Evaluating the Effect of Metal Bipolar Plate Coating on the Performance of Proton Exchange Membrane Fuel Cells
Abstract
:1. Introduction
- It must be light in weight;
- Does not melt and produce metallic ions;
- A high mechanical strength less than 200 Nm−2;
- High corrosion resistance with corrosion current at 0.1 V and H2 purge < 16 Acm−2;
- Cost-effective mass production: US$ 10 kW−1
- High corrosion resistance with corrosion current at 0.6 V and air purge < 16 Acm−2;
- Interfacial contact resistance (ICR) @140 Ncm−2 = 20 m cm2;
- The ohmic resistance must be low and steady all through the operation;
- The surface tension must be high with a water contact angle close to 90 °C (i.e., high dehydration).
1.1. Materials for Bipolar Plate
1.2. Graphite Bipolar Plate
1.3. Polymer Composite Bipolar Plate
1.4. Metallic Bipolar Plates
- It causes a rise in the contact resistance among the metallic bipolar plate and GDL.
- There will be an important change to the plate surface, hereby decreasing the area of contact with the GDL.
- The metal ions diffusion in the membrane and significant trapping of the metal ions in the catalyst sites will lead to ionic conductivity diminution and fuel cell malfunction.
2. Coating with Metal Nitrides
2.1. Coating with Metal Carbides
2.2. Coating with Metal Oxide
2.3. Carbon Based Coating (Graphene Carbon–Based Coating)
2.4. Amorphous Carbon-Based Coating
2.5. Graphite Carbon-Based Coating
2.6. Coating with Composites on Metallic Bipolar Plates
3. Coating of Open Porous Cellular Metal Foam Flow Channel
4. Impacts or Effects of Ions Contamination on PEMFC Performance
- It reduces proton conductivity, allows the reactant to overlap the membrane and increases ohmic resistance.
- Reduces both the mechanical and chemical balance of the membrane.
- Pattern of oxide layers with lofty resistivity.
- Poisons the catalyst.
- Prohibits oxygen transportation by reducing the hydrophobicity, thus resulting in performance degradation.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olabi, A.G. Hydrogen and Fuel Cell developments: An introduction to the special issue on The 8th International Conference on Sustainable Energy and Environmental Protection (SEEP 2015), 11–14 August 2015, Paisley, Scotland, UK. Int. J. Hydrogen Energy 2016, 41, 16323–16329. [Google Scholar] [CrossRef]
- Olabi, A.G. The 3rd international conference on sustainable energy and environmental protection SEEP 2009 the guest editor’s introduction. Energy 2010, 35, 4508–4509. [Google Scholar] [CrossRef]
- Olabi, A.G. Selahaddin Orhan Akansu, Nafiz Kahraman. Fuel cell and energy storage systems: A special issue section on “The 9th International Conference on Sustainable Energy and Environmental Protection (SEEP 2016), 22–25 September 2016, Kayseri, Turkey”. Int. J. Hydrogen Energy 2017, 42, 25544–25549. [Google Scholar] [CrossRef]
- Olabi, A.G. Renewable and energy storage system. Energy 2017, 136, 1–6. [Google Scholar] [CrossRef]
- Wilberforce, T.; El-Hassan, Z.; Khatib, F.N.; Al Makky, A.; Baroutaji, A.; Carton, J.G.; Thompson, J.; Olabi, A.G. Modelling and simulation of Proton Exchange Membrane fuel cell with serpentine bipolar plate using MATLAB. Int. J. Hydrogen Energy 2017, 25639–25662. [Google Scholar] [CrossRef]
- Planes, E.; Flandin, L.; Alberola, N. Polymer composites bipolar plates for PEMFCs. Energy Procedia 2012, 20, 311–323. [Google Scholar] [CrossRef]
- Wilberforce, T.; Khatib, F.N.; Al Makky, A.; Baroutaji, A.; Olabi, A.G. Characterisation of Proton Exchange Membrane Fuel Cell Through Design of Experiment (DOE). In Proceedings of the SEEP 2017, Bled, Slovenia, 27–30 June 2017. [Google Scholar] [CrossRef]
- Bai, C.; Ger, M.; Wu, M. Corrosion behaviors and contact resistances of the low-carbon steel bipolar plate with a chromized coating containing carbides and nitrides. Int. J. Hydrogen Energy 2009, 34, 6778–6789. [Google Scholar] [CrossRef]
- Wilberforce, T.; Al Makky, A.; Baroutaji, A.; Sambi, R.; Olabi, A.G. Optimization of bipolar plate through computational fluid dynamics simulation and modelling using nickle open pore cellular foam material. In Proceedings of the International Conference on Renewable Energies and Power Quality (ICREPQ’17), Malaga, Spain, 4–6 April 2017. [Google Scholar]
