Cracks Formation in Lithium-Rich Cathode Materials for Lithium-Ion Batteries during the Electrochemical Process
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Preparation
2.2. Characterization
2.3. Electrochemical Measurements
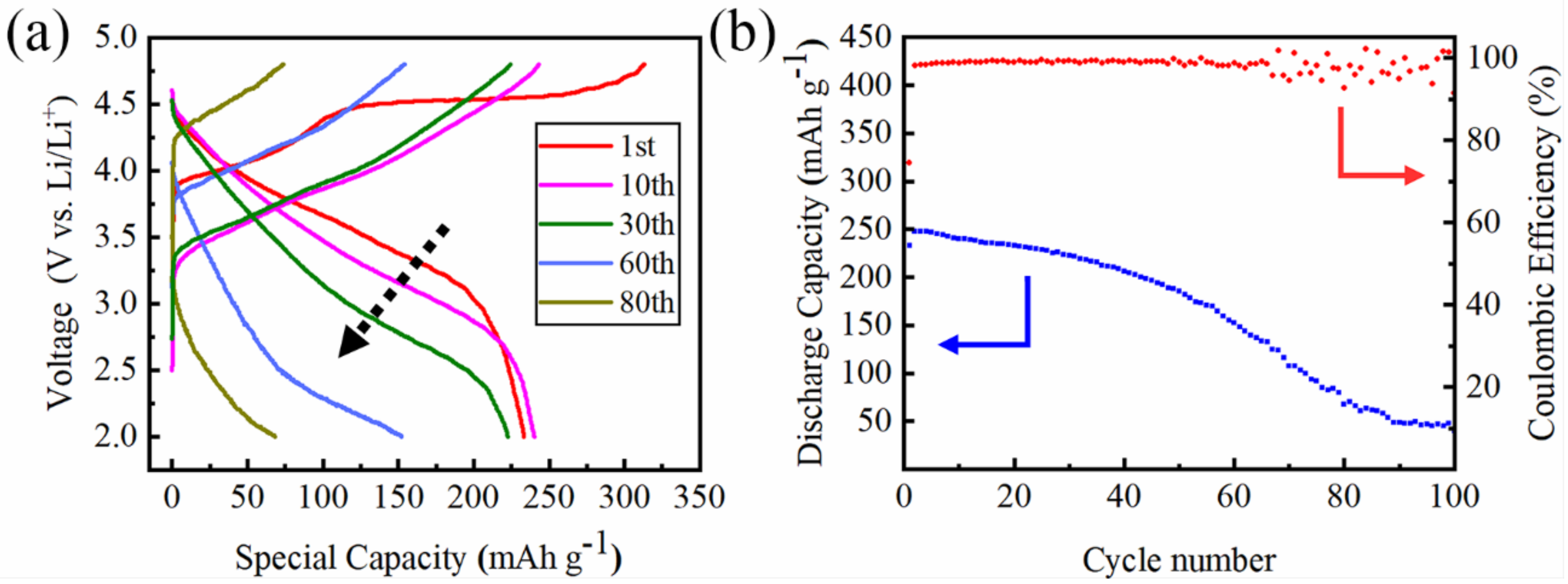
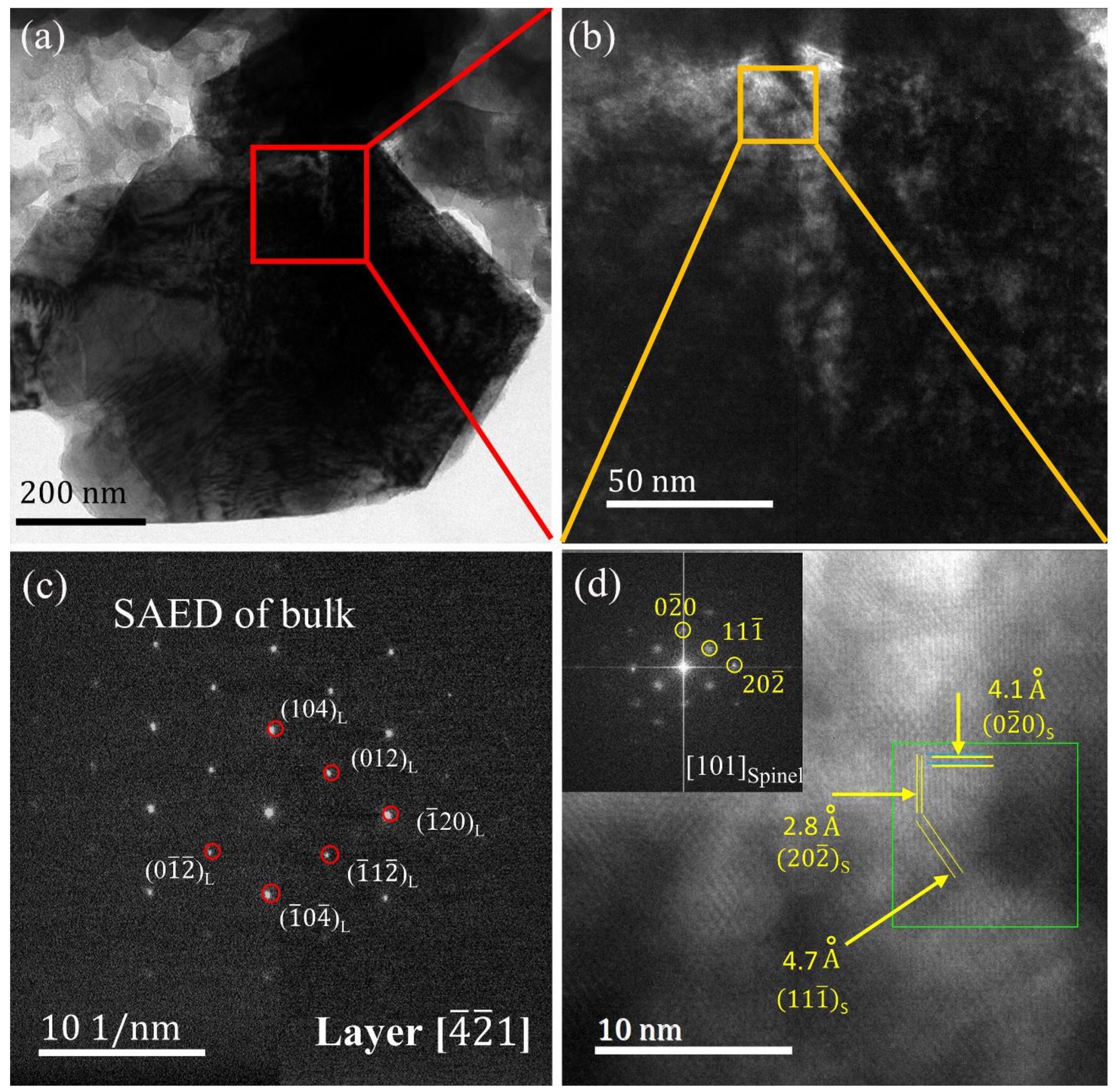
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclatures
| Li-rich | lithium-rich |
| SEM | scanning electron microscope |
| TEM | transmission electron microscopy |
| mA·g−1 | milliampere per gram |
| mA·h·g−1 | milliampere hour per gram |
| V | voltage |
| XRD | X-ray diffraction |
| STEM | scanning transmission electron microscopy |
| 3D | three dimensional |
| mmol | milimolar |
| FE-SEM | field-emission scanning electron microscopy |
| EDS | energy dispersive spectrometer |
| HR-TEM | high-resolution transmission electron microscopy |
| PVDF | polyvinylidene |
| NMP | N-methyl-2-pyrrolidone |
| M | molar per liter |
| EC | ethylene carbonate |
| DEC | dimethyl carbonate |
| C-rate | a measure of the rate at which a battery is charged or discharged relative to its capacity |
| CC | constant current |
| Li+ | lithium ion |
| EDX | energy-dispersive X-ray |
| 2θ | measuring the angle of scattering angle in XRD |
| TM | transition metal |
| Rm | of a kind of space group, group number 166 |
| I(003) | the intensity of (003) peak in XRD pattern |
| SAED | selected area electron diffraction |
| FFT | fast Fourier transform |
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. Engl. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Ultimate Limits to Intercalation Reactions for Lithium Batteries. Chem. Rev. 2014, 114, 11414–11443. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-M. Building a Better Battery. Science 2010, 330, 1485–1486. [Google Scholar] [CrossRef] [PubMed]
- Koksbang, R.; Barker, J.; Shi, H.; Saidi, M.Y. Cathode materials for lithium rocking chair batteries. Solid State Ion. 1996, 84, 1–21. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Li, W.; Song, B.; Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 2017, 46, 3006–3059. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Song, B.; Li, W. A perspective on nickel-rich layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 2017, 6, 125–139. [Google Scholar] [CrossRef]
