Effects of Supply Power and Environmental Conditions on Urea Coolant Melting Phenomena in Urea Supply Lines in Vehicles
Abstract
:1. Introduction
2. Research Method
2.1. Structure of the Heating System in the Urea Supply Line
2.2. Heat Transfer Circuit of the Urea Supply Line
2.3. Numerical Analysis Setting
3. Results and Discussion
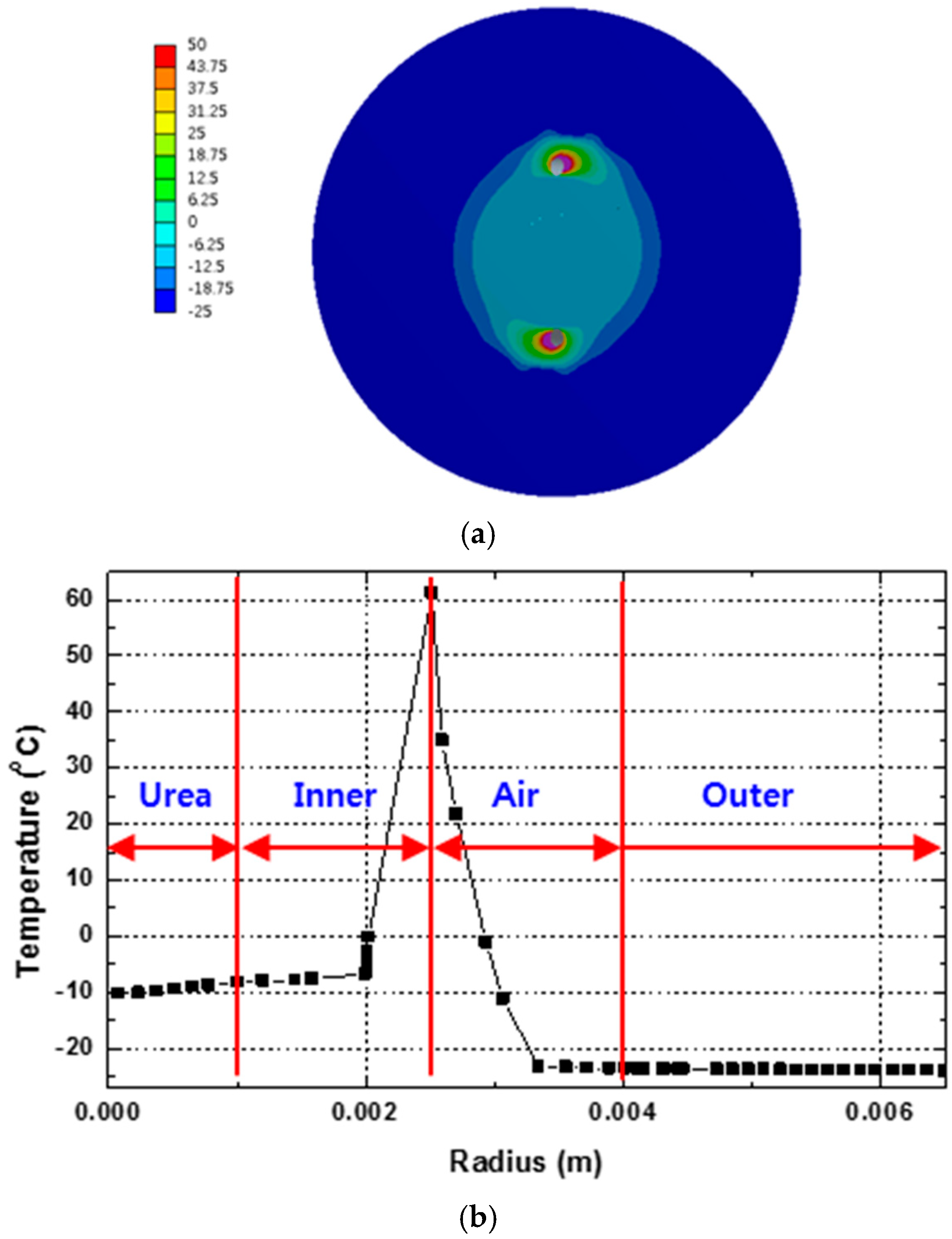