- Wilberforce, T.; Al Makky, A.; Baroutaji, A.; Sambi, R.; Olabi, A.G. Computational Fluid Dynamic Simulation and modelling (CFX) of Flow Plate in PEM fuel cell using Aluminum Open Pore Cellular Foam Material. In Proceedings of the IEEE Power and Energy Conference (TPEC), College Station, TX, USA, 9–10 February 2017. [Google Scholar] [CrossRef]
- Wilberforce, T.; Alaswad, A.; Palumbo, A.; Dassisti, M.; Olabi, A.G. Advances in stationary and portable fuel cell applications. Int. J. Hydrogen Energy 2016, 41, 16509–16522. [Google Scholar] [CrossRef]
- Fu, Y.; Lin, G.; Hou, M.; Wu, B.; Shao, Z.; Yi, B. Technical communication Carbon-based films coated 316L stainless steel as bipolar plate for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 405–409. [Google Scholar] [CrossRef]
- Feng, K.; Li, Z.; Sun, H.; Yu, L.; Cai, X.; Wu, Y.; Chu, P.K. Short communication C/CrN multilayer coating for polymer electrolyte membrane fuel cell metallic bipolar plates. J. Power Sources 2013, 222, 351–358. [Google Scholar] [CrossRef]
- Jung, H.; Huang, S.; Ganesan, P.; Popov, B.N. Performance of gold-coated titanium bipolar plates in unitized regenerative fuel cell operation. J. Power Sources 2009, 194, 972–975. [Google Scholar] [CrossRef]
- Lavigne, O.; Alemany-dumont, C.; Normand, B.; Berthon-fabry, S.; Metkemeijer, R. Thin chromium nitride PVD coatings on stainless steel for conductive component as bipolar plates of PEM fuel cells: Ex-situ and in-situ performances evaluation. HE 2012, 37, 10789–10797. [Google Scholar] [CrossRef]
- Derieth, T.; Bandlamudi, G.; Beckhaus, P.; Kreuz, C.; Mahlendorf, F.; Heinzel, A. Development of highly filled graphite compounds as bipolar plate materials for low and high temperature PEM fuel cells Development of Highly Filled Graphite Compounds as Bipolar Plate Materials for Low and High Temperature PEM Fuel Cells. J. New Mater. Electrochem. Syst. 2008, 11, 21–29. [Google Scholar]
- Cunningham, B.D.; Baird, D.G. Development of bipolar plates for fuel cells from graphite filled wet-lay material and a compatible thermoplastic laminate skin layer. J. Power Sources 2007, 168, 418–425. [Google Scholar] [CrossRef]
- Dihrab, S.S.; Sopian, K.; Alghoul, M.A.; Sulaiman, M.Y. Review of the membrane and bipolar plates materials for conventional and unitized regenerative fuel cells. Renew. Sustain. Energy Rev. 2009, 13, 1663–1668. [Google Scholar] [CrossRef]
- Huang, J.; Baird, D.G.; Mcgrath, J.E. Development of fuel cell bipolar plates from graphite filled wet-lay thermoplastic composite materials. J. Power Sources 2005, 150, 110–119. [Google Scholar] [CrossRef]
- Scholta, J. Investigations on novel low-cost graphite composite bipolar plates. J. Power Sources 1999, 84, 231–234. [Google Scholar] [CrossRef]
- Karimi, S.; Fraser, N.; Roberts, B.; Foulkes, F.R. A review of metallic bipolar plates for proton exchange membrane fuel cells: Materials and fabrication methods. Adv. Mater. Sci. Eng. 2012, 2012. [Google Scholar] [CrossRef]
- Gül, F.; San, B.; Isik-gulsac, I.; Okur, O. Analysis of the polymer composite bipolar plate properties on the performance of PEMFC (polymer electrolyte membrane fuel cells) by RSM (response surface methodology). Energy 2013, 55, 1067–1075. [Google Scholar] [CrossRef]
- Gül, F.; San, B.; Tekin, G. A review of thermoplastic composites for bipolar plate applications. Int. J. Energy Res. 2013, 283–309. [Google Scholar] [CrossRef]
- San, F.G.; Okur, O. The effect of compression molding parameters on the electrical and physical properties of polymer composite bipolar plates. Int. J. Hydrogen Energy 2017, 42. [Google Scholar] [CrossRef]
- Alaswad, A.; Benyounis, K.Y.; Olabi, A.G. Optimization Techniques in Material Processing. Engineering 2016. [Google Scholar] [CrossRef]
- Yeetsorn, R.; Fowler, M.; Tzoganakis, C.; Yuhua, W. Polypropylene Composites for Polymer Electrolyte Membrane Fuel Cell Bipolar Plates. Macromol. Symp. 2008, 34–43. [Google Scholar] [CrossRef]
- San, F.G.; Isik-Gulsac, I. Effect of surface wettability of polymer composite bipolar plates on polymer electrolyte membrane fuel cell performances. Int. J. Hydrogen Energy 2013, 38. [Google Scholar] [CrossRef]
