Biological Pretreatment of Mexican Caribbean Macroalgae Consortiums Using Bm-2 Strain (Trametes hirsuta) and Its Enzymatic Broth to Improve Biomethane Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Macroalgae Consortium Biomass and Characterization
2.2. Fungal Strain and Culture Conditions
2.3. Fungal Broth Production (Fb)
2.4. Biologic Pretreatment
2.5. Fourier Transforms Infrared (FT-IR) Analysis
2.6. Scanning Electron Microscopy (SEM)
2.7. Biochemical Methane Potential (BMP) Tests
2.8. Analytical Methods
2.9. Statistical Analysis
2.10. Numerical Calculations
3. Results and Discussion
3.1. Biological Pretreatment
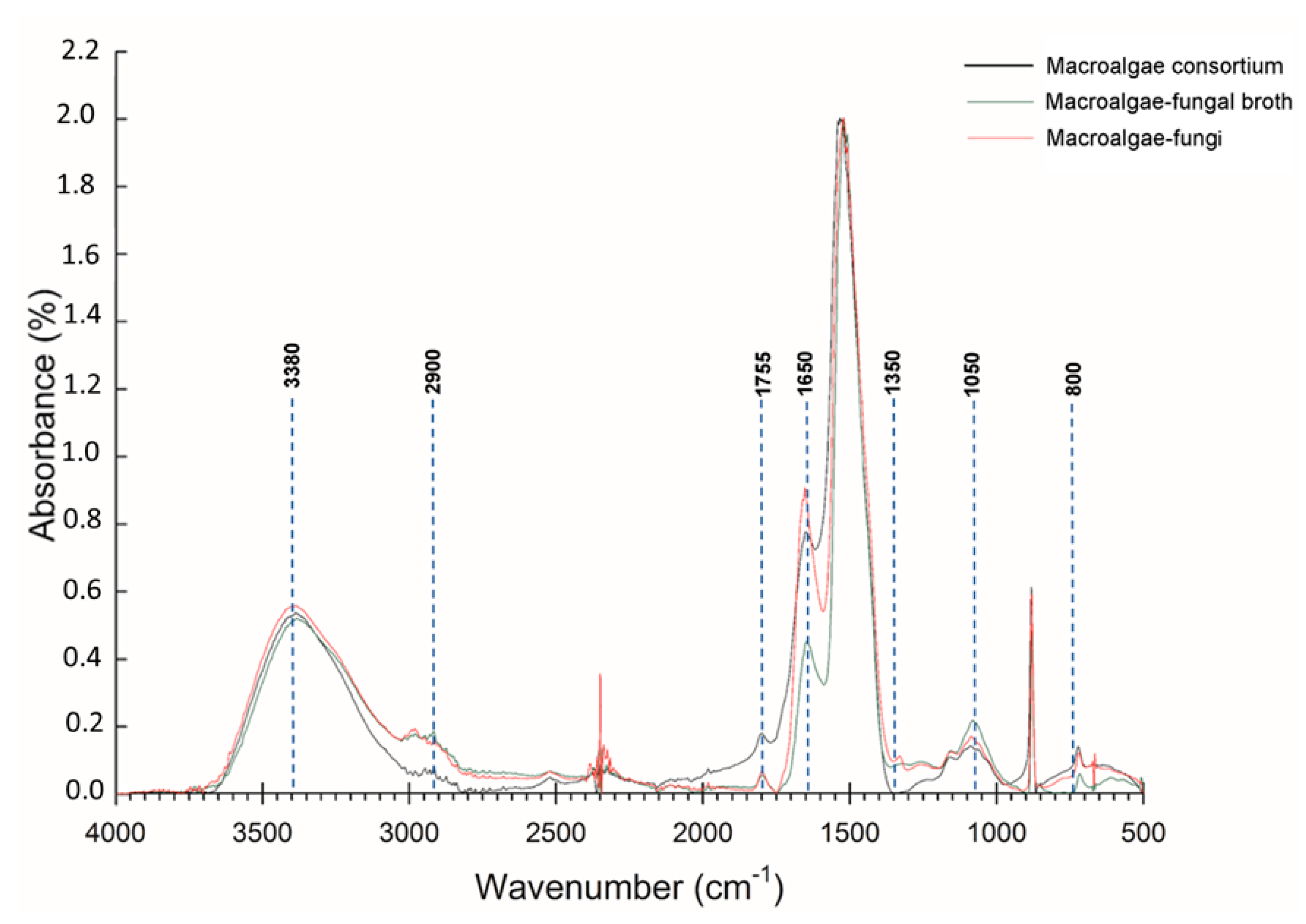
3.2. FT-IR and SEM Analysis Biology Pretreatment
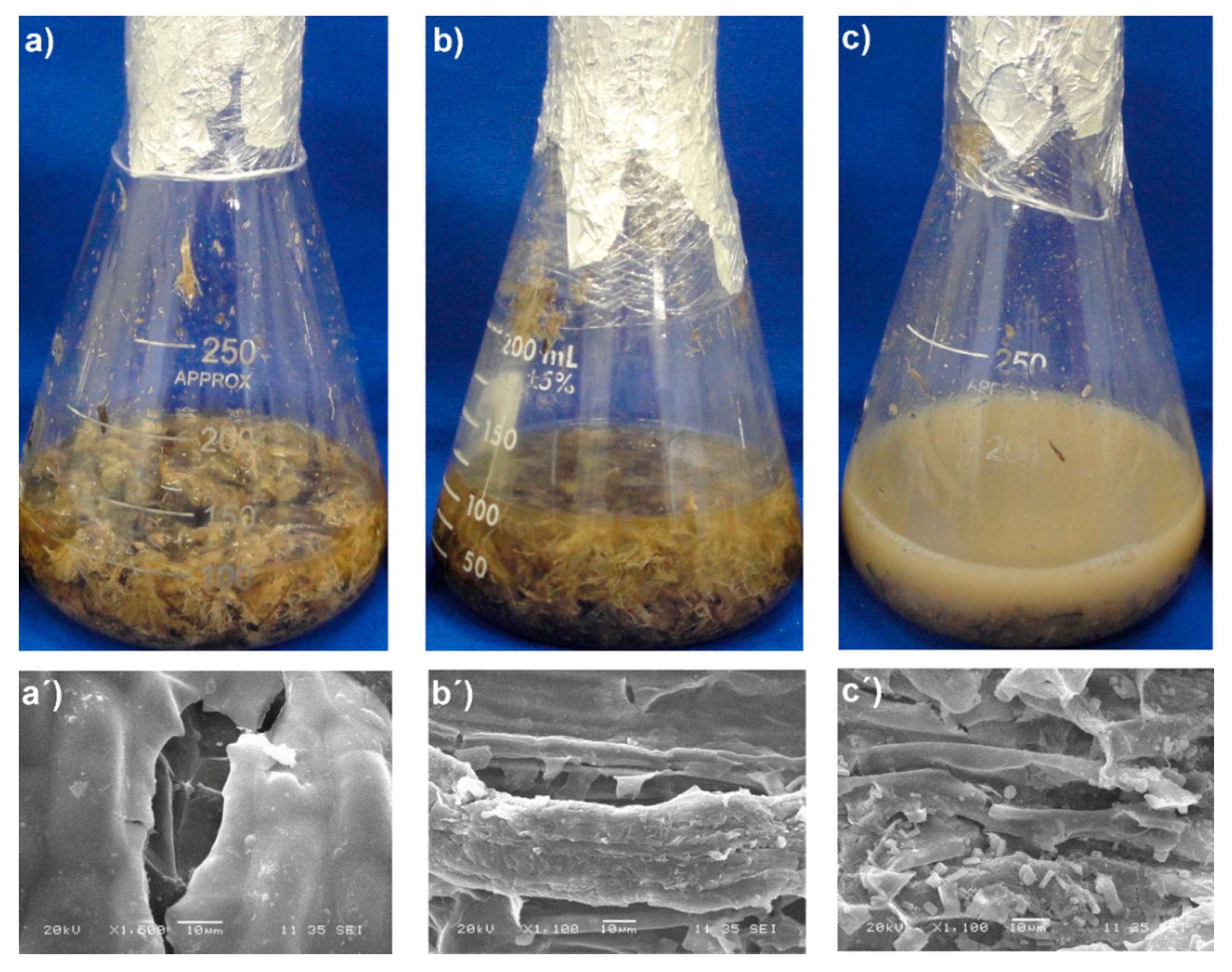
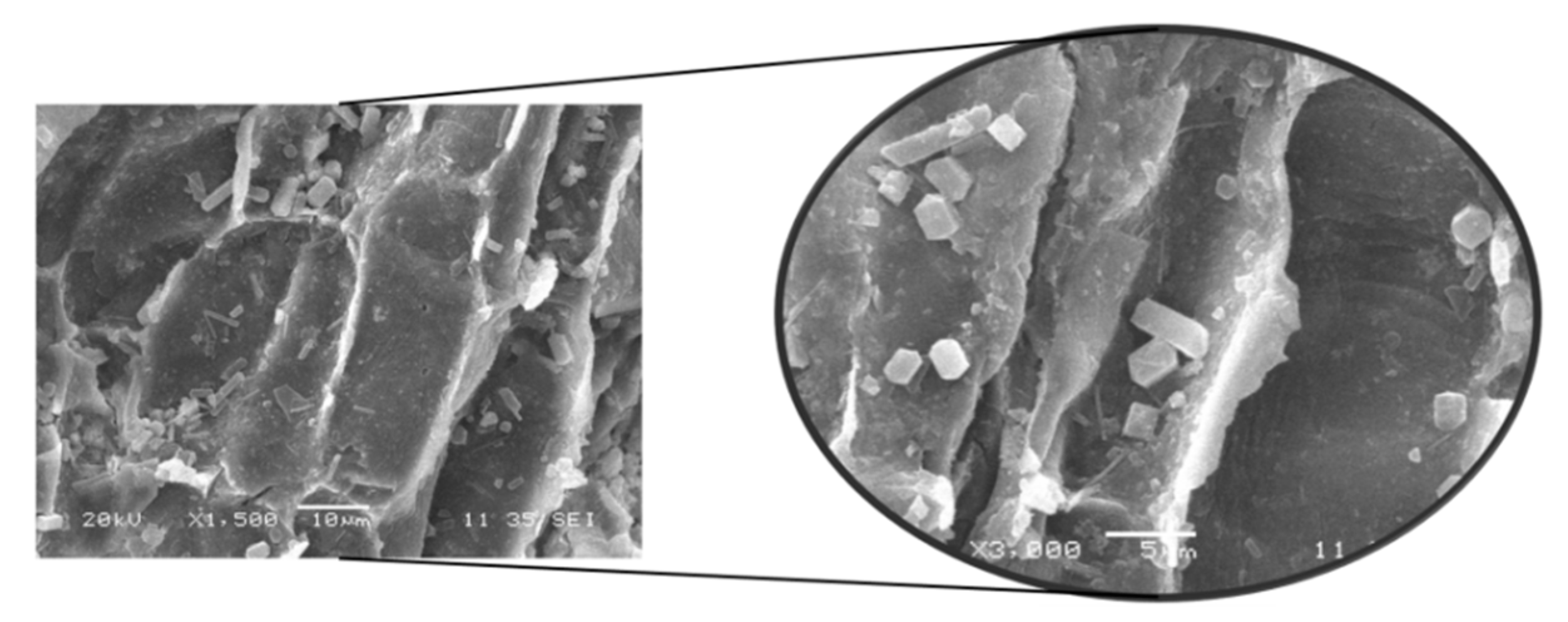
3.3. SEM Analysis of Untreated and Pretreated Macroalgae Consortium
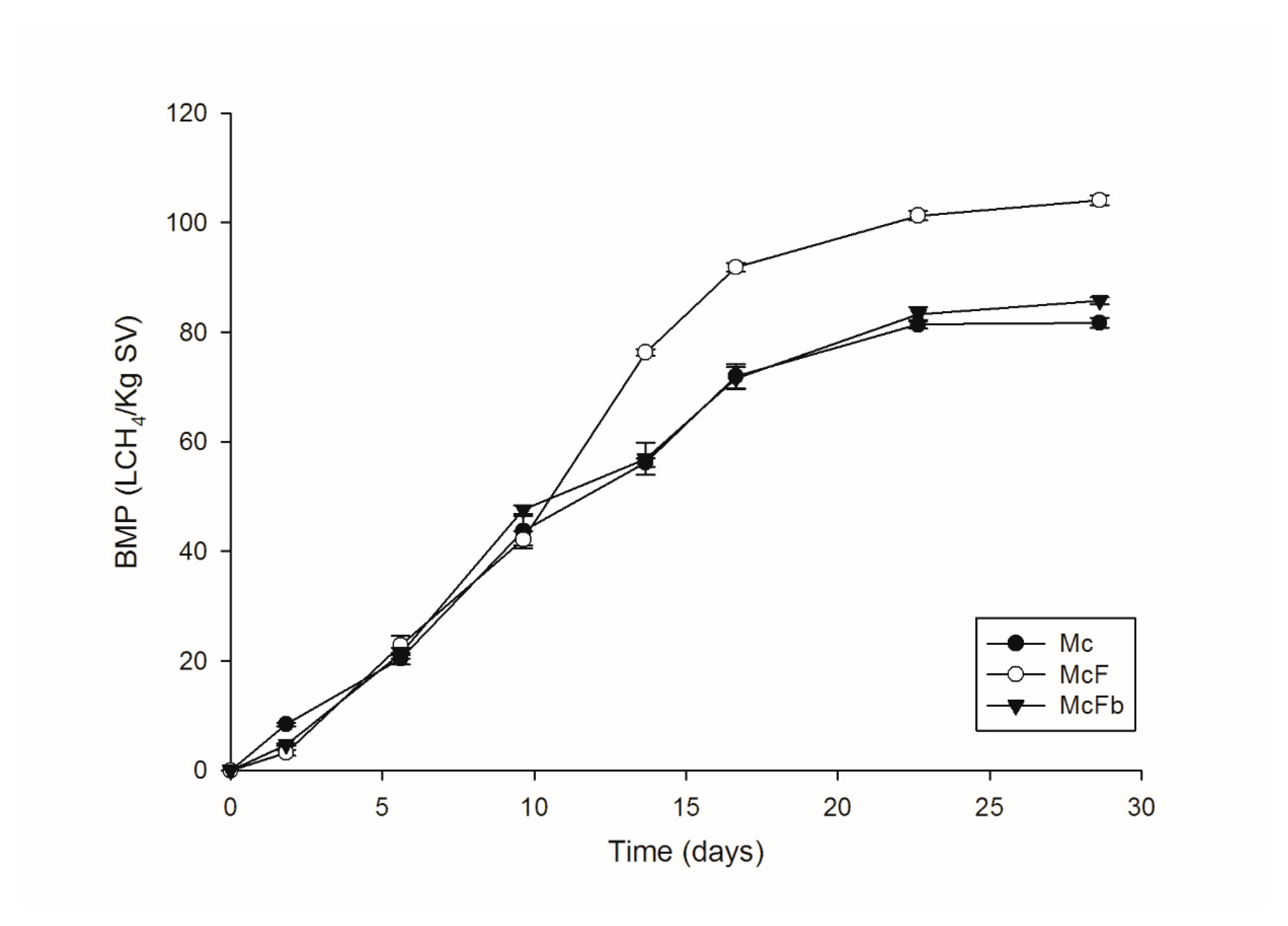
3.4. Production of Biogas in BMP Test
Macroalgae Consortiums and BMP Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause-Jensen, D.; Duarte, C.M. Substantial role of Macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Barbot, Y.N.; Al-Ghaili, H.; Benz, R. A review on the valorization of macroalgal wastes for biomethane production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Harvey, P.J. Golden tides: Problem or golden opportunity? The valorisation of sargassum from beach inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Potential of seaweed as a feedstock for renewable gaseous fuel production in Ireland. Renew. Sustain. Energy Rev. 2017, 68, 136–146. [Google Scholar] [CrossRef]
