Simulation-Optimization Framework for Synthesis and Design of Natural Gas Downstream Utilization Networks
Abstract
:1. Introduction
- (i)
- It analyses different production processes namely LNG, GTL, and methanol along with different design alternatives for each of the main processing units.
- (ii)
- It considers both the maximization profit to reflect the economic perspective, and minimization of CO2 emission to reflect the environmental perspective.
2. Process Description
2.1. Syngas Preparation Unit (A)
2.2. Liquefaction Unit (B)
2.3. N2 Rejection Unit (C)
2.4. Hydrogen Unit (D)
2.5. FT Synthesis Unit (E)
2.6. Methanol Synthesis Unit (F)
2.7. FT Upgrading Unit (G)
2.8. Methanol Upgrading Unit (H)
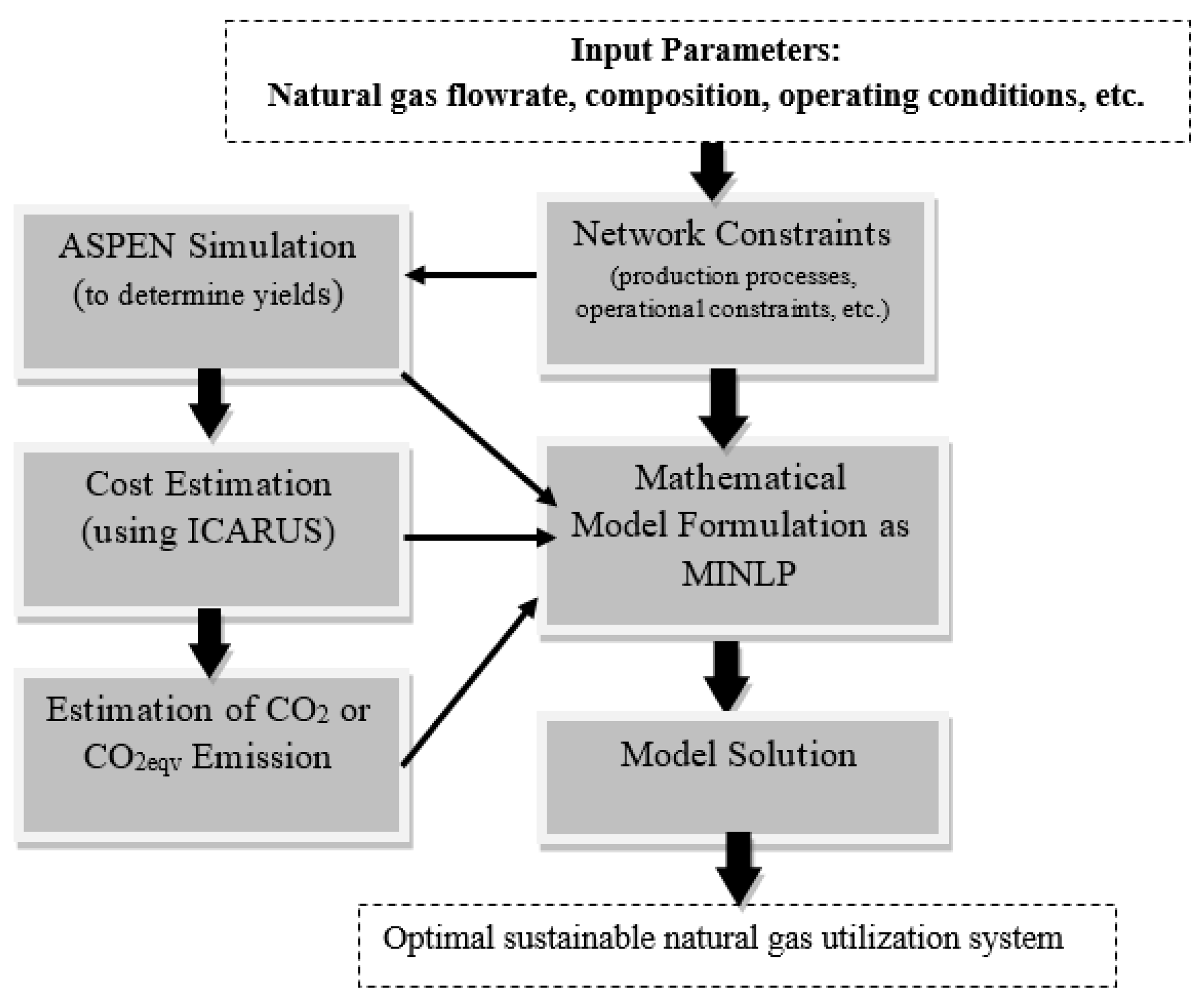
3. Problem Statement and overall Methodology
4. Mathematical Programming Model
4.1. Overall Mass Balance and Yield Model
4.2. Supply and Demand Constraints
4.3. Capacity Constraint for Processing Units
4.4. Objective Function
5. Case Study
5.1. Economic Planning Using Formulated Model
5.2. Sustainable Planning Using Formulated Model
6. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
Nomenclature
| Sets |
| i {A,…,H} = processing units j = technology/configuration |
| m = operational mode |
| k = product = the set of technology/configuration for processing unit i = the set of operating modes for processing unit i, |
| Binary variables |
| = Binary variable for selection of technology j in processing unit i at operational mode m |
| Continuous variables |
| |
| |
| |
| Other Parameters |
| under technology j and operating mode m |
| of product k we = cost of CO2 emision CO2ijm = CO2 emision in unit i for technology j in operating mode m in operating mode m in operating mode m |