- Eun, H.; Sik, Y.; Su, S. Feasibility study on carbon-felt-reinforced thermoplastic composite materials for PEMFC bipolar plates. Compos. Struct. 2017, 180, 378–385. [Google Scholar] [CrossRef]
- Adloo, A.; Sadeghi, M.; Masoomi, M.; Naja, H. High performance polymeric bipolar plate based on polypropylene/graphite/graphene/nano-carbon black composites for PEM fuel cells. Renew. Energy 2016, 99. [Google Scholar] [CrossRef]
- Heinzel, A.; Mahlendorf, F.; Niemzig, O.; Kreuz, C. Injection moulded low cost bipolar plates for PEM fuel cells. J. Power Sources 2004, 131, 35–40. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Zarmehri, E.; Sadeghi, M.; Mehrabani-zeinabad, A. Construction of Composite Polymer Bipolar Plate for Pem Fuel Cell. Iran. J. Energy Environ. 2013, 4, 357–360. [Google Scholar] [CrossRef]
- Sun, H.; Cooke, K.; Eitzinger, G.; Hamilton, P.; Pollet, B. Development of PVD coatings for PEMFC metallic bipolar plates. Thin Solid Films 2013, 528, 199–204. [Google Scholar] [CrossRef]
- Mathur, R.B.; Dhakate, S.R.; Gupta, D.K.; Dhami, T.L.; Aggarwal, R.K. Effect of different carbon fillers on the properties of graphite composite bipolar plate. J. Mater. Process. Technol. 2007, 3, 184–192. [Google Scholar] [CrossRef]
- Wilberforce, T.; El-Hassan, Z.; Khatib, F.N.; Al Makky, A.; Mooney, J.; Barouaji, A.; Carton, J.G.; Olabi, A.G. Development of Bi-polar plate design of PEM fuel cell using CFD techniques ScienceDirect Development of Bi-polar plate design of PEM fuel cell using CFD techniques. Int. J. Hydrogen Energy 2017. [Google Scholar] [CrossRef]
- Lee, T.; Santamaria, A.D.; Wan, J.; Yamazaki, K. Alloy selection and die design for stamped Proton Exchange Membrane Fuel Cell (PEMFC) bipolar plates. Procedia CIRP 2014, 14, 275–280. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.; Liang, X.; Luo, F.; Cheng, R.; Gong, F. Fabrication of metallic bipolar plate for proton exchange membrane fuel cells by using polymer powder medium based flexible forming. J. Mater. Process. Technol. 2018, 262, 32–40. [Google Scholar] [CrossRef]
- Ahmadi, F.; Elyasi, M.; Talebi, H. Evaluation of effective parameters on stamping of metallic bipolar plates. Procedia Eng. 2017, 183, 322–329. [Google Scholar] [CrossRef]
- Mehta, V.; Cooper, J.S. Review and analysis of PEM fuel cell design and manufacturing. J. Power Sources 2003, 114, 32–53. [Google Scholar] [CrossRef]
- Antunes, R.A.; Cristina, M.; Oliveira, L.; Ett, G.; Ett, V. Corrosion of metal bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2010, 35, 3632–3647. [Google Scholar] [CrossRef]
- Mahdavi, A.; Ranjbar, A.A.; Rahimi-esbo, M. Numerical simulation based design for an innovative PEMFC cooling flow field with metallic bipolar plates. Appl. Energy 2018, 228, 656–666. [Google Scholar] [CrossRef]
- Cooper, J.S. Design analysis of PEMFC bipolar plates considering stack manufacturing and environment impact. J. Power Sources 2004, 129, 152–169. [Google Scholar] [CrossRef]
- Hornung, R.; Kappelt, G. Bipolar plate materials development using Fe-based alloys for solid polymer fuel cells. J. Power Sources 1998, 72, 20–21. [Google Scholar] [CrossRef]
- Kumagai, M.; Myung, S.; Kuwata, S. Corrosion behavior of austenitic stainless steels as a function of pH for use as bipolar plates in polymer electrolyte membrane fuel cells. Electrochim. Acta 2008, 53, 4205–4212. [Google Scholar] [CrossRef]
- Hentall, P.L.; Lakeman, J.B.; Mepsted, G.O.; Moore, J.M.; Adcock, P.L. New materials for polymer electrolyte membrane fuel cell current collectors. J. Power Sources 1999, 80, 235–241. [Google Scholar] [CrossRef]
- Wang, H.; Sweikart, M.A.; Turner, J.A. Stainless steel as bipolar plate material for polymer electrolyte membrane fuel cells. J. Power Sources 2003, 115, 243–251. [Google Scholar] [CrossRef]
- Davies, D.P.; Adcock, P.L.; Turpin, M.; Rowen, S.J. Stainless steel as a bipolar plate material for solid polymer fuel cells. J. Power Sources 2000, 86, 237–242. [Google Scholar] [CrossRef]
- Davies, D.P.; Adcock, P.L.; Turpin, M.; Rowen, S.J. Bipolar plate materials for solid polymer fuel cells. J. Appl. Electrochem. 2000, 30, 101–105. [Google Scholar] [CrossRef]
- Wang, Y.; Northwood, D.O. An investigation into TiN-coated 316L stainless steel as a bipolar plate material for PEM fuel cells. J. Power Sources 2007, 165, 293–298. [Google Scholar] [CrossRef]