- Kalluri, S.; Yoon, M.; Jo, M.; Park, S.; Myeong, S.; Kim, J.; Dou, S.X.; Guo, Z.; Cho, J. Surface Engineering Strategies of Layered LiCoO2 Cathode Material to Realize High-Energy and High-Voltage Li-Ion Cells. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Tang, D.; Sun, Y.; Yang, Z.; Ben, L.; Gu, L.; Huang, X. Surface structure evolution of LiMn2O4 cathode material upon charge/discharge. Chem. Mater. 2014, 26, 3535–3543. [Google Scholar] [CrossRef]
- Ma, J.; Hu, P.; Cui, G.; Chen, L. Surface and Interface Issues in Spinel LiNi0.5Mn1.5O4: Insights into a Potential Cathode Material for High Energy Density Lithium Ion Batteries. Chem. Mater. 2016, 28, 3578–3606. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Zheng, J.; Myeong, S.; Cho, W.; Yan, P.; Xiao, J.; Wang, C.; Cho, J.; Zhang, J.-G. Li- and Mn-Rich Cathode Materials: Challenges to Commercialization. Adv. Energy Mater. 2017, 7, 1601284. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.-H.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112–3125. [Google Scholar] [CrossRef]
- Koga, H.; Croguennec, L.; Menetrier, M.; Douhil, K.; Belin, S.; Bourgeois, L.; Suard, E.; Weill, F.; Delmas, C. Reversible Oxygen Participation to the Redox Processes Revealed for Li1.20Mn0.54Co0.13Ni0.13O2. J. Electrochem. Soc. 2013, 160, A786–A792. [Google Scholar] [CrossRef]
- Hong, J.; Lim, H.-D.; Lee, M.; Kim, S.-W.; Kim, H.; Oh, S.-T.; Chung, G.-C.; Kang, K. Critical Role of Oxygen Evolved from Layered Li-Excess Metal Oxides in Lithium Rechargeable Batteries. Chem. Mater. 2012, 24, 2692–2697. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.; Li, B. Recent progress in Li-rich layered oxides as cathode materials for Li-ion batteries. RSC Adv. 2014, 4, 63268–63284. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, H. Initial Coulombic efficiency improvement of the Li1.2Mn0.567Ni0.166Co0.067O2 lithium-rich material by ruthenium substitution for manganese. J. Mater. Chem. 2012, 22, 15507–15510. [Google Scholar] [CrossRef]
- Zang, Y.; Ding, C.-X.; Wang, X.-C.; Wen, Z.-Y.; Chen, C.-H. Molybdenum-doped lithium-rich layered-structured cathode material Li1.2Ni0.2Mn0.6O2 with high specific capacity and improved rate performance. Electrochim. Acta 2015, 168, 234–239. [Google Scholar] [CrossRef]