3.1. Urea Melting Phenomena in the Supply Line
3.2. Effect of Ambient Conditions
4. Conclusions
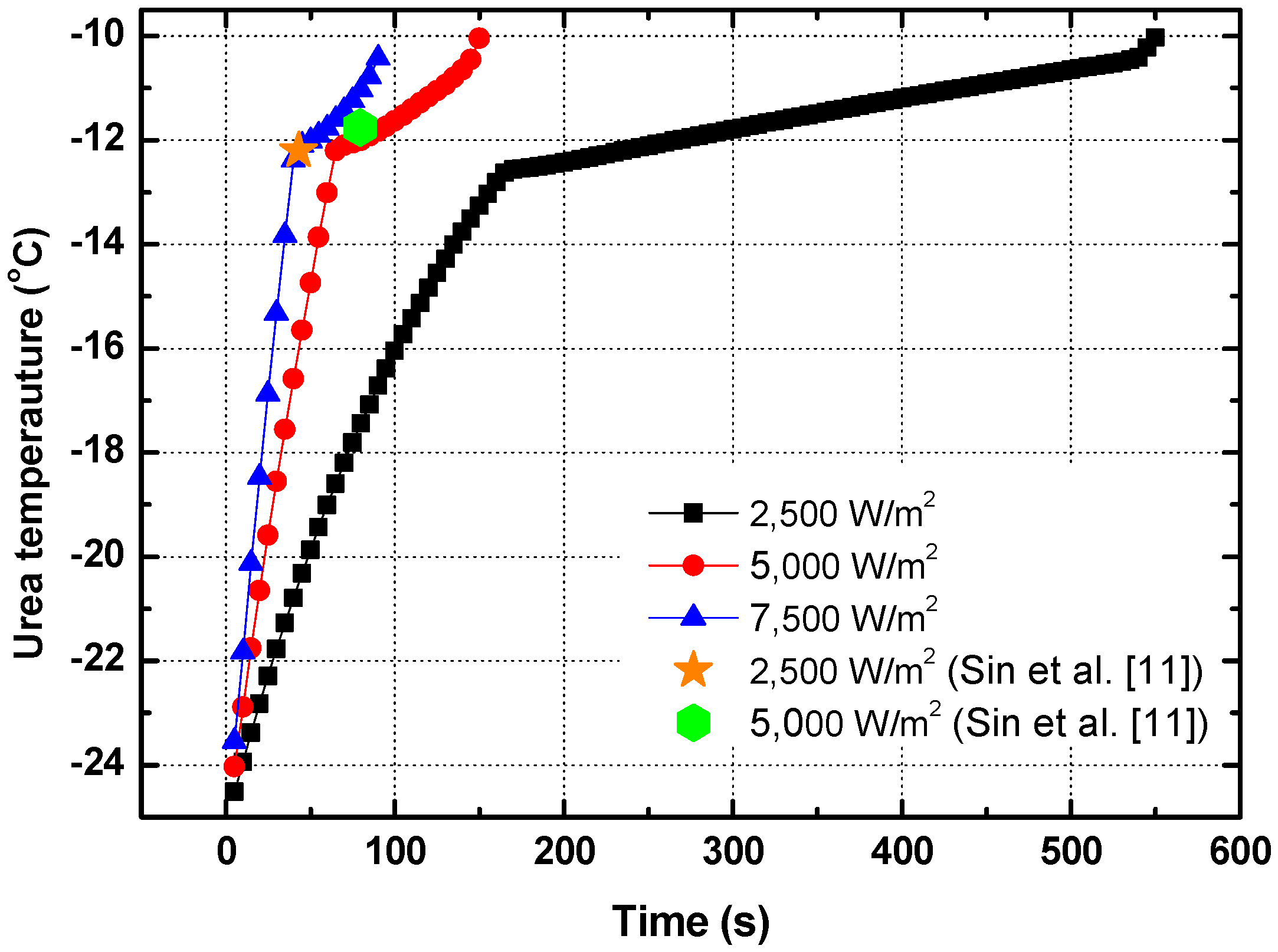
- There is a limit to the power supply to the heater because of the limitation of the output of the vehicle battery. Nevertheless, the urea melted in less than 600 s at Tamb = −25 °C, even at the lowest power supply ().