- Montingelli, M.E.; Benyounis, K.Y.; Quilty, B.; Stokes, J.; Olabi, A.G. Optimisation of biogas production from the Macroalgae Laminaria sp. at different periods of harvesting in Ireland. Appl. Energy 2016, 177, 671–682. [Google Scholar] [CrossRef]
- Tapia-Tussell, R.; Pérez-Brito, D.; Torres-Calzada, C.; Cortés-Velázquez, A.; Alzate-Gaviria, L.; Chablé-Villacís, R.; Solís-Pereira, S. Laccase Gene Expression and Vinasse Biodegradation by Trametes hirsuta Strain Bm-2. Molecules 2015, 20, 15147–15157. [Google Scholar] [CrossRef] [PubMed]
- Hom-Diaz, A.; Passos, F.; Ferrer, I.; Vicent, T.; Blánquez, P. Enzymatic pretreatment of microalgae using fungal broth from Trametes versicolor and commercial laccase for improved biogas production. Algal Res. 2016, 19, 184–188. [Google Scholar] [CrossRef]
- Tapia-Tussell, R.; Pérez-Brito, D.; Rojas-Herrera, R.; Cortes-Velazquez, A.; Rivera-Muñoz, G.; Solis-Pereira, S. New laccase-producing fungi isolates with biotechnological potential in dye decolorization. Afr. J. Biotechnol. 2011, 10, 10134–10142. [Google Scholar]
- Tien, M.; Kirk, T.K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc. Natl. Acad. Sci. USA 1984, 81, 2280–2284. [Google Scholar] [CrossRef] [PubMed]
- Kirk, T.K.; Croan, S.; Tien, M.; Murtagh, K.E.; Farrell, R.L. Production of multiple ligninases by Phanerochaete chrysosporium: Effect of selected growth conditions and use of a mutant strain. Enzym. Microb. Technol. 1986, 8, 27–32. [Google Scholar] [CrossRef]
