Deacetylation Followed by Fractionation of Yellow Poplar Sawdust for the Production of Toxicity-Reduced Hemicellulosic Sugar for Ethanol Fermentation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Composition Change in the Liquid Phase Derived from Deacetylated YPS
2.2. Optimization of the Deacetylation Process Conditions
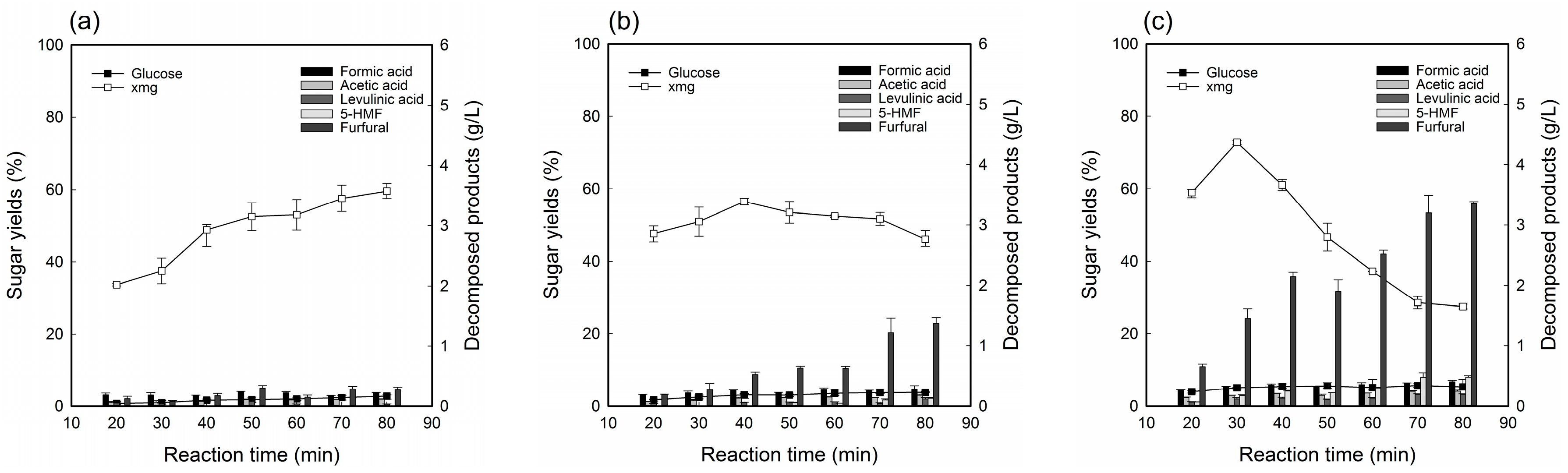
2.3. Optimization of Dilute Sulfuric Acid Fractionation Conditions with Deacetylated YPS for TRH Hydrolyzate Production
2.4. Ethanol Fermentation with Toxicity-Reduced Hemicellulosic Hydrolyzate
2.5. Overall Mass Balance
3. Materials and Methods
3.1. Raw Materials
3.2. Deacetylation
3.3. Dilute Sulfuric Acid Fractionation of Deacetylated YPS
3.4. Microorganism and Inoculum Preparation
3.5. Ethanol Fermentation of Toxicity-Reduced Hemicellulosic Hydrolyzateml
3.6. Compositional Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Conde-Mejía, C.; Jiménez-Gutiérrez, A.; El-Halwagi, M. A comparison of pretreatment methods for bioethanol production from lignocellulosic materials. Process Saf. Environ. Prot. 2012, 90, 189–202. [Google Scholar] [CrossRef]
- Yan, K.; Liu, Y.; Lu, Y.; Chai, J.; Sun, L. Catalytic application of layered double hydroxide-derived catalysts for the conversion of biomass-derived molecules. Catal. Sci. Technol. 2017, 7, 1622–1645. [Google Scholar] [CrossRef]
- Yan, K.; Jarvis, C.; Gu, J.; Yan, Y. Production and catalytic transform ation of levulinic acid: A platform for speciality chem icals and fuels. Renew. Sustain. Energy Rev. 2015, 51, 986–997. [Google Scholar] [CrossRef]
- Jorgensen, H.; Kristensen, J.B.; Felby, C. Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuels Bioprod. Biorefin. 2007, 1, 119–134. [Google Scholar] [CrossRef]
- Lim, W.S.; Kim, J.Y.; Kim, H.Y.; Choi, J.W.; Choi, I.G.; Lee, J.W. Structural properties of pretreated biomass from different acid pretreatments and their effects on simultaneous saccharification and ethanol fermentation. Bioresour. Technol. 2013, 139, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Long, J.; Wang, T.; Shu, R.; Zhang, Q.; Ma, L. Process intensification effect of ball milling on the hydrothermal pretreatment for corn straw enzymolysis. Energy Convers. 2015, 101, 481–488. [Google Scholar] [CrossRef]
- De Assis Castro, R.C.; Fonseca, B.F.; dos Santos, H.T.L.; Ferreira, I.S.; Mussatto, S.I.; Roberto, I.C. Alkaline deacetylation as a strategy to improve sugars recovery and ethanol production from rice straw hemicellulose and cellulose. Ind. Crop. Prod. 2017, 106, 65–73. [Google Scholar] [CrossRef]
- Mussatto, S.I. Biomass pretreatment with acids. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier Inc.: Waltham, MA, USA, 2016; pp. 169–185. [Google Scholar]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 6, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.J.; Peinado, S.; Mateo, S.; Fonseca, B.G.; Sanchez, S. Improving bioethanol production from olive pruning biomass by deacetylation step prior acid hydrolysis and fermentation processes. Bioresour. Technol. 2016, 220, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.A.; Carneiro, L.M.; Roberto, I.C. Treatment of rice straw hemicellulosic hydrolyzates with advanced oxidative processes: A new and promising detoxification method to improve the bioconversion process. Biotechnol. Biofuels 2013, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmqvist, E.; Hahn-Hagerdal, B. Fermentation of lignocellulosic hydrolyzates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.; Amann, M.; Hirth, T.; Rupp, S.; Zibek, S. Development and optimization of single and combined detoxification processes to improve the fermentability of lignocellulose hydrolyzates. Bioresour. Technol. 