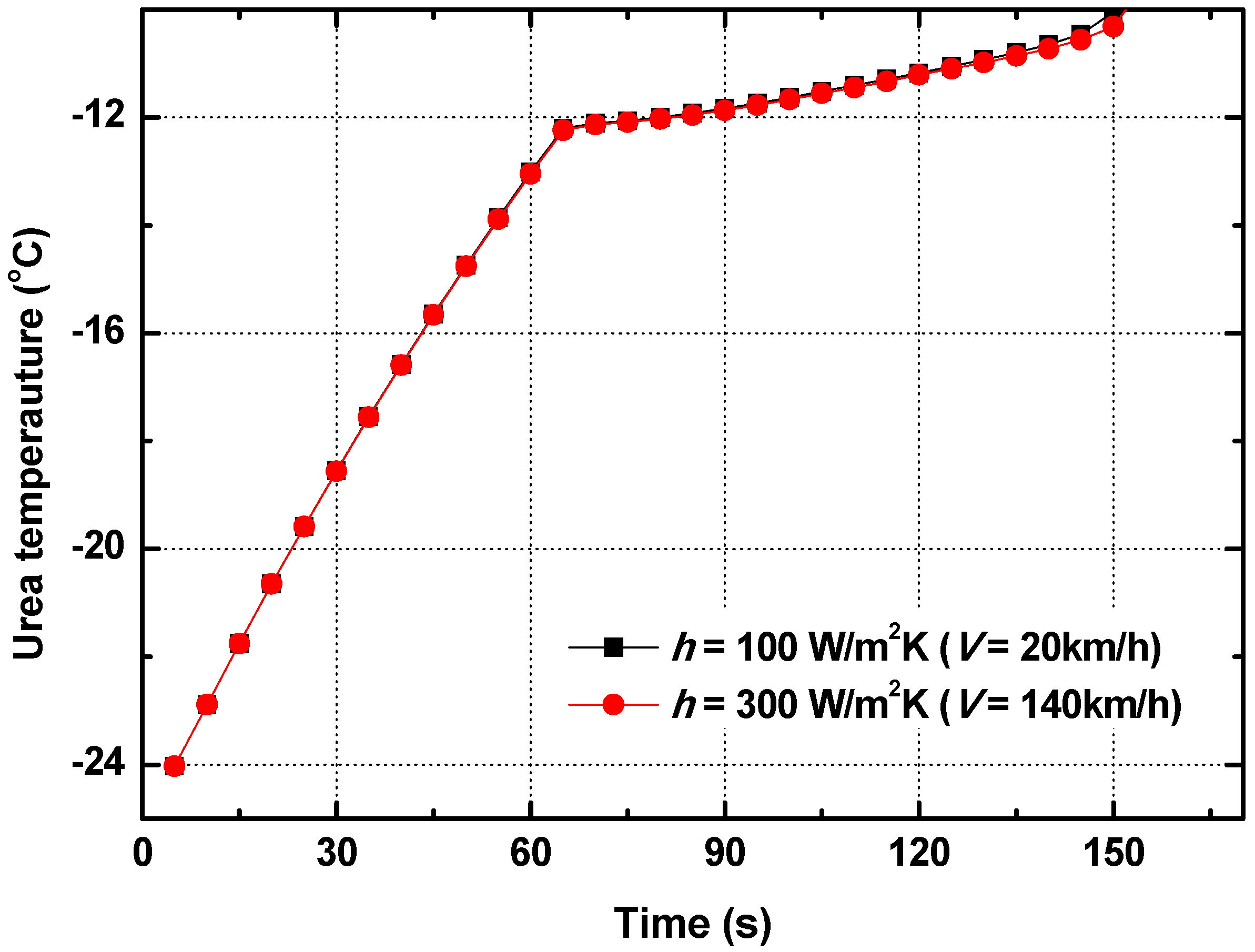
- When the vehicle speed increased, the convective heat transfer coefficient on the external surface of the tube also increased. However, the thermal resistance of convective heat transfer on the external surface was not a large portion of the total thermal resistance of the urea supply tube. As a result, the urea melting time did not change, even though the vehicle speed increased.