- Chung, C.; Chen, S.; Chiu, P.; Chang, M.; Hung, T.; Ko, T. Carbon film-coated 304 stainless steel as PEMFC bipolar plate. J. Power Sources 2008, 176, 276–281. [Google Scholar] [CrossRef]
- Lafront, A.; Ghali, E.; Morales, A.T. Corrosion behavior of two bipolar plate materials in simulated PEMFC environment by electrochemical noise technique. Electrochim. Acta 2007, 52, 5076–5085. [Google Scholar] [CrossRef]
- Dang, N.; Su, D.; Gu, J.; Ho, D. Corrosion protection of CrN/TiN multi-coating for bipolar plate of polymer electrolyte membrane fuel cell. Thin Solid Films 2011, 519, 6787–6791. [Google Scholar] [CrossRef]
- Wu, B.; Fu, Y.; Xu, J.; Lin, G.; Hou, M. Chromium nitride films on stainless steel as bipolar plate for proton exchange membrane fuel cell. J. Power Sources 2009, 194, 976–980. [Google Scholar] [CrossRef]
- Tian, R. Chromium nitride/Cr coated 316L stainless steel as bipolar plate for proton exchange membrane fuel cell. J. Power Sources 2011, 196, 1258–1263. [Google Scholar] [CrossRef]
- Cui, J.; Jing, B.; Xu, X.; Wang, L.; Cheng, F.; Li, S.; Wen, Z.; Ji, S.; Sun, J. Performance of niobium nitride-modified AISI316L stainless steel as bipolar plates for direct formic acid fuel cells. Int. J. Hydrog. Energy 2017, 42, 11830–11837. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, L.; Guo, L.; Wang, Z.; Zhao, J.; Tuan, W.; Niihara, K. TiN-coated titanium as the bipolar plate for PEMFC by multi-arc ion plating. Int. J. Hydrogen Energy 2011, 36, 9155–9161. [Google Scholar] [CrossRef]
- Li, M.; Luo, S.; Zeng, C. Corrosion behavior of TiN coated type 316 stainless steel in simulated PEMFC environments. Corros. Sci. 2004, 46, 1369–1380. [Google Scholar] [CrossRef]
- Wang, L.; Northwood, D.O.; Nie, X.; Housden, J.; Leyland, A.; Matthews, A. Corrosion properties and contact resistance of TiN, TiAlN and CrN coatings in simulated proton exchange membrane fuel cell environments. J. Power Sources 2010, 195, 3814–3821. [Google Scholar] [CrossRef]
- Larsson, M.; Hollman, P.; Hedenqvist, P.; Hogmark, S. Deposition and microstructure of PVD TiN-NbN multilayered coatings by combined reactive electron beam evaporation and DC sputtering. Surf. Coat. Technol. 1996, 86–87, 351–356. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Sun, J.; Lv, Y.; Li, S.; Ji, S.; Wen, Z. Niobium nitride modified AISI 304 stainless steel bipolar plate for proton exchange membrane fuel cell. J. Power Sources 2012, 199, 195–200. [Google Scholar] [CrossRef]
- Choe, C.; Choi, H.; Hong, W.; Lee, J. Tantalum nitride coated AISI 316L as bipolar plate for polymer electrolyte membrane fuel cell. Int. J. Hydrogen Energy 2011, 37, 405–411. [Google Scholar] [CrossRef]
- Soldera, F.; Gago, R. Electrochemical behavior of nanocrystalline Ta / TaN multilayer on 316L stainless steel: Novel bipolar plates for proton exchange membrane. J. Power Sources 2016, 322, 1–9. [Google Scholar] [CrossRef]
- Hung, Y.; Tawfik, H.; Mahajan, D. Durability and characterization studies of chromium carbide coated aluminum fuel cell stack. Int. J. Hydrog. Energy 2016, 41, 12273–12284. [Google Scholar] [CrossRef]
- Pileggi, R.; Tului, M.; Stocchi, D.; Lionetti, S. Surface & Coatings Technology Tribo-corrosion behaviour of chromium carbide based coatings deposited by HVOF. Surf. Coat. Technol. 2015, 268, 247–251. [Google Scholar] [CrossRef]
- Huang, N.B.; Yu, H.; Xu, L.S.; Zhan, S.; Sun, M.; Kirk, D.W. Corrosion kinetics of 316L stainless steel bipolar plate with chromiumcarbide coating in simulated PEMFC cathodic environment. Results Phys. 2016, 6, 730–736. [Google Scholar] [CrossRef]
- Ren, Y.J.; Zeng, C.L. Corrosion protection of 304 stainless steel bipolar plates using TiC films produced by high-energy micro-arc alloying process. J. Power Sources 2007, 171, 778–782. [Google Scholar] [CrossRef]
- Ravnikar, D.; Shanker, R.; Dahotre, N.B.; Grum, J. Electrochemical and DFT studies of laser-alloyed TiB2/TiC/Al coatings on aluminium alloy. Corros. Sci. 2018, 136, 18–27. [Google Scholar] [CrossRef]
- Soltani, R.; Sohi, M.H.; Ansari, M.; Haghighi, A.; Ghasemi, H.M.; Haftlang, F. Evaluation of niobium carbide coatings produced on AISI L2 steel via thermo-reactive diffusion technique. Vaccum 2017, 146, 44–51. [Google Scholar] [CrossRef]