- Wolfenden, B.S.; Willson, R.L. Radical-cations as reference chromogens in kinetic studies of ono-electron transfer reactions: Pulse radiolysis studies of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate). J. Chem. Soc. Perkin Trans. 2 1982, 0, 805–812. [Google Scholar] [CrossRef]
- Valero, D.; Montes, J.A.; Rico, J.L.; Rico, C. Influence of headspace pressure on methane production in Biochemical Methane Potential (BMP) tests. Waste Manag. 2016, 48, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Poggi-Varaldo, H.; Valdés, L.; Esparza-Garcia, F.; Fernández-Villagómez, G. Solid substrate anaerobic co-digestion of paper mill sludge, biosolids, and municipal solid waste. Water Sci. Technol. 1997, 35, 197–204. [Google Scholar]
- Association, A.P.H.; Association, A.W.W.; Federation, W.P.C.; Federation, W.E. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1915; Volume 2. [Google Scholar]
- Zapata-Castillo, P.; Villalonga-Santana, L.; Islas-Flores, I.; Rivera-Muñoz, G.; Ancona-Escalante, W.; Solís-Pereira, S. Synergistic action of laccases from Trametes hirsuta Bm2 improves decolourization of indigo carmine. Lett. Appl. Microbiol. 2015, 61, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sulaiman, O.; Hashim, R.; Peng, L.C.; Singh, R.P. Evaluating biopulping as an alternative application on oil palm trunk using the white-rot fungus Trametes versicolor. Int. Biodeterior. Biodegrad. 2013, 82, 96–103. [Google Scholar] [CrossRef]