2013, 133, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H. Pretreatment of lignocellulosic biomass. In Bioprocessing Technologies in Integrated Biorefinery for Production of Biofuels, Biochemicals, and Biopolymers from Biomass; Yang, S.T., El-Enshasy, H.A., Thongchul, N., Martin, Y., Eds.; Wiley: New York, NY, USA, 2013; pp. 91–109. [Google Scholar]
- Guo, B.; Zhang, Y.; Yu, G.; Lee, W.H.; Jin, Y.S.; Morgenroth, E. Two-stage acidic-alkaline hydrothermal pretreatment of lignocellulose for the high recovery of cellulose and hemicellulose sugars. Appl. Biochem. Biotechnol. 2013, 169, 1069–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Song, Y.; Liu, D. Enzymatic hydrolysis and simultaneous saccharification and fermentation of alkali/peracetic acid-pretreated sugarcane bagasse for ethanol and 2,3-butanediol production. Enzym. Microbiol. Technol. 2011, 49, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Gurpilhares, D.B.; Hasmann, F.A.; Pessoa, A.; Roberto, I.C. The behavior of key enzymes of xylose metabolism on the xylitol production by Candida guilliermondii grown in hemicellulosic hydrolyzate. J. Ind. Microbiol. Biotechnol. 2009, 36, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shekiro, J.; Franden, M.A.; Wang, W.; Zhang, M.; Kuhn, E.; Johnson, D.K.; Tucker, M.P. The impacts of deacetylation prior to dilute acid pretreatment on the bioethanol process. Biotechnol. Biofuels 2012, 5, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Kundu, C.; Lee, J.W. Optimization conditions for oxalic acid pretreatment of deacetylated yellow poplar for ethanol production. J. Ind. Eng. Chem. 2015, 32, 298–304. [Google Scholar] [CrossRef]
- Kundu, C.; Lee, H.J.; Lee, J.W. Enhanced bioethanol production from yellow poplar by deacetylation and oxalic acid pretreatment without detoxification. Bioresour. Technol. 2015, 178, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Brannt, W.T. A Practical Treatise on the Manufacture of Vinegar; Henry Carey Baird & Co.: Lancaster, PA, USA, 1914; pp. 316–317. [Google Scholar]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolyzates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Dunlop, A. Furfural formation and behavior. Ind. Eng. Chem. Res. 1948, 40, 204–209. [Google Scholar] [CrossRef]
- Ulbricht, R.J.; Northup, S.J.; Thomas, J.A. A review of 5-hydroxymethylfurfural (HMF) in parenteral solutions. Toxicol. Sci. 1984, 4, 843–853. [Google Scholar] [CrossRef]
- Guo, G.L.; Chen, W.H.; Chen, W.H.; Men, L.C.; Hwang, W.S. Characterization of dilute acid pretreatment of silvergrass for ethanol production. Bioresour. Technol. 2008, 99, 6046–6053. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wua, S.; Liu, L. Enzymatic saccharification of dilute acid pretreated eucalyptus chips for fermentable sugar production. Bioresour. Technol. 2012, 110, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Tmpleton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; NREL/TP-510–42623; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Tmpleton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510–42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012.