- Orjuelag, A.; Rincón, R.; Jairo, J. Corrosion resistance of niobium carbide coatings produced on AISI 1045 steel via thermo-reactive diffusion deposition. Surf. Coat. Technol. 2014, 259, 667–675. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Xu, S.; Munroe, P.; Xie, Z. A nanocrystalline zirconium carbide coating as a functional corrosion-resistant barrier for polymer electrolyte membrane fuel cell application. J. Power Sources 2015, 297, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Turner, J.A. SnO2: F coated ferritic stainless steels for PEM fuel cell bipolar plates. J. Power Sources 2007, 170, 387–394. [Google Scholar] [CrossRef]
- Wang, H.; Turner, J.A.; Li, X.; Bhattacharya, R. SnO2: F coated austenite stainless steels for PEM fuel cell bipolar plates. J. Power Sources 2007, 171, 567–574. [Google Scholar] [CrossRef]
- Lv, J.; Tongxiang, L.; Chen, W. ScienceDirect The effects of molybdenum and reduced graphene oxide on corrosion resistance of amorphous nickel e phosphorus as bipolar plates in PEMFC environment. Int. J. Hydrogen Energy 2016, 41, 9738–9745. [Google Scholar] [CrossRef]
- Jang, H.; Kim, J.; Kang, H.; Bae, D.; Chang, H.; Choi, H. Applied Surface Science Reduced graphene oxide as a protection layer for Al. Appl. Surf. Sci. 2017, 407, 1–7. [Google Scholar] [CrossRef]
- Liu, J.; Hua, L.; Li, S.; Yu, M. Graphene dip coatings: An effective anticorrosion barrier on aluminum. Appl. Surf. Sci. 2015, 327, 241–245. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Schiller, T.; Medhekar, N.; Birbilis, N. Exploring graphene as a corrosion protection barrier. Corros. Sci. 2012, 56, 1–4. [Google Scholar] [CrossRef]
- Stoot, A.C.; Camilli, L.; Spiegelhauer, S.; Yu, F.; Bøggild, P. Multilayer graphene for long-term corrosion protection of stainless steel bipolar plates for polymer electrolyte membrane fuel cell. J. Power Sources 2015, 293, 846–851. [Google Scholar] [CrossRef]
- Mišković-Stanković, V.; Jevremović, I.; Jung, I.; Rhee, K.Y. Electrochemical study of corrosion behavior of graphene coatings on copper and aluminum in a chloride solution. Carbon 2014, 75, 335–344. [Google Scholar] [CrossRef]
- Mingge, W.; Congda, L.; Tao, H.; Guohai, C.; Donghui, W.; Haifeng, Z.; Dong, Z.; Aiying, W. Surface & Coatings Technology Chromium interlayer amorphous carbon film for 304 stainless steel bipolar plate of proton exchange membrane fuel cell. Surf. Coat. Technol. 2016, 307, 374–381. [Google Scholar] [CrossRef]
- Show, Y. Electrically conductive amorphous carbon coating on metal bipolar plates for PEFC. Surf. Coat. Technol. 2007, 202, 1252–1255. [Google Scholar] [CrossRef]
- Feng, K.; Shen, Y.; Sun, H.; Liu, D.; An, Q.; Cai, X.; Chu, P.K. Conductive amorphous carbon-coated 316L stainless steel as bipolar plates in polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 2009, 34, 6771–6777. [Google Scholar] [CrossRef]
- Yi, P.; Peng, L.; Feng, L.; Gan, P.; Lai, X. Performance of a proton exchange membrane fuel cell stack using conductive amorphous carbon-coated 304 stainless steel bipolar plates. J. Power Sources 2010, 195, 7061–7066. [Google Scholar] [CrossRef]
- Bi, F.; Peng, L.; Yi, P.; Lai, X. Multilayered Zr–C/a-C film on stainless steel 316L as bipolar plates for proton exchange membrane fuel cells. J. Power Sources 2016, 314, 58–65. [Google Scholar] [CrossRef]
- Wang, W.; He, S.; Lan, C. Electrochimica Acta Protective graphite coating on metallic bipolar plates for PEMFC applications. Electrochim. Acta 2012, 62, 30–35. [Google Scholar] [CrossRef]
- Sisan, M.M.; Sereshki, M.A.; Khorsand, H.; Siadati, M.H. Carbon coating for corrosion protection of SS-316L and AA-6061 as bipolar plates of PEM fuel cells. J. Alloys Compd. 2014, 613, 288–291. [Google Scholar] [CrossRef]
- Fu, Y.; Hou, M.; Xu, H.; Hou, Z.; Ming, P. Ag–polytetrafluoroethylene composite coating on stainless steel as bipolar plate of proton exchange membrane fuel cell. J. Power Sources 2008, 182, 580–584. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Z.; Huang, K. Composite coatings with in situ formation for Fe–Ni–Cr alloy as bipolar plate of PEMFC. Int. J. Hydrog. Energy 2013, 38, 11379–11391. [Google Scholar] [CrossRef]