- Yang, H.; Wang, K.; Song, X.; Xu, F.; Sun, R.-C. Enhanced enzymatic hydrolysis of triploid poplar following stepwise acidic pretreatment and alkaline fractionation. Process Biochem. 2012, 47, 619–625. [Google Scholar] [CrossRef]
- Ju, Y.-H.; Huynh, L.-H.; Kasim, N.S.; Guo, T.-J.; Wang, J.-H.; Fazary, A.E. Analysis of soluble and insoluble fractions of alkali and subcritical water treated sugarcane bagasse. Carbohydr. Polym. 2011, 83, 591–599. [Google Scholar] [CrossRef]
- Silva, T.A.L.; Zamora, H.D.Z.; Varão, L.H.R.; Prado, N.S.; Baffi, M.A.; Pasquini, D. Effect of Steam Explosion Pretreatment Catalysed by Organic Acid and Alkali on Chemical and Structural Properties and Enzymatic Hydrolysis of Sugarcane Bagasse. Waste Biomass Valoriz. 2017, 1–11. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar]
- Fillat, Ú.; Ibarra, D.; Eugenio, M.E.; Moreno, A.D.; Tomás-Pejó, E.; Martín-Sampedro, R. Laccases as a Potential Tool for the Efficient Conversion of Lignocellulosic Biomass: A Review. Fermentation 2017, 3, 17. [Google Scholar] [CrossRef]
- Carrere, H.; Passos, F.; Antonopoulou, G.; Rouches, E.; Affes, R.; Battimelli, A.; Ferrer, I.; Steyer, J.; Lyberatos, G. Enhancement of Anaerobic Digestion Performance: Which Pretreatment for which Waste? In Proceedings of the 5th International Conference on Engineering for Waste and Biomass Valorisation (WasteEng14), Rio de Janeiro, Brazil, 25–28 August 2014. [Google Scholar]