| Superscripts |
| L = lower bound |
| U = upper bound |
Acronyms
| CH4 | Methane |
| LNG | Liquefied natural gas |
| GTL | Gas to liquids |
| LP | Linear programming |
| MILP | Mixed integer linear programming |
| NLP | Nonlinear programming |
| MINLP | Mixed integer nonlinear programming |
| GHG | Greenhouse gas |
| CNG | Compressed natural gas |
| GTS | Gas to solid |
| GTW | Gas to wire |
| CBM | Coal bed methane |
| FT | Fischer-Tropsch |
| DME | Dimethylether |
| RWGSR | Reverse water gas shift reaction |
References
- Thomas, S. Review of ways to transport natural gas energy from countries which do not need the gas for domestic use. Energy 2003, 2814, 1461–1477. [Google Scholar] [CrossRef]
- BC Ministry of Energy and Mines. British Columbia’s Natural Gas Strategy Fuelling B.C.’s Economy for the Next Decade and Beyond; BC Ministry of Energy and Mines: Vancouver, BC, Canada, 2012. Available online: http://www.gov.bc.ca/ener/popt/down/natural_gas_strategy.pdf (accessed on 24 September 2017).
- BC Ministry of Energy, Mines, and Natural Gas. British Columbia’s Liquefied Natural Gas Strategy: One Year Update; BC Ministry of Energy, Mines, and Natural Gas: Vancouver, BC, Canada, 2013; pp. 1–16. Available online: https://lnginbc.gov.bc.ca/app/uploads/sites/16/2016/07/BCs-LNG-Strategy-One-Year-Update-2013_web130207.pdf (accessed on 24 September 2017).
- ExxonMobil. Outlook for Energy: A View to 2040; ExxonMobil: Irving, TX, USA, 2017; Available online: http://cdn.exxonmobil.com/~/media/global/files/outlook-for-energy/2016/2016-outlook-for-energy.pdf (accessed on 24 September 2017).