| Solid-to-Liquid Ratio | Ammonia Concentration (%) | Compositions (g/L) | |||
|---|---|---|---|---|---|
| Glucose | XMG 1 | Acetic Acid | Formic Acid | ||
| 1:4 | 2 | 0.36 | 1.91 | 8.16 | 0.73 |
| 4 | 0.19 | 0.30 | 6.20 | 0.67 | |
| 6 | 0.17 | 0.28 | 5.63 | 0.60 | |
| 8 | 0.21 | 0.30 | 4.84 | 0.59 | |
| 10 | 0.23 | 0.29 | 4.54 | 0.59 | |
| 1:6 | 2 | 0.21 | 0.32 | 4.94 | 0.31 |
| 4 | 0.12 | 0.17 | 4.21 | 0.39 | |
| 6 | 0.13 | 0.17 | 3.72 | 0.36 | |
| 8 | 0.15 | 0.18 | 3.47 | 0.38 | |
| 10 | 0.17 | 0.18 | 3.31 | 0.37 | |
| 1:8 | 2 | 0.15 | 0.34 | 3.67 | 0.21 |
| 4 | 0.10 | 0.12 | 3.08 | 0.26 | |
| 6 | 0.11 | 0.13 | 2.78 | 0.27 | |
| 8 | 0.12 | 0.13 | 2.64 | 0.29 | |
| 10 | 0.13 | 0.14 | 2.45 | 0.27 | |
| 1:10 | 2 | 0.14 | 0.49 | 3.17 | 0.18 |
| 4 | 0.11 | 0.09 | 2.51 | 0.22 | |
| 6 | 0.10 | 0.10 | 2.19 | 0.23 | |
| 8 | 0.10 | 0.09 | 1.99 | 0.20 | |
| 10 | 0.08 | 0.04 | 0.36 | 0.00 | |
| S/L Ratio | Ammonia Concentrations (%) | Solid Remaining (%) | Composition of Deacetylated YPS (%) | Removal through Deacetylation Treatment (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucan | XMG 1 | Acetyl Group | AIL 2 | AIA 3 | Glucan | XMG 1 | Acetyl Group | AIL 2 | AIA 3 | |||
| Initial | 100.00 | 38.52 | 14.32 | 4.42 | 18.86 | 3.51 | ||||||
| 1:4 | 2 | 69.09 | 49.28 | 18.13 | 0.45 | 25.43 | 0.00 | 11.61 | 12.53 | 92.97 | 6.84 | 100.00 |
| 4 | 68.32 | 50.17 | 17.89 | 0.33 | 25.93 | 0.00 | 11.02 | 14.65 | 94.90 | 6.07 | 100.00 | |
| 6 | 70.34 | 49.73 | 17.72 | 0.30 | 25.03 | 0.05 | 9.19 | 12.96 | 95.23 | 6.65 | 99.00 | |
| 8 | 66.97 | 50.73 | 17.85 | 0.26 | 25.39 | 0.00 | 11.80 | 16.52 | 96.06 | 9.84 | 100.00 | |
| 10 | 75.82 | 49.31 | 17.38 | 0.52 | 25.63 | 0.21 | 2.94 | 7.98 | 91.08 | 0.00 | 95.46 | |
| 1:6 | 2 | 77.02 | 49.49 | 17.97 | 0.49 | 25.97 | 0.23 | 1.05 | 3.35 | 91.46 | 0.00 | 94.95 |
| 4 | 75.09 | 48.93 | 17.26 | 0.24 | 25.82 | 0.51 | 4.62 | 9.49 | 95.92 | 0.00 | 89.09 | |
| 6 | 73.93 | 48.69 | 17.02 | 0.24 | 24.67 | 0.60 | 6.55 | 12.13 | 95.99 | 3.30 | 87.36 | |
| 8 | 71.74 | 47.61 | 16.58 | 0.44 | 24.36 | 0.66 | 11.33 | 16.94 | 92.86 | 7.34 | 86.51 | |
| 10 | 68.83 | 50.53 | 17.70 | 0.39 | 27.51 | 0.00 | 9.71 | 14.92 | 93.93 | 0.00 | 100.00 | |
| 1:8 | 2 | 74.13 | 49.33 | 18.04 | 1.36 | 24.90 | 0.47 | 5.07 | 6.61 | 77.19 | 2.13 | 90.07 |