- Zang, Y.; Sun, X.; Tang, Z.-F.; Xiang, H.-F.; Chen, C.-H. Vanadium-doped lithium-rich layered-structured cathode material Li1.2Ni0.2Mn0.6O2 with a high specific capacity and improved rate performance. RSC Adv. 2016, 6, 30194–30198. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, E.; Yang, F.; Corbett, J.; Sun, Z.; Lyu, Y.; Yu, X.; Liu, Y.; Yang, X.-Q.; Li, H. Structural integrity-Searching the key factor to suppress the voltage fade of Li-rich layered cathode materials through 3D X-ray imaging and spectroscopy techniques. Nano Energy 2016, 28, 164–171. [Google Scholar] [CrossRef]
- Lyu, Y.; Hu, E.; Xiao, D.; Wang, Y.; Yu, X.; Xu, G.; Ehrlich, S.N.; Amine, K.; Gu, L.; Yang, X.-Q.; et al. Correlations between Transition-Metal Chemistry, Local Structure, and Global Structure in Li2Ru0.5Mn0.5O3 Investigated in a Wide Voltage Window. Chem. Mater. 2017, 29, 9053–9065. [Google Scholar] [CrossRef]
- Su, N.; Lyu, Y.; Gu, R.; Guo, B. Al2O3 coated Li1.2Ni0.2Mn0.2Ru0.4O2 as cathode material for Li-ion batteries. J. Alloys Compd. 2018, 741, 398–403. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, P.; Gu, M.; Xiao, J.; Browning, N.D.; Yan, P.; Wang, C.; Zhang, J.-G. Structural and Chemical Evolution of Li- and Mn-Rich Layered Cathode Material. Chem. Mater. 2015, 27, 1381–1390. [Google Scholar] [CrossRef]
- Hu, E.; Lyu, Y.; Xin, H.L.; Liu, J.; Han, L.; Bak, S.-M.; Bai, J.; Yu, X.; Li, H.; Yang, X.-Q. Explore the Effects of Microstructural Defects on Voltage Fade of Li- and Mn-Rich Cathodes. Nano Lett. 2016, 16, 5999–6007. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Ramasse, Q.M.; Ophus, C.; Duncan, H.; Hage, F.; Chen, G. Unravelling structural ambiguities in lithium- and manganese-rich transition metal oxides. Nat. Commun. 2015, 6, 8711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, B.; Liu, Z.; Lai, M.O.; Lu, L. Structural evolution and the capacity fade mechanism upon long-term cycling in Li-rich cathode material. Phys. Chem. Chem. Phys. 2012, 14, 12875–12883. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Nie, A.; Zheng, J.; Zhou, Y.; Lu, D.; Zhang, X.; Xu, R.; Belharouak, I.; Zu, X.; Xiao, J.; et al. Evolution of lattice structure and chemical composition of the surface reconstruction layer in Li1.2Ni0.2Mn0.6O2 cathode material for lithium ion batteries. Nano Lett. 2015, 15, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Kalnaus, S.; Meisner, R.A.; Rhodes, K.J.; Li, J.; Payzant, E.A.; Wood, D.L., III; Daniel, C. Structural transformation of a lithium-rich Li1.2Co0.1Mn0.55Ni0.15O2 cathode during high voltage cycling resolved by in situ X-ray diffraction. J. Sources 2013, 229, 239–248. [Google Scholar] [CrossRef]
- Gu, M.; Belharouak, I.; Zheng, J.; Wu, H.; Xiao, J.; Genc, A.; Amine, K.; Thevuthasan, S.; Baer, D.R.; Zhang, J.-G.; et al. Formation of the Spinel Phase in the Layered Composite Cathode Used in Li-Ion Batteries. ACS Nano 2013, 7, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Gu, M.; Xiao, J.; Zuo, P.; Wang, C.; Zhang, J.-G. Corrosion/Fragmentation of Layered Composite Cathode and Related Capacity/Voltage Fading during Cycling Process. Nano Lett. 2013, 13, 3824–3830. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Li, D.; Sato, Y.; Arao, M.; Watanabe, M.; Hatano, M.; Horie, H.; Ohsawa, Y. Cyclic deterioration and its improvement for Li-rich layered cathode material Li[Ni0.17Li0.2Co0.07Mn0.56]O2. J. Power Sources 2010, 195, 567–573. [Google Scholar] [CrossRef]
- Kim, J.H.; Myung, S.T.; Sun, Y.K. Molten salt synthesis of LiNi0.5Mn1.5O4 spinel for 5 V class cathode material of Li-ion secondary battery. Electrochim. Acta 2004, 49, 219–227. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, X.; Zhao, T.; Li, L.; Xie, M.; Chen, R. Multifunctional AlPO4 Coating for Improving Electrochemical Properties of Low-Cost Li[Li0.2Fe0.1Ni0.15Mn0.55]O2 Cathode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 3773–3781. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Manthiram, A. Structural stability of chemically delithiated layered (1-z)Li[Li1/3Mn2/3] O2-zLi[Mn0.5−yNi0.5−yCo2y]O2 solid solution cathodes. J. Power Sources 2008, 183, 749–754. [Google Scholar] [CrossRef]
- Liu, J.; Reeja-Jayan, B.; Manthiram, A. Conductive Surface Modification with Aluminum of High Capacity Layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 Cathodes. J. Phys. Chem. C 2010, 114, 9528–9533. [Google Scholar] [CrossRef]
- Wang, C.-C.; Jarvis, K.A.; Ferreira, P.J.; Manthiram, A. Effect of Synthesis Conditions on the First Charge and Reversible Capacities of Lithium-Rich Layered Oxide Cathodes. Chem. Mater. 2013, 25, 3267–3275. [Google Scholar] [CrossRef]