- Wood, D.A.; Nwaoha, C.; Towler, B.F. Gas-to-liquids (GTL): A review of an industry offering several routes for monetizing natural gas. J. Nat. Gas Sci. Eng. 2012, 9, 196–208. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Beyond Oil and Gas: The Methanol Economy; WILEY-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Al-Sobhi, S.A.; Elkamel, A. Simulation and optimization of natural gas processing and production network consisting of LNG, GTL, and methanol facilities. J. Nat. Gas Sci. Eng. 2015, 23, 500–508. [Google Scholar] [CrossRef]
- Al-Sobhi, S.A.; Shaik, M.A.; Elkamel, A.; Erenay, F.S. Integrating simulation in optimal synthesis and design of natural gas upstream processing networks. Ind. Eng. Chem. Res. 2017. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.; Christiansen, L.J. Concepts in Syngas Manufacture; World Scientific: Singapore, 2011; Volume 10. [Google Scholar]
- Ramberg, D.J.; Chen, Y.H.H.; Paltsev, S.; Parsons, J.E. The economic viability of gas-to-liquids technology and the crude oil-natural gas price relationship. Energy Econ. 2017, 63, 13–21. [Google Scholar] [CrossRef]
- Aasberg-Petersen, K.; Bak Hansen, J.H.; Christensen, T.S.; Dybkjaer, I.; Christensen, P.S.; Stub Nielsen, C.; Rostrup-Nielsen, J.R. Technologies for large-scale gas conversion. Appl. Catal. A Gen. 2001, 221, 379–387. [Google Scholar] [CrossRef]
- Luyben, W.L. Design and Control of the Dry Methane Reforming Process. Ind. Eng. Chem. Res. 2014, 53, 14423–14439. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. New aspects of syngas production and use. Catal. Today 2000, 63, 159–164. [Google Scholar] [CrossRef]
- Wilhelm, D.J.; Simbeck, D.R.; Karp, A.D.; Dickenson, R.L. Syngas production for gas-to-liquids applications: Technologies, issues and outlook. Fuel Process. Technol. 2001, 71, 139–148. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Syngas in perspective. Catal. Today 2002, 71, 243–247. [Google Scholar] [CrossRef]
- Aasberg-Petersen, K.; Christensen, T.S.; Nielsen, C.S.; Dybkjær, I. Recent developments in autothermal reforming and pre-reforming for synthesis gas production in GTL applications. Fuel Process. Technol. 2003, 83, 253–261. [Google Scholar] [CrossRef]
- Bakkerud, P.K. Update on synthesis gas production for GTL. Catal. Today 2005, 106, 30–33. [Google Scholar] [CrossRef]
- Julia, L.M.; Ortiz-Espinoza, A.P.; El-Halwagi, M.M.; Jime, A. Techno-Economic Assessment and Environmental Impact of Shale Gas Alternatives to Methanol. ACS Sustain. Chem. Eng. 2014, 2, 2338–2344. [Google Scholar]
- Noureldin, M.M.B.; Elbashir, N.O.; El-Halwagi, M.M. Optimization and selection of reforming approaches for syngas generation from natural/shale gas. Ind. Eng. Chem. Res. 2014, 53, 1841–1855. [Google Scholar] [CrossRef]
- Tusiani, M.; Shearer, G. LNG, A Nontechnical Guide; PennWell Corporation: Tulsa, OK, USA, 2007. [Google Scholar]
- Mokhatab, S.; Economides, M.J. Onshore LNG Production Process Selection. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 24–27 September 2006; Volume 1, pp. 1–11. [Google Scholar]
- International Gas Union (IGU). International Gas Union (IGU) World LNG Report; IGU: Barcelona, Spain, 2017. [Google Scholar]
- Kidnay, A.J.; Parrish, W.R. Fundamentals of Natural Gas Processing; Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Gas Processors Suppliers Association (GPSA). Engineering Data Book, 12th ed.; GPSA: Tulsa, OK, USA, 2004. [Google Scholar]
- Kuo, J.C.; Wang, K.H.; Chen, C. Pros and cons of different Nitrogen Removal Unit (NRU) technology. J. Nat. Gas Sci. Eng. 2012, 7, 52–59. [Google Scholar] [CrossRef]
- Mueller-Langer, F.; Tzimas, E.; Kaltschmitt, M.; Peteves, S. Techno-economic assessment of hydrogen production processes for the hydrogen economy for the short and medium term. Int. J. Hydrog. Energy 2007, 32, 3797–3810. [Google Scholar] [CrossRef]
- Silveira, J.L. Sustainable Hydrogen Production Processes: Energy, Economic and Ecological Issues; Green Energy and Technology Series; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Dry, M.E. The Fischer-Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Steynberg, A.P.; Espinoza, R.L.; Jager, B.; Vosloo, A.C. High temperature Fischer–Tropsch synthesis in commercial practice. Appl. Catal. A Gen. 1999, 186, 41–54. [Google Scholar] [CrossRef]
- De Klerk, A. Fischer-Tropsch Refining; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Velasco, J.A.; Lopez, L.; Velásquez, M.; Boutonnet, M.; Cabrera, S.; Järås, S. Gas to liquids: A technology for natural gas industrialization in Bolivia. J. Nat. Gas Sci. Eng. 2010, 2, 222–228. [Google Scholar] [CrossRef]
- Espinoza, R.L.; Steynberg, A.P.; Jager, B.; Vosloo, A.C. Low temperature Fischer–Tropsch synthesis from a Sasol perspective. Appl. Catal. A Gen. 1999, 186, 13–26. [Google Scholar] [CrossRef]
- Jager, B.; Espinoza, R.L. Advances in Low-Temperature Fischer-Tropsch Synthesis. Catal. Today 1995, 23, 17–28. [Google Scholar] [CrossRef]
- ASPEN Plus V7.3; Aspen Technology, Inc.: Bedford, MA, USA, 2011.