- Mawdsley, J.R.; Carter, J.D.; Wang, X.; Niyogi, S.; Fan, C.Q.; Koc, R.; Osterhout, G. Composite-coated aluminum bipolar plates for PEM fuel cells. J. Power Sources 2013, 231, 106–112. [Google Scholar] [CrossRef]
- Husby, H.; Edvard, O.; Oedegaard, A.; Seland, F. ScienceDirect Carbon-polymer composite coatings for PEM fuel cell bipolar plates. Int. J. Hydrogen Energy 2013, 39, 951–957. [Google Scholar] [CrossRef]
- Tsai, B.; Tseng, C.; Liu, Z.; Wang, C.; Lee, C.; Yang, C.; Lo, S. Effects of flow field design on the performance of a PEM fuel cell with metal foam as the flow distributor. Int. J. Hydrogen Energy 2012, 37, 13060–13066. [Google Scholar] [CrossRef]
- Carton, J.G.; Olabi, A.G. Design of experiment study of the parameters that affect performance of three flow plate configurations of a proton exchange membrane fuel cell. Energy 2010, 35, 2796–2806. [Google Scholar] [CrossRef] [Green Version]
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Progress in material science. Prog. Mat. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Carton, J.G.; Olabi, A.G. Representative model and flow characteristics of open pore cellular foam and potential use in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2015, 40, 5726–5738. [Google Scholar] [CrossRef] [Green Version]
- Baroutaji, A.; Carton, G.J.; Olabi, A.G. Design and development of Proton Exchange Membrane Fuel Cell Using Open Pore Cellular Foam as Flow Plate Material. J. Energy Challenges Mech. 2014, 1. [Google Scholar]
- Lee, Y.-H.; Li, S.-M.; Tseng, C.-J.; Su, C.-Y.; Lin, S.-C.; Jhuang, J.-W. Graphene as corrosion protection for metal foam flow distributor in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2017, 42, 22201–22207. [Google Scholar] [CrossRef]
- Rossi, S.; Calovi, M.; Fedel, M. Progress in Organic Coatings Corrosion protection of aluminum foams by cataphoretic deposition of organic coatings. Prog. Org. Coat. 2017, 109, 144–151. [Google Scholar] [CrossRef]
- Simoncini, A.; Ucciardello, N.; Tagliaferri, V. Thermal and mechanical improvement of aluminum open-cells foams through electrodeposition of copper and graphene. Manuf. Rev. 2016, 3. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Sudagar, J.; Gao, F.; Feng, P. Preparation and Corrosion Resistance of Electroless Ni-P Coating on Open-Cell Aluminum Foams. Int. J. Electrochem. Sci. 2012, 7, 5951–5961. [Google Scholar]
- Aal, A.A.; Aly, M.S. Electroless Ni–Cu–P plating onto open cell stainless steel foam. Appl. Surf. Sci. 2009, 255, 6652–6655. [Google Scholar] [CrossRef]
- Baroutaji, A.; Stokes, J. Application of Open Pore Cellular Foam for air breathing PEM fuel cell ScienceDirect Application of Open Pore Cellular Foam for air breathing PEM fuel cell. Int. J. Hydrogen Energy 2017. [Google Scholar] [CrossRef]
- Pozio, A.; Zaza, F.; Masci, A.; Silva, R.F. Bipolar plate materials for PEMFCs: A conductivity and stability study. J. Power Sources 2008, 179, 631–639. [Google Scholar] [CrossRef]
- Yousfi-steiner, N.; Moc, P. A review on polymer electrolyte membrane fuel cell catalyst degradation and starvation issues: Causes, consequences and diagnostic for mitigation. J. Power Sources 2009, 194, 130–145. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Taheri, A.; Chan, S.H. A review on ions induced contamination of polymer electrolyte membrane fuel cells, poisoning mechanisms and mitigation approaches. J. Ind. Eng. Chem. 2016, 34. [Google Scholar] [CrossRef]
- Taherian, R. A review of composite and metallic bipolar plates in proton exchange membrane fuel cell: Materials, fabrication, and material selection. J. Power Sources 2014, 265, 370–390. [Google Scholar] [CrossRef]
- Sulek, M.; Adams, J.; Kaberline, S.; Ricketts, M.; Waldecker, J.R. In situ metal ion contamination and the effects on proton exchange membrane fuel cell performance. J. Power Sources 2011, 196, 8967–8972. [Google Scholar] [CrossRef]
- Okada, T.; Ayato, Y.; Yuasa, M.; Sekine, I. The Effect of Impurity Cations on the Transport Characteristics of Perfluorosulfonated Ionomer Membranes. J. Phys. Chem. B 1999, 3315–3322. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Qian, W.; Zhang, S.; Wessel, S.; Cheng, T.T.H.; Shen, J.; Wu, S. Chloride contamination effects on proton exchange membrane fuel cell performance and durability. J. Power Sources 2011, 196, 6249–6255. [Google Scholar] [CrossRef]