- Adams, J.; Ross, A.Á.; Anastasakis, K.; Hodgson, E.; Gallagher, J.; Jones, J.; Donnison, I. Seasonal variation in the chemical composition of the bioenergy feedstock Laminaria digitata for thermochemical conversion. Bioresour. Technol. 2011, 102, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Alves, M.; Costa, J. Optimization of biogas production from Sargassum sp. using a design of experiments to assess the co-digestion with glycerol and waste frying oil. Bioresour. Technol. 2015, 175, 480–485. [Google Scholar] [PubMed]
- Ross, A.; Jones, J.; Kubacki, M.; Bridgeman, T. Classification of Macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Wall, D.M.; Herrmann, C.; Xia, A.; Murphy, J.D. What is the gross energy yield of third generation gaseous biofuel sourced from seaweed? Energy 2015, 81, 352–360. [Google Scholar] [CrossRef]
- Hanssen, J.F.; Indergaard, M.; Østgaard, K.; Bævre, O.A.; Pedersen, T.A.; Jensen, A. Anaerobic digestion of Laminaria spp. and Ascophyllum nodosum and application of end products. Biomass 1987, 14, 1–13. [Google Scholar] [CrossRef]
- Apostolidis, E.; Karayannakidis, P.D.; Kwon, Y.-I.; Lee, C.M.; Seeram, N.P. Seasonal variation of phenolic antioxidant-mediated α-glucosidase inhibition of Ascophyllum nodosum. Plant Foods Hum. Nutr. 2011, 66, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Seasonal variation of chemical composition and biomethane production from the brown seaweed Ascophyllum nodosum. Bioresour. Technol. 2016, 216, 219–226. [Google Scholar] [CrossRef] [PubMed]
| Component Name | Average (%) |
|---|---|
| Lignin | 15.6 ± 0.51 |
| Cellulose | 31.2 ± 1.2 |
| Hemicellulose | 10.5 ± 0.83 |
| Phenols | 18.7 ± 1.5 |
| Extractable in solvent | 3.1 ± 0.04 |
| Ash | 35.5 ± 1.9 |