- Steynberg, A.; Dry, M. Fischer-Tropsch Technology; Elsevier B.V.: Amsterdam, The Netherlands, 2004. [Google Scholar]
- International Energy Agency (IEA). World CO2 Emissions from Fuel Combustion Database Documentation; International Energy Agency: Paris, France, 2017. [Google Scholar]
- British Petroleum Company (BP). British Petroleum Statistical Review of World Energy; British Petroleum Co.: London, UK, June 2017. [Google Scholar]
- Energy Information Administration (EIA). Monthly Natural Gas Liquids Reports; Energy Information Administration: Washington, DC, USA, 2015.
- Linear, Interactive, and General Optimizer (LINGO) Software, LINDO System Inc.: Chicago, IL, USA, 2013.
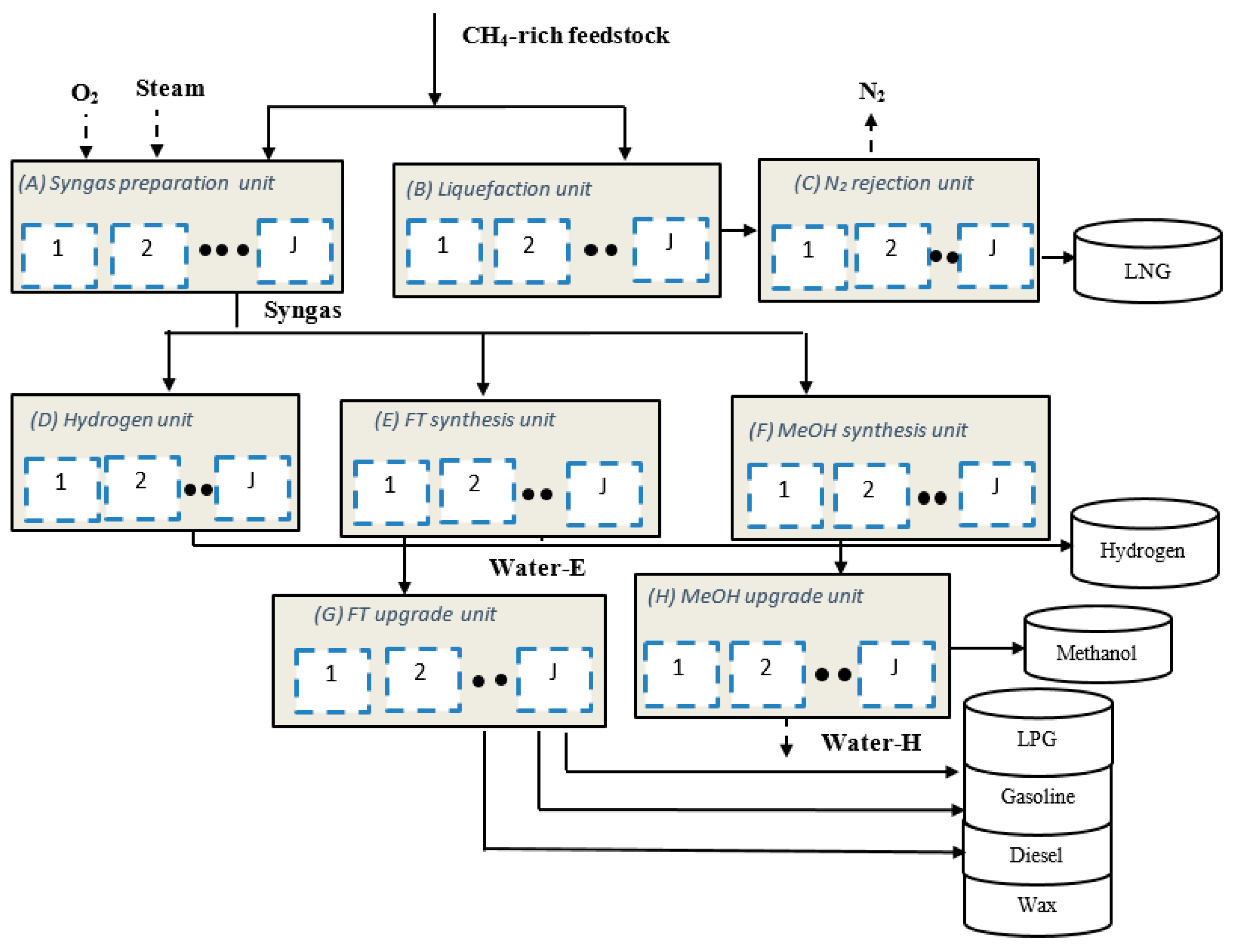
| Major Processing Unit (i) | Possible Processes/Technologies | Considered Technology | Operating Modes (Mi) |
| Syngas preparation unit (A) |
|
|
|
| Liquefaction unit (B) |
|
|
|
| N2 rejection unit (C) |
|
|
|
| Hydrogen unit (D) |
|
|
|
| FT synthesis unit (E) |
|
|
|
| Methanol synthesis unit (F) |
|
|
|
| Product upgrading unit (G&H) |
|
|
|
| Natural Gas Feedstock | $ 4.4 per MMBtu |
|---|---|
| LNG | $7 per thousand cubic feet |
| LPG | $2.5 per gallon |
| Gasoline | $2.8 per gallon |
| Diesel | $3 per gallon |
| Wax | $2 per gallon |
| Methanol | $500 per ton |
| Products | Natural Gas (kg/h) | Yield * | Min. Demand (kg/h) | Max. Demand (kg/h) | LP Model Output (kg/h) |
|---|---|---|---|---|---|
| LNG | 1,044,157 | 0.690 | 1,000,000 | 1,200,000 | 1,090,000 |
| Losses associated with LNG | 232,343 | 0.152 | 230,000 | 235,000 | 230,000 |
| LPG | 114,000 | 0.075 | 110,000 | 120,000 | 120,000 |
| Gasoline | 227,911 | 0.150 | 220,000 | 230,000 | 230,000 |
| Diesel | 174,730 | 0.110 | 170,000 | 180,000 | 180,000 |
| Wax | 99,900 | 0.070 | 95,000 | 100,000 | 100,000 |
| Losses associated with GTL | 735,159 | 0.480 | 733,000 | 735,200 | 733,000 |
| Methanol | 870,000 | 0.574 | 850,000 | 900,000 | 900,000 |
| Losses associated with methanol | 419,200 | 0.270 | 400,000 | 410,000 | 400,000 |
| Available NG supply (kg/h) | 1,515,000 | - | - | - | - |
| LNG | 100% | 70% | 50% | 30% |