| 4 | 67.58 | 51.03 | 17.95 | 0.91 | 25.13 | 0.00 | 13.12 | 17.80 | 86.50 | 9.95 | 100.00 | |
| 6 | 68.95 | 50.01 | 17.67 | 0.00 | 24.97 | 0.37 | 10.48 | 14.92 | 100.00 | 8.71 | 92.73 | |
| 8 | 71.10 | 51.19 | 17.90 | 0.00 | 26.61 | 0.30 | 5.51 | 11.13 | 100.00 | 0.00 | 93.92 | |
| 10 | 68.93 | 50.89 | 17.81 | 0.00 | 27.85 | 0.65 | 8.93 | 14.27 | 100.00 | 0.00 | 87.24 | |
| 1:10 | 2 | 68.24 | 50.15 | 18.14 | 1.33 | 27.39 | 0.51 | 11.16 | 13.56 | 79.47 | 0.90 | 90.08 |
| 4 | 71.04 | 49.94 | 17.79 | 0.00 | 26.44 | 0.92 | 7.90 | 11.75 | 100.00 | 0.41 | 81.38 | |
| 6 | 67.71 | 51.72 | 18.07 | 0.00 | 25.82 | 0.20 | 9.09 | 14.56 | 100.00 | 7.30 | 96.14 | |
| 8 | 71.30 | 51.07 | 17.70 | 0.00 | 25.43 | 0.40 | 5.47 | 11.87 | 100.00 | 3.86 | 91.87 | |
| 10 | 65.50 | 51.32 | 17.93 | 0.00 | 24.91 | 0.54 | 12.73 | 17.99 | 100.00 | 13.49 | 89.92 | |
| Components in Solid Residue | Contents (%) | Components in Liquid Phase | Concentrations in Hydrolyzate (g/L) | ||
|---|---|---|---|---|---|
| Deacetylated | Non-Deacetylated | Deacetylated | Non-Deacetylated | ||
| Solid remaining | 67.94 | 66.89 | Glucose | 1.36 | 1.01 |
| Glucan | 54.15 | 46.28 | XMG 4 | 11.31 | 3.37 |
| XMG 1 | 9.18 | 13.82 | arabinose | N/D 5 | 0.29 |
| Arabinan | 0.16 | 0.71 | Formic acid | 0.22 | 0.65 |
| AIL 2 | 29.00 | 25.96 | Acetic acid | 0.09 | 1.35 |
| AIA 3 | 1.08 | 0.66 | Levulinic acid | N/D 5 | 0.27 |
| Acetyl group | 0.23 | 3.70 | 5-HMF | N/D | 0.03 |
| Sum | 93.80 | 90.98 | Furfural | N/D | 0.31 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J.; Kim, T.H.; Oh, K.K. Deacetylation Followed by Fractionation of Yellow Poplar Sawdust for the Production of Toxicity-Reduced Hemicellulosic Sugar for Ethanol Fermentation. Energies 2018, 11, 404. https://doi.org/10.3390/en11020404
Kim SJ, Kim TH, Oh KK. Deacetylation Followed by Fractionation of Yellow Poplar Sawdust for the Production of Toxicity-Reduced Hemicellulosic Sugar for Ethanol Fermentation. Energies. 2018; 11(2):404. https://doi.org/10.3390/en11020404
Chicago/Turabian StyleKim, Seong Ju, Tae Hyun Kim, and Kyeong Keun Oh. 2018. "Deacetylation Followed by Fractionation of Yellow Poplar Sawdust for the Production of Toxicity-Reduced Hemicellulosic Sugar for Ethanol Fermentation" Energies 11, no. 2: 404. https://doi.org/10.3390/en11020404