| Trial | Methane Content (%) | BMP L CH4·kg VS−1 |
|---|---|---|
| Macroalgae consortium | 40 ± 1.0 | 81 ± 1.2 |
| Macroalgae consortium + enzymatic broth | 46 ± 0.5 | 86 ± 0.7 |
| Macroalgae consortium + fungi | 52 ± 1.0 | 104 ± 1.4 |
| Component Name | Average (ppm) |
|---|---|
| Fe | 584.9 ± 3 |
| Mn | 12.9 ± 2 |
| Cu | 7.5 ± 0.2 |
| Zn | 60.1 ± 0.1 |
| Na | 45,210 ± 25 |
| K | 28,500 ± 55 |
| Cd | <0.1 |
| Mg | 4806.1 ± 20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia-Tussell, R.; Avila-Arias, J.; Domínguez Maldonado, J.; Valero, D.; Olguin-Maciel, E.; Pérez-Brito, D.; Alzate-Gaviria, L. Biological Pretreatment of Mexican Caribbean Macroalgae Consortiums Using Bm-2 Strain (Trametes hirsuta) and Its Enzymatic Broth to Improve Biomethane Potential. Energies 2018, 11, 494. https://doi.org/10.3390/en11030494
Tapia-Tussell R, Avila-Arias J, Domínguez Maldonado J, Valero D, Olguin-Maciel E, Pérez-Brito D, Alzate-Gaviria L. Biological Pretreatment of Mexican Caribbean Macroalgae Consortiums Using Bm-2 Strain (Trametes hirsuta) and Its Enzymatic Broth to Improve Biomethane Potential. Energies. 2018; 11(3):494. https://doi.org/10.3390/en11030494
Chicago/Turabian StyleTapia-Tussell, Raúl, Julio Avila-Arias, Jorge Domínguez Maldonado, David Valero, Edgar Olguin-Maciel, Daisy Pérez-Brito, and Liliana Alzate-Gaviria. 2018. "Biological Pretreatment of Mexican Caribbean Macroalgae Consortiums Using Bm-2 Strain (Trametes hirsuta) and Its Enzymatic Broth to Improve Biomethane Potential" Energies 11, no. 3: 494. https://doi.org/10.3390/en11030494