- Paiva, T.I.; Hashimoto, T.; Plancha, M.J.; Thompson, G.E.; Rangel, C.M. The effect of chloride as catalyst layer contaminant on the degradation of PEMFCs. In Proceedings of the HYCELTEC’2013-IV Iberian Symposium on Hydrogen, Fuel Cells and Advanced Batteries, Estoril, Portugal, 26–28 June 2013. [Google Scholar]
- Cheng, X.; Shi, Z.; Glass, N.; Zhang, L.; Zhang, J.; Song, D.; Liu, Z.; Wang, H.; Shen, J. A review of PEM hydrogen fuel cell contamination: Impacts, mechanisms, and mitigation. J. Power Sources 2007, 165, 739–756. [Google Scholar] [CrossRef]
- Benesch, R.; Salman, S.; Jacksier, T. The Effect of Fuel and Oxidant Contaminants on the Performance of PEM Fuel Cells. In Proceedings of the 16th World Hydrogen Energy Conference, Lyon, France, 13–16 June 2006. [Google Scholar]
- Jia, R.; Dong, S.; Hasegawa, T.; Ye, J.; Dauskardt, R.H. Contamination and moisture absorption effects on the mechanical properties of catalyst coated membranes in PEM fuel cells. Int. J. Hydrogen Energy 2012, 37, 6790–6797. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, S.; Gao, Y.; Jiang, L.; Sun, H.; Sun, G. Effect of RuO2. xH2O in anode on the performance of direct methanol fuel cells. Int. J. Hydrogen Energy 2010, 35, 11254–11260. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, S.; Jiang, L.; Xia, Z.; Sun, H.; Sun, G. High Pt utilization catalyst prepared by ion exchange method for direct methanol fuel cells. Int. J. Hydrogen Energy 2012, 37, 14543–14548. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Kingsbury, R.S.; Call, D.F.; Coronell, O. Impact of solution composition on the resistance of ion exchange membranes. J. Membr. Sci. 2018, 554, 39–47. [Google Scholar] [CrossRef]

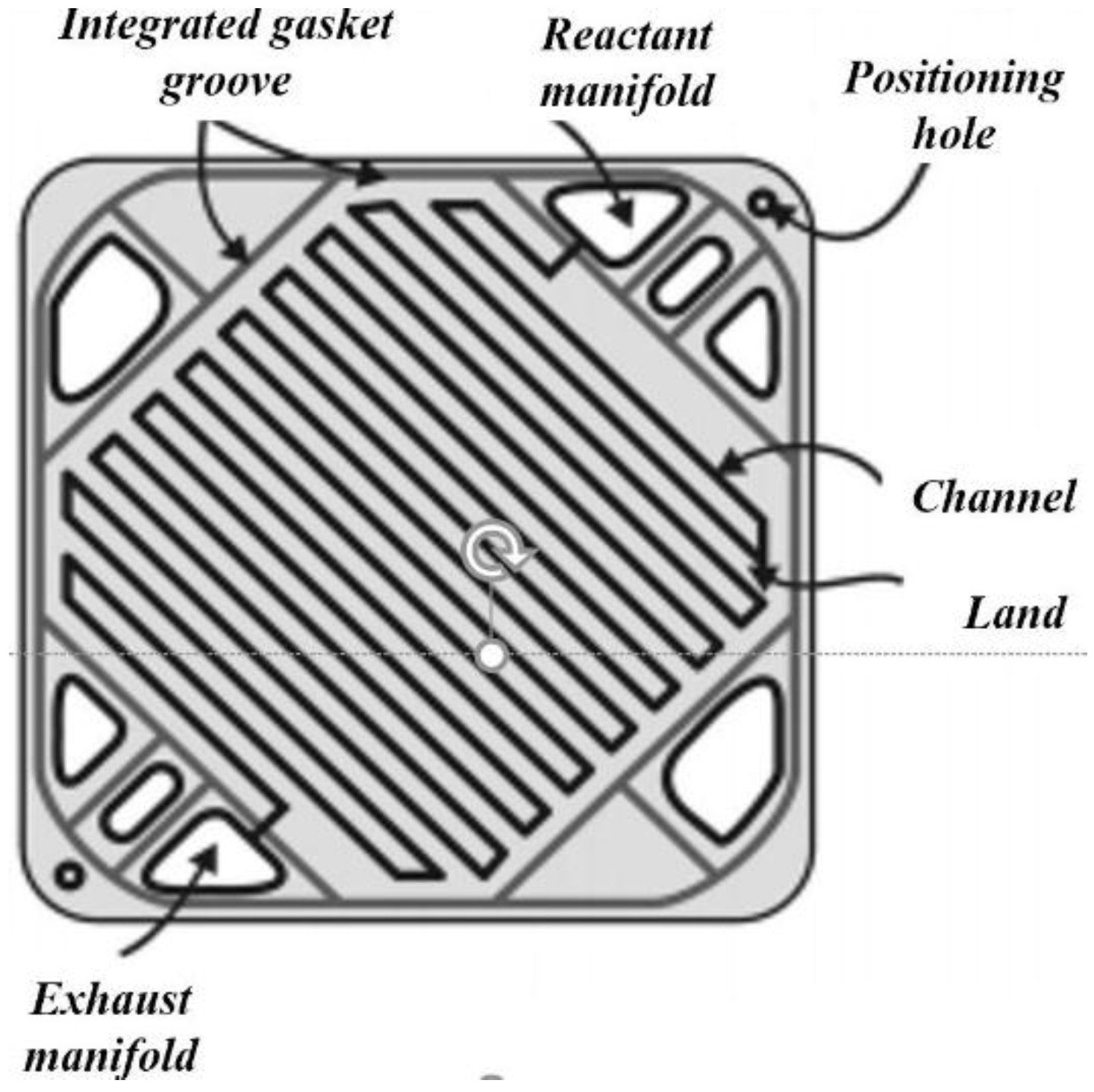



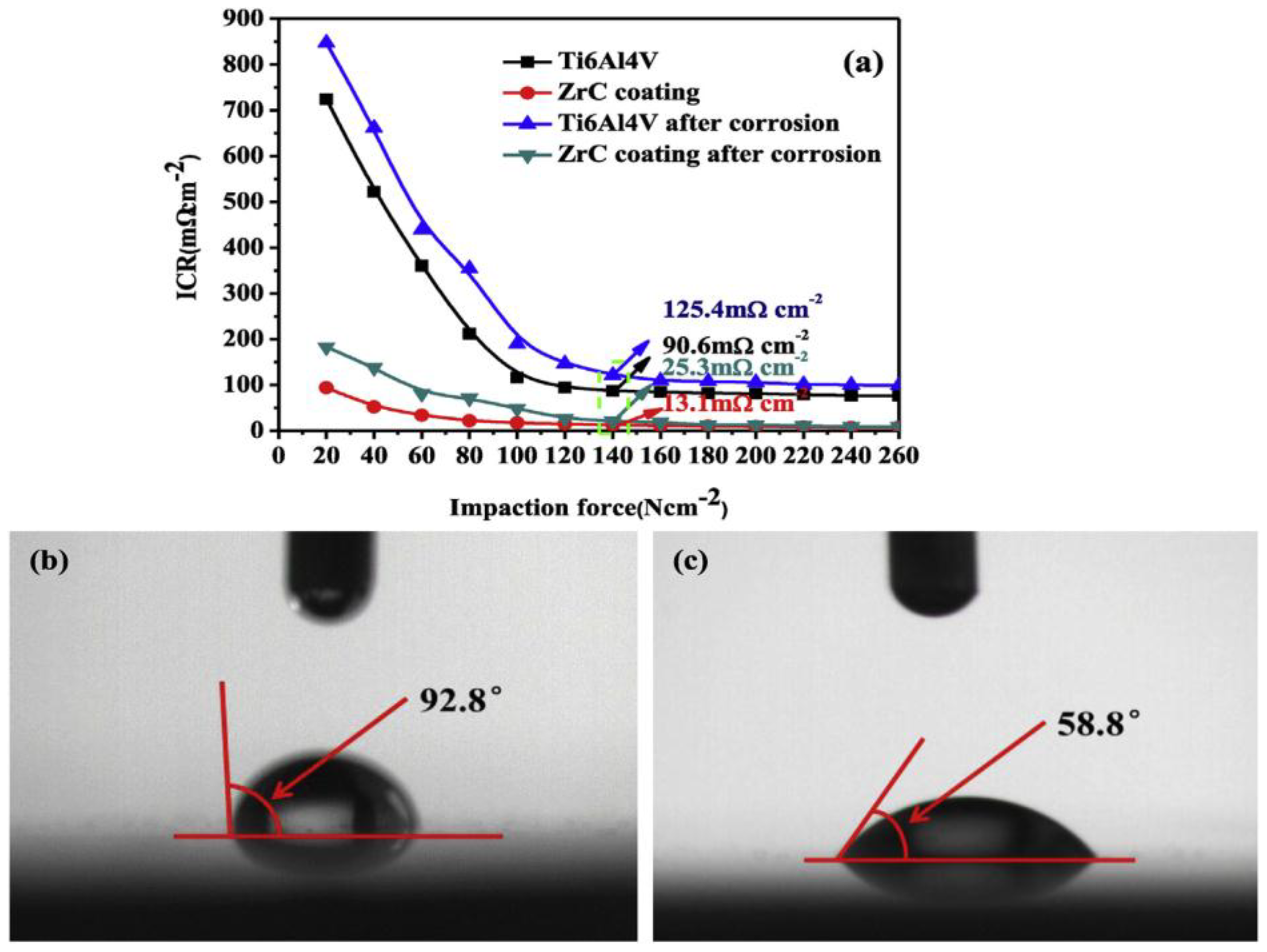




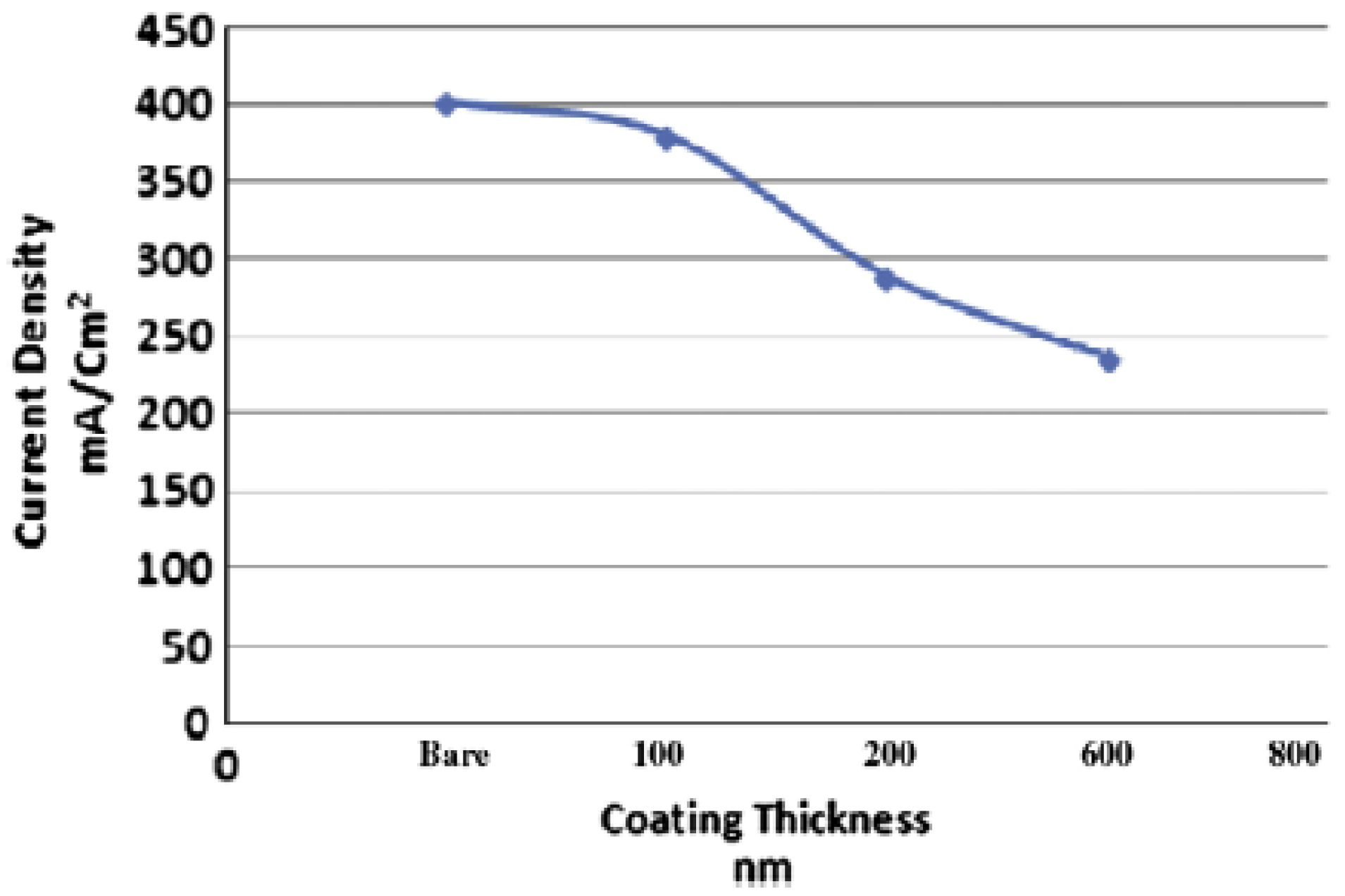



| Property | Unit | 2017 | 2020 |
|---|---|---|---|
| Weight | KgKW−1 | <0.4 | 0.4 |
| H2 permeation rate | cm3(cm2s)−1 | <1.3 × 10−14 | 1.3 × 10−14 |
| Cost | $KW−1 | 3 | 3 |
| Corrosion at anode & cathode | µAcm−2 | <1 | <1 |
| Electrical conductivity | Scm−1 | >100 | >100 |
| Flexural strength (ASTM D790-10) | MPa | >25 | >25 |
| Area specific resistance | Ω-cm2 | 0.02 | 0.01 |
| Characteristics | Graphite | Polymer Composite | Metals |
|---|---|---|---|
| Advantages | Minimum contact resistance. Low density. High corrosion resistance. Good thermal and electrical conductivity. | Excellent corrosion resistance. Eradication of machining process. Low contact resistance. | Cost effectiveness. High formability and machinability. Good electrical and thermal conductivity. |
| Disadvantages | Low mechanical strength. Time consuming and expensive to machine. | Low electrical conductivity. | Poor chemical stability. Prone to corrosion. |
| Characteristics | Thermoset/Filler Composite | Thermoplastic/Filler Composite |
|---|---|---|
| Advantages | - High temperature operation - Quick cycle time - Introduction of flow field in the course of moulding - Little contact resistance | - Injection moulding lends itself to manufacturing automation - Quick cycle time - Introduction of flow field in the course of moulding - Minimum contact resistance |
| Disadvantages | - Poor electrical conductivity | - Poor electrical conductivity during standard thermoplastics - Restricted to low temperature - Injection moulding difficult at high filler loading |
| Processing options | - Compression moulding - Post-moulding computer numberic control (CNC) milling of blank | - Injection moulding - Compression moulding - Post-moulding CNC milling of blank |
| Substrate Material | Coating Method | Coating Process | Electrolyte | Reference |
|---|---|---|---|---|
| SS316L | Titanium-nitride (TiN) layering. | Physical vapor deposition (PVD) | 0.5 M H2SO4 at 70 °C | [49] |
| Chromium and Titanium nitride (CrN/TiN) layering | Magnetron sputtering | 1 M corrosive H2SO4 + 2 ppm HF solution at 70 °C | [52] | |
| Chromium nitride (CrN) layering | PBAIP | 0.5 M H2SO4 + 5 ppm F− solution at 25 °C | [53] | |
| Chromium nitride/Cr layering | Physical vapor deposition (PVD) | 0.005 M H2SO4 + 2 ppm F− solution at 70 °C | [54] | |