|---|---|---|---|---|
| Total capital cost, $M | 18.45 | 14.30 | 11.60 | 8.90 |
| Amortized capital cost, $M/year | 2.24 | 1.74 | 1.42 | 1.08 |
| Total operating cost, $M/year | 248.10 | 174 | 124 | 74.5 |
| Total utilities cost, $M/year | 229.60 | 161 | 115 | 18.9 |
| Desired rate of return, %/year | 10 | 10 | 10 | 10 |
| Lifetime of the project, year | 20 | 20 | 20 | 20 |
| LNG mass flow rate, kg/h | 1,128,350 | 789,845 | 564,175 | 338,505 |
| LNG yield | 0.88 | 0.62 | 0.44 | 0.26 |
| Objective function, $M | 196 | 134 | 92.60 | 51.10 |
| Methanol | 100% | 70% | 50% | 30% |
|---|---|---|---|---|
| Total capital cost, $M | 44.8 | 33.7 | 25 | 19.3 |
| Amortized capital cost, $M/year | 5.45 | 4.10 | 3.04 | 2.35 |
| Total operating cost, $M/year | 71.5 | 58.5 | 50.4 | 42.3 |
| Total utilities cost, $M/year | 12.0 | 50.6 | 43.4 | 36.2 |
| Desired rate of return, %/year | 10 | 10 | 10 | 10 |
| Lifetime of the project, year | 20 | 20 | 20 | 20 |
| Methanol mass flowrate, kg/h | 688,053 | 481,615 | 344,011 | 206,401 |
| Water mass flowrate, kg/h | 480 | 336 | 240 | 144 |
| Methanol yield | 0.67 | 0.47 | 0.33 | 0.20 |
| Objective function, $M | 1100 | 845 | 568 | 291 |
| GTL LTFT | 100% | 70% | 50% | 30% |
|---|---|---|---|---|
| Total capital cost, $M | 86.4 | 64 | 44.0 | 30.4 |
| Amortized capital cost, $M/year | 10.5 | 7.8 | 5.35 | 3.7 |
| Total operating cost, $M/year | 31.6 | 22.9 | 16.2 | 10.6 |
| Total utilities cost, $M/year | 24.3 | 17.0 | 11.7 | 1.96 |
| Desired rate of return, %/year | 10 | 10 | 10 | 10 |
| lifetime of the project, year | 20 | 20 | 20 | 20 |
| LPG mass flowrate, kg/h | 12,850 | 4471 | 2725 | 2395 |
| Gasoline mass flowrate, kg/h | 83,664 | 57,696 | 41,179 | 27,773 |
| Diesel mass flowrate, kg/h | 162,909 | 114,612 | 76,502 | 40,226 |
| Wax mass flowrate, kg/h | 610,756 | 443,272 | 310,548 | 188,175 |
| Water mass flowrate, kg/h | 65,799 | 34,693 | 32,462 | 19,477 |
| LPG yield | 0.012 | 0.004 | 0.003 | 0.002 |
| Gasoline yield | 0.081 | 0.056 | 0.040 | 0.027 |
| Diesel yield | 0.158 | 0.111 | 0.074 | 0.040 |
| Wax yield | 0.592 | 0.430 | 0.301 | 0.182 |
