (%) | Catalyst | Sugars | Ash | Mw | Mn | PDI | Phenolic OH/Methoxy (mmol·g−1) | Aliphatic OH (mmol·g−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (w/w) | ||||||||||||
| G | 187 | 60 | 65 | 1.10% a | n.a. | n.a. | 2240 | 1150 | 1.94 | 4.29 | 3.77 | [35] |
| F | 187 | 60 | 65 | 1.10% a | n.a. | n.a. | 3050 | 1330 | 2.29 | 4.00 | 3.45 | [35] |
| H | 170 | 60 | 75 | 1.0% a | 1.63 | n.a. | n.a. | n.a. | n.a. | 2.81/7.96 | 4.55 | [78] |
| G | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 3288 | 1381 | 2.38 | 3.86 | 3.64 | [79] |
| G b | 170 | 50 | 60 | 1.0% a | 1.2 | - | 8875 | 1661 | 5.35 | n.a. | n.a. | [76] |
| G c | 170 | 50 | 60 | 1.0% a | 6.3 | 1.6 | n.a. | n.a. | n.a. | n.a. | n.a. | [76] |
| G | 170 | 60 | 65 | 1.1% | 1.9 | 1.4 | 3500 | 1300 | 2.6 | 2.8 | n.a. | [85] |
| O | 180 | 60 | 50 | 1.20% a | 0.5 | 3.2 | 3081 | 1065 | 2.89 | 2.99 | 0.75 | [84] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitsos, C.; Rova, U.; Christakopoulos, P. Organosolv Fractionation of Softwood Biomass for Biofuel and Biorefinery Applications. Energies 2018, 11, 50. https://doi.org/10.3390/en11010050
Nitsos C, Rova U, Christakopoulos P. Organosolv Fractionation of Softwood Biomass for Biofuel and Biorefinery Applications. Energies. 2018; 11(1):50. https://doi.org/10.3390/en11010050
Chicago/Turabian StyleNitsos, Christos, Ulrika Rova, and Paul Christakopoulos. 2018. "Organosolv Fractionation of Softwood Biomass for Biofuel and Biorefinery Applications" Energies 11, no. 1: 50. https://doi.org/10.3390/en11010050
APA StyleNitsos, C., Rova, U., & Christakopoulos, P. (2018). Organosolv Fractionation of Softwood Biomass for Biofuel and Biorefinery Applications. Energies, 11(1), 50. https://doi.org/10.3390/en11010050