| Niobium nitride diffusion (Nb–N layering) | Plasma surface diffusion alloying (PSDA) | 0.05 M H2SO4 + 2 ppm HF at 50 °C | [55] | |
| Titanium-nitride (TiN) coating | Not mentioned | 0.01 M HCl + 0.01 M Na2SO4 solutions | [57] | |
| Titanium aluminium nitride (TiAlN)/TiN/CrN layering | Electron beam physical vapour deposition (EBPV) | 1 M H2SO4 purged with either H2 or O2 | [58] | |
| Tantalum nitride (TaN) layering | Magnetron sputtering | 0.05 M H2SO4 + 0.2 ppm HF solution at 80 °C | [61] | |
| Tantalum/Tantalum nitride (Ta/TaN) | Magnetron sputtering | 0.5 M H2SO4 + 2 ppm HF solution at room temperature and 80 °C | [62] | |
| a-C film layering | CFUBMSIP | 0.5 M H2SO4 + 2 ppm HF solution at 80 °C | [81] | |
| Graphite film layering | Not mentioned | 0.5 M H2SO4 solution | [84] | |
| Ag–polytetrafluoroethylene (PTFE), composite film layering | Bi-pulse electroplating method | 0.5 M H2SO4 + 5 ppm F solution at 80 °C | [86] | |
| Carbon polymer composite coating | Spraying and hot-pressing techniques | 1mM H2SO4 solution at 70 °C | [89] | |
| SS304 | Nickel layering | Chemical vapour deposition (CVD) | 0.5 M H2SO4 | [50] |
| Niobium nitride layering | Plasma surface diffusion alloying method | 0.05 M H2SO4 + 2 ppm F− solution at 70 °C | [60] | |
| Titanium carbide layering | HEMA technique | 1 M H2SO4 solution at room temperature | [66] | |
| Cr interlayer/a-C | Direct current magnetron sputtering | 0.5 M H2SO4 + 5 ppm HF solution at room temp. and 80 °C | [79] | |
| Titanium plate | Titanium nitride layering | MIP technique | 0.5 M H2SO4 + 2 ppm HF solution | [56] |
| Amorphous carbon (a-C) films layering | RF-PECVD method | Not mentioned | [80] | |
| Al6061 | Chromium carbide coating | HVOF thermal spray method | 0.5 M H2SO4 + 2 ppm HF solution at 70 °C | [63] |
| AISI304 | Chromium carbide coating | HVOF thermal spray method | 1 M NaCl solution | [64] |
| AISI 1045 | Niobium carbide layering | Thermo-reactive deposition/diffusion technique (TRD) | 3 wt.% NaCl solution | [69] |
| AISI441, AISI444, and AISI446 | Tin(IV) Oxide (SnO2) films layering | Chemical vapour deposition (CVD) | 1 M H2SO4 + 2 ppm F− solution at 70 °C | [71] |
| 316L, 317L and 349TM | Tin(IV) Oxide (SnO2) films layering | Chemical vapour deposition (CVD | 1 M H2SO4 + 2 ppm F− solution at 70 °C | [72] |
| Al | Graphene oxide layering | Polyvinyl alcohol (PVA) | 0.5 M H2SO4 solution | [74] |
| Graphene coating | Dip coating | 0.5M NaCl solution | [75] | |
| Composite coating | Wet spraying | 0.001 M H2SO4 with 0.1 ppm NaF (pH = 3) | [88] | |
| Cu and Ni | Graphene coating | CVD | 0.1M NaCl solution | [76] |
| Ni/SS | Graphene layering | CVD | 3.5 wt.% NaCl solution | [77] |
| SS-316L and AA-6061 | Graphite layering | PVD | 1 M H2SO4 at 70 °C | [85] |
| Ni open pore metal foam | Graphene layering | CVD | 3 M H2SO4 solution at room temperature, 50 °C and 80 °C | [95] |
| PTFE coating | CoBlast™ | 0.5 M H2SO4 + 2 ppm HF solution at 70 °C | ||
| Al open pore metal foam | Organic coating | Cataphoretic deposition | NaCl solution | [96] |
| Copper and Graphene layering | Electro-deposition | 1.25 M CuSO4, 0.61 M H2SO4 and Cl-50 ppm solutions | [97] | |
| NI-P layering | Hypophosphite techniques | 3.5 wt.% NaCl solution at room temperature | [98] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ijaodola, O.; Ogungbemi, E.; Khatib, F.N.; Wilberforce, T.; Ramadan, M.; El Hassan, Z.; Thompson, J.; Olabi, A.G. Evaluating the Effect of Metal Bipolar Plate Coating on the Performance of Proton Exchange Membrane Fuel Cells. Energies 2018, 11, 3203. https://doi.org/10.3390/en11113203
Ijaodola O, Ogungbemi E, Khatib FN, Wilberforce T, Ramadan M, El Hassan Z, Thompson J, Olabi AG. Evaluating the Effect of Metal Bipolar Plate Coating on the Performance of Proton Exchange Membrane Fuel Cells. Energies. 2018; 11(11):3203. https://doi.org/10.3390/en11113203
Chicago/Turabian StyleIjaodola, Oluwatosin, Emmanuel Ogungbemi, Fawwad Nisar. Khatib, Tabbi Wilberforce, Mohamad Ramadan, Zaki El Hassan, James Thompson, and Abdul Ghani Olabi. 2018. "Evaluating the Effect of Metal Bipolar Plate Coating on the Performance of Proton Exchange Membrane Fuel Cells" Energies 11, no. 11: 3203. https://doi.org/10.3390/en11113203