- Kim, Y. Lithium Nickel Cobalt Manganese Oxide Synthesized Using Alkali Chloride Flux: Morphology and Performance as a Cathode Material for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2012, 4, 2329–2333. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Ma, J.; Jiang, N.; Xia, D. Effects of Particle Size on Voltage Fade for Li-Rich Mn-Based Layered Oxides. ACS Omega 2018, 3, 11136–11143. [Google Scholar] [CrossRef]
- Kuppan, S.; Shukla, A.K.; Membreno, D.; Nordlund, D.; Chen, G. Revealing Anisotropic Spinel Formation on Pristine Li- and Mn-Rich Layered Oxide Surface and Its Impact on Cathode Performance. Adv. Energy Mater. 2017, 7, 1602010. [Google Scholar] [CrossRef]
- Li, J.; Shunmugasundaram, R.; Doig, R.; Dahn, J.R. In Situ X-ray Diffraction Study of Layered Li-Ni-Mn-Co Oxides: Effect of Particle Size and Structural Stability of Core-Shell Materials. Chem. Mater. 2016, 28, 162–171. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Gu, M.; Xiao, J.; Zhang, J.-G.; Wang, C.-M. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.S.; Jun, D.-W.; Myung, S.-T.; Sun, Y.-K. Structural Stability of LiNiO2 Cycled above 4.2 V. ACS Energy Lett. 2017, 2, 1150–1155. [Google Scholar] [CrossRef]
- Yoon, C.S.; Ryu, H.-H.; Park, G.-T.; Kim, J.-H.; Kim, K.-H.; Sun, Y.-K. Extracting maximum capacity from Ni-rich Li[Ni0.95Co0.025Mn0.025]O2 cathodes for high-energy-density lithium-ion batteries. J. Mater. Chem. A 2018, 6, 4126–4132. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, T.; Ma, Z.; Gu, R.; Chen, R.; Lyu, Y.; Nie, A.; Guo, B. Cracks Formation in Lithium-Rich Cathode Materials for Lithium-Ion Batteries during the Electrochemical Process. Energies 2018, 11, 2712. https://doi.org/10.3390/en11102712
Cheng T, Ma Z, Gu R, Chen R, Lyu Y, Nie A, Guo B. Cracks Formation in Lithium-Rich Cathode Materials for Lithium-Ion Batteries during the Electrochemical Process. Energies. 2018; 11(10):2712. https://doi.org/10.3390/en11102712
Chicago/Turabian StyleCheng, Tao, Zhongtao Ma, Run Gu, Riming Chen, Yingchun Lyu, Anmin Nie, and Bingkun Guo. 2018. "Cracks Formation in Lithium-Rich Cathode Materials for Lithium-Ion Batteries during the Electrochemical Process" Energies 11, no. 10: 2712. https://doi.org/10.3390/en11102712