| Objective function, $M | 1780 | 1400 | 935 | 513 |
| GTL HTFT | 100% | 70% | 50% | 30% |
|---|---|---|---|---|
| Total capital cost, $M | 90.5 | 57.7 | 47.2 | 34.4 |
| Amortized capital cost, $M/year | 11.0 | 7.02 | 5.74 | 4.18 |
| Total operating cost, $M/year | 4560 | 1510 | 1150 | 923 |
| Total utilities cost, $M/year | 4170 | 1400 | 1060 | 852 |
| Desired rate of return, %/year | 10 | 10 | 10 | 10 |
| lifetime of the project, year | 20 | 20 | 20 | 20 |
| LPG mass flowrate, kg/h | 67,898 | 308,035 | 202,597 | 205,452 |
| Gasoline mass flowrate, kg/h | 308,035 | 223,772 | 136,950 | 137,400 |
| Diesel mass flowrate, kg/h | 202,597 | 158,931 | 103,560 | 94,112 |
| Wax mass flowrate, kg/h | 205,452 | 77,533 | 50,213 | 47,776 |
| Water mass flowrate, kg/h | 124,745 | 87,321 | 62,504 | 61,502 |
| LPG yield | 0.066 | 0.299 | 0.196 | 0.199 |
| Gasoline yield | 0.2987 | 0.217 | 0.133 | 0.133 |
| Diesel yield | 0.196 | 0.154 | 0.100 | 0.091 |
| Wax yield | 0.199 | 0.075 | 0.049 | 0.046 |
| Objective function, $M | 282 | 1960 | 1410 | 1480 |
| Utilization Option/Percentage | 100% | 70% | 50% | 30% |
|---|---|---|---|---|
| LNG | 0.01471095 | −7.23 × 107 | −1.21 × 108 | −1.69 × 108 |
| Methanol | −1.14 × 104 | −7.95 × 103 | −5.68 × 103 | −3.41 × 103 |
| LTFT | 3.94 × 105 | 1.57 × 105 | 1.96 × 105 | 1.17 × 105 |
| HTFT | 1.94 × 106 | 1.36 × 106 | 9.72 × 105 | 9.95 × 105 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sobhi, S.A.; Elkamel, A.; Erenay, F.S.; Shaik, M.A. Simulation-Optimization Framework for Synthesis and Design of Natural Gas Downstream Utilization Networks. Energies 2018, 11, 362. https://doi.org/10.3390/en11020362
Al-Sobhi SA, Elkamel A, Erenay FS, Shaik MA. Simulation-Optimization Framework for Synthesis and Design of Natural Gas Downstream Utilization Networks. Energies. 2018; 11(2):362. https://doi.org/10.3390/en11020362
Chicago/Turabian StyleAl-Sobhi, Saad A., Ali Elkamel, Fatih S. Erenay, and Munawar A. Shaik. 2018. "Simulation-Optimization Framework for Synthesis and Design of Natural Gas Downstream Utilization Networks" Energies 11, no. 2: 362. https://doi.org/10.3390/en11020362