- The urea melting time was largely affected by the ambient temperature. When the ambient temperature was −50 °C, the urea did not melt in the case due to the match of the heat balance between the heat supply and heat loss to the external surface of the tube. Therefore, the power supply should be controlled for urea melting by the ambient temperature.
Author Contributions
Acknowledgments
Conflicts of Interest
Nomenclature
| Cavg | Average specific heat of urea |
| CL | Specific heat of liquid urea |
| CS | Specific heat of solid urea |
| H | Enthalpy of urea |
| HTR | Enthalpy of urea between solid/liquid temperature |
| he | Convective heat transfer coefficient on external surface |
| kair | Conductivity of air |
| ki | Conductivity of TPE |
| ko | Conductivity of PPA |
| kurea | Conductivity of urea |
| L | Length of tube |
| Nu | Nusselt number |
| Pr | Prandtl number |
| Heat flux | |
| Rc | Resistance of copper |
| Re | Reynolds number |
| ri1 | Inner diameter of inner pipe |
| ri2 | Outer diameter of inner pipe |
| rair | Inner diameter of outer pipe |
| rout | Outer diameter of outer pipe |
| T | Temperature |
| V | Voltage |
| Density of urea |
References
- A Cleaner and Safer Future with SCR. AutoTechnology 2004, 4, 28–30. [CrossRef]
- Willems, F.; Cloudt, R.; Van Den Eijnden, E.; Van Genderen, M.; Verbeek, R.; De Jager, B.; Boomsma, W. Is closed-loop SCR control required to meet future emission targets? In SAE Technical Paper; SAE International: Hong Kong, China, 2007. [Google Scholar]
- Lee, S.-Y.; Kim, M.-Y.; Lee, C.-H.; Park, Y.-B. Numerical Investigation of the Urea Melting and Heat Transfer Characteristics with Three Different Types of Coolant Heaters. Trans. Korean Soc. Automot. Eng. 2012, 20, 125–132. [Google Scholar] [CrossRef]
- Van Helden, R.; Verbeek, R.; Willems, F.; van der Welle, R. Optimization of Urea SCR deNOx Systems for HD Diesel Engines. In SAE Technical Paper; SAE International: Hong Kong, China, 2004. [Google Scholar]
- Choi, B.-C.; Kim, Y.K.; Jhung, W.-N.; Lee, C.-H.; Hwang, C.-Y. Experimental investigation on melting characteristics of frozen urea-water-solutions for a diesel SCR de-NOx-system. Appl. Therm. Eng. 2013, 50, 1235–1245. [Google Scholar] [CrossRef]
- Birkhold, F.; Meingast, U.; Wassermann, P.; Deutschmann, O. Modeling and simulation of the injection of urea-water-solution for automotive SCR DeNOx-systems. Appl. Catal. B Environ. 2007, 70, 119–127. [Google Scholar] [CrossRef]
- Aus der Wiesche, S. Numerical heat transfer and thermal engineering of AdBlue (SCR) tanks for combustion engine emission reduction. Appl. Therm. Eng. 2007, 27, 1790–1798. [Google Scholar] [CrossRef]
- AdBlue, Technical leaflet, M 6221 e, November 2006. Available online: http://www.gabriels.be/sites/gabriels/files/pdf/technische_fiche_adblue-_engels.pdf (accessed on 29 April 2018).
- ANSYS, Release 18.0 User’s Guide. 2017. Available online: https://support.ansys.com/AnsysCustomerPortal/en_us/Knowledge+Resources/Tutorials+&+Training+Materials (accessed on 29 April 2018).
- Incropera, F.; DeWitt, D.; Bergman, T.; Lavine, A. Fundamentals of Heat and Mass Transfer, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 423–428. [Google Scholar]
- Sin, Y.; Kim, H.; Han, S.; Choo, S. Thawing characteristics of the urea solution in accordance with the heating voltage and ambient temperature. In Proceeding of KSAE; The Korea Society of Automotive Engineers: Seoul, Korea, 2016; pp. 109–111. [Google Scholar]
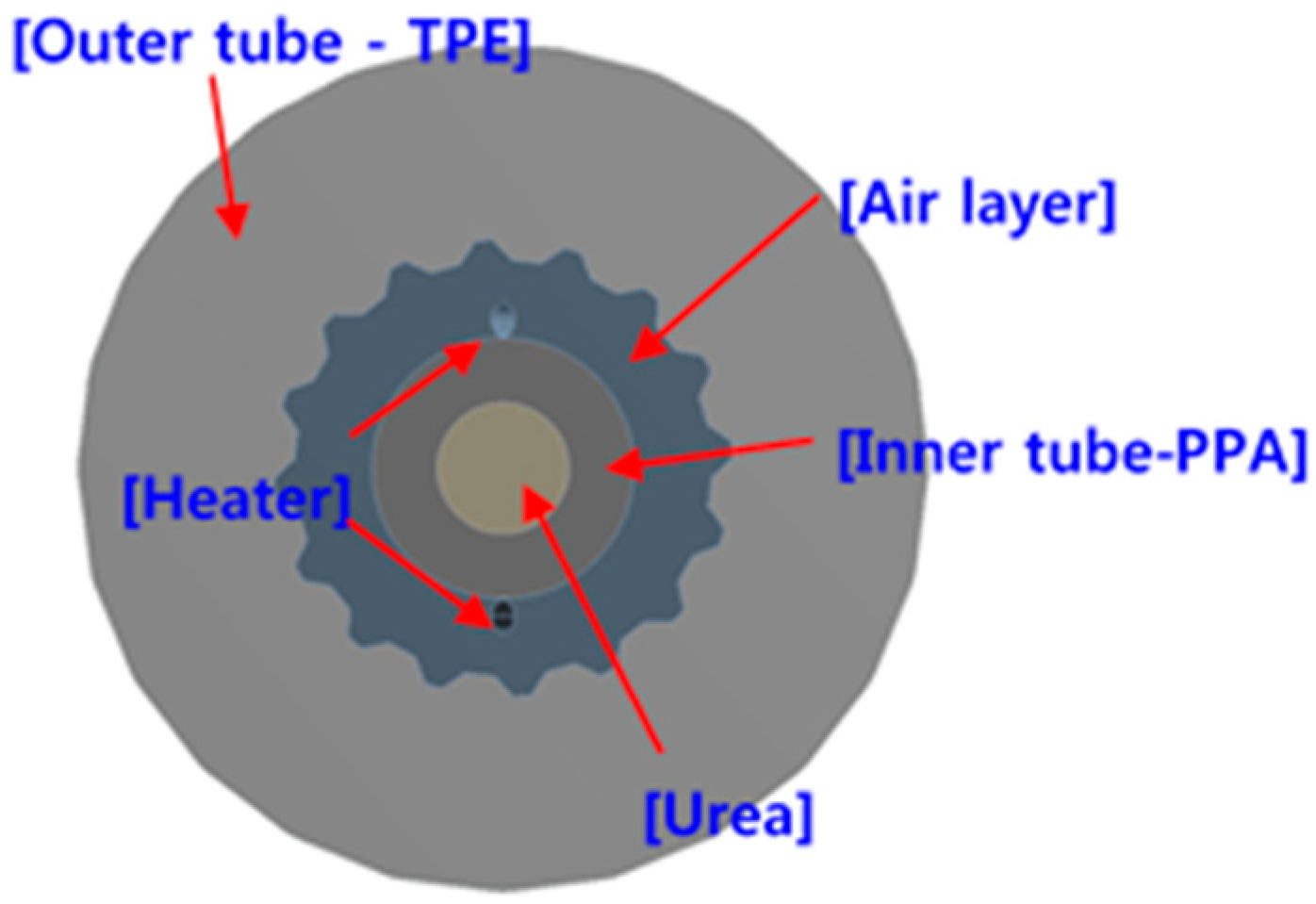
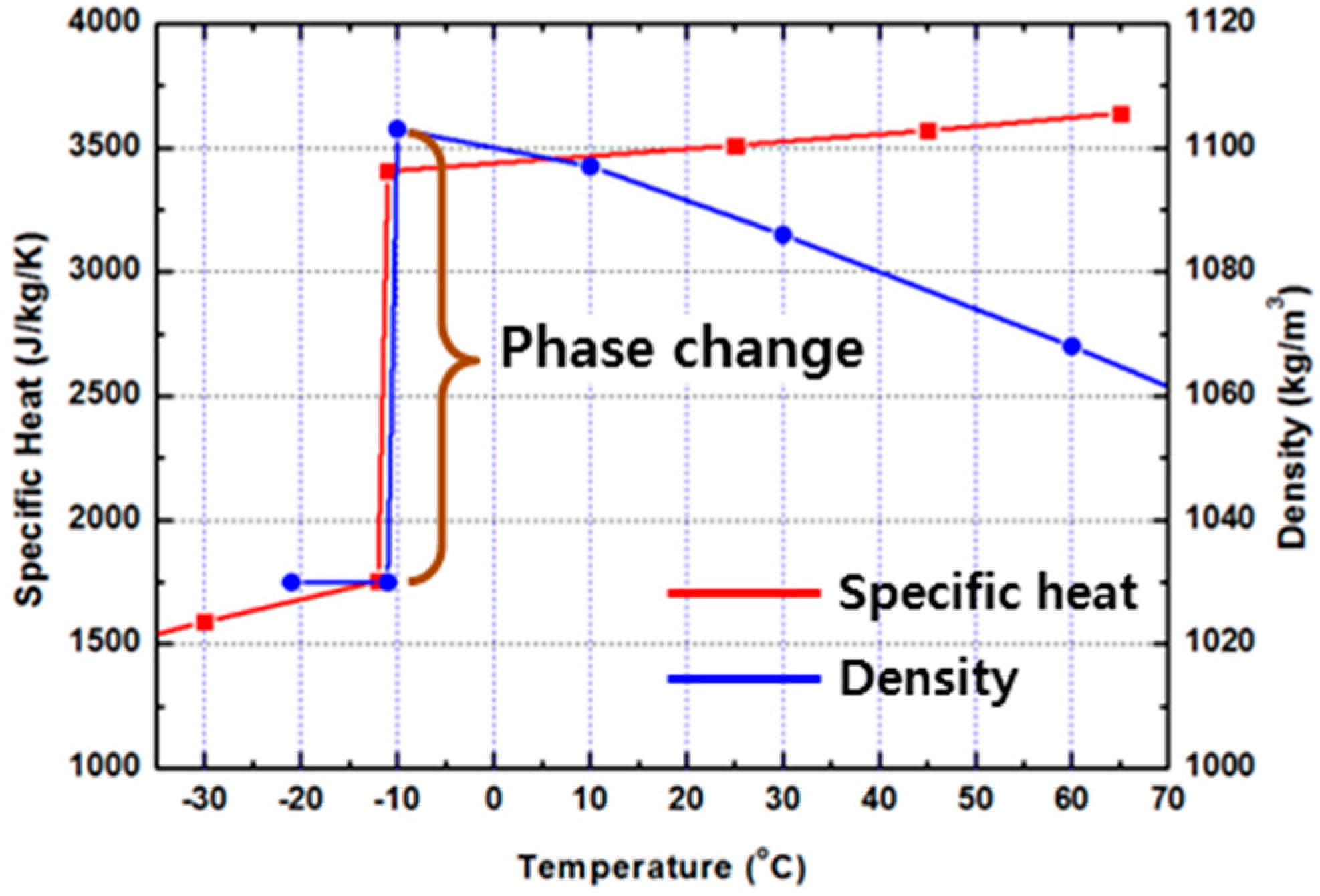
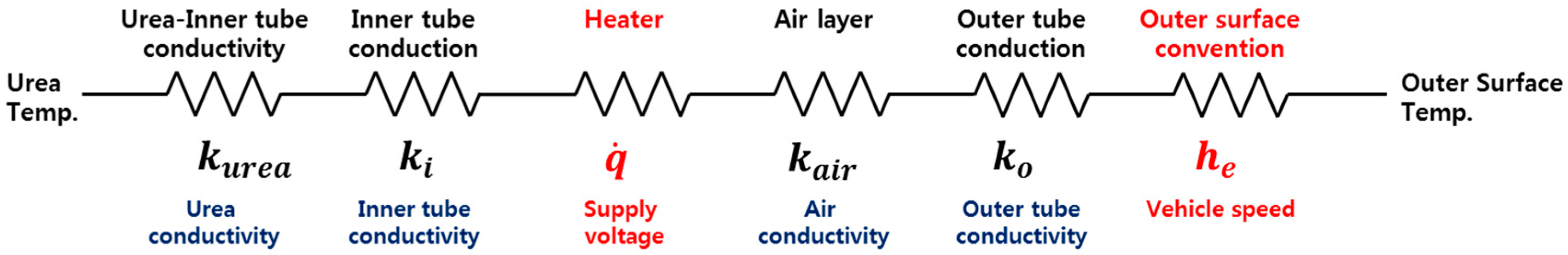
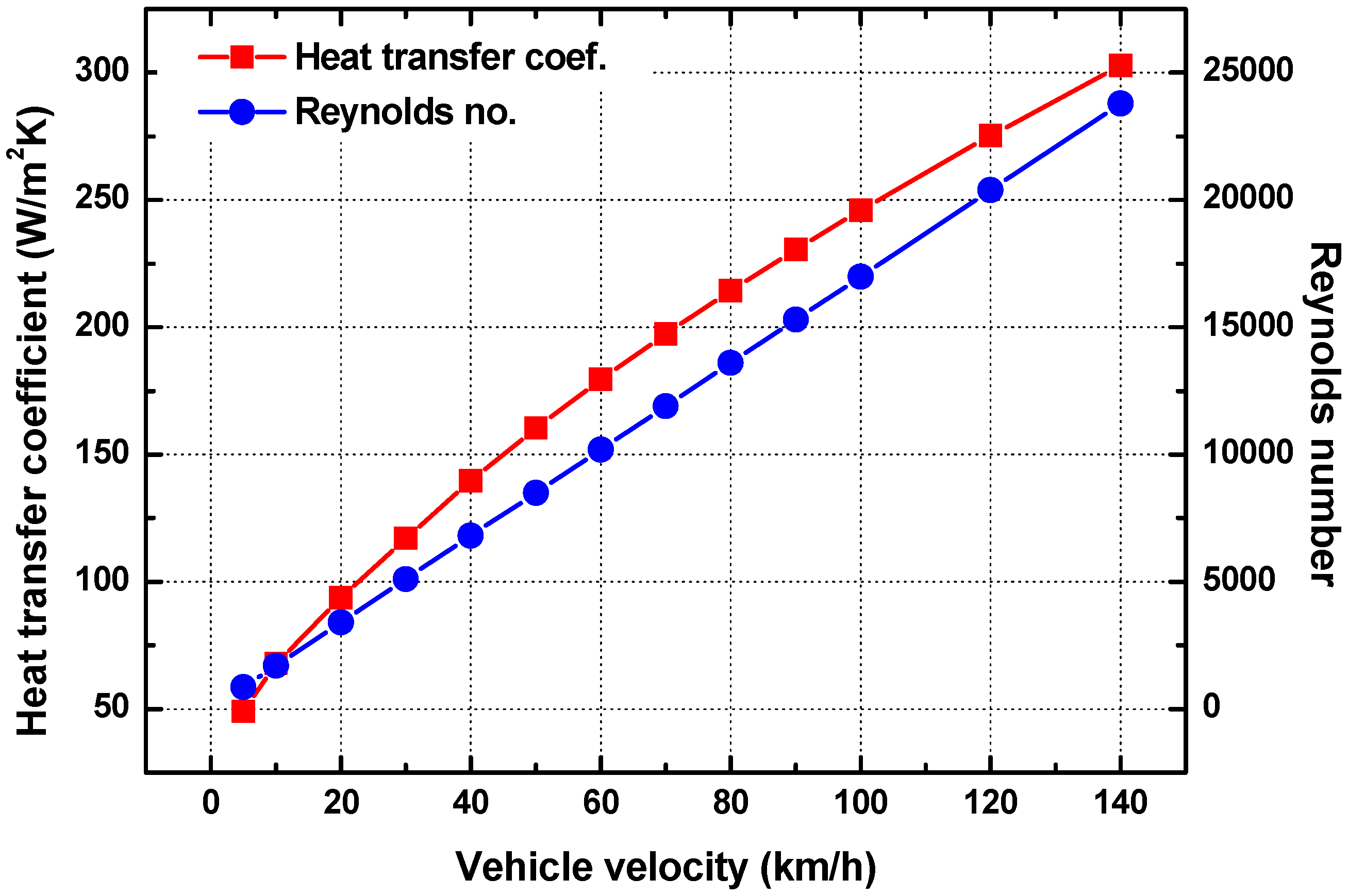



| Conductivity of TPE | 2.61 W/m∙K | Conductivity of air | 0.0242 W/m∙K |
| Conductivity of PPA | 3.00 W/m∙K | Conductivity of urea, liquid | 0.570 W/m∙K |
| Latent heat of urea | 152.86 kJ/kg | Conductivity of urea, solid | 0.750 W/m∙K |
| No. of Grid | 1,915,630 | Heat Flux Variation | 2500 W/m2 to 7500 W/m2 |
| Total analysis time | 600 s | Initial temp. variation | −25 °C to −50 °C |
| Time step | 5 s | Outer heat transfer coef. | 100 W/m2∙K to 300 W/m2∙K |
| Location | Thermal Resistance |
|---|---|
| Thermal resistance in urea | 4.24 |
| Thermal resistance in inner pipe | 0.845 |
| Thermal resistance in air gap | 69.8 |
| Thermal resistance in outer pipe | 0.688 |
| Thermal resistance in external surface (h = 100 W/m2∙K) | 4.90 |
| Thermal resistance in external surface (h = 300 W/m2∙K) | 1.63 |
| Convective Heat Transfer Coefficient on External Surface | Thermal Resistance in External Surface | Total Thermal Resistance |
|---|---|---|
| h = 10 W/m2∙K | 49.0 | 124.6 |
| h = 100 W/m2∙K | 4.90 | 80.51 |
| h = 300 W/m2∙K | 1.63 | 77.24 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, J.; Chon, M.S.; Choo, S.-H.; Park, J.S. Effects of Supply Power and Environmental Conditions on Urea Coolant Melting Phenomena in Urea Supply Lines in Vehicles. Energies 2018, 11, 1099. https://doi.org/10.3390/en11051099
Cha J, Chon MS, Choo S-H, Park JS. Effects of Supply Power and Environmental Conditions on Urea Coolant Melting Phenomena in Urea Supply Lines in Vehicles. Energies. 2018; 11(5):1099. https://doi.org/10.3390/en11051099
Chicago/Turabian StyleCha, Junepyo, Mun Soo Chon, Seong-Hwa Choo, and Jun Su Park. 2018. "Effects of Supply Power and Environmental Conditions on Urea Coolant Melting Phenomena in Urea Supply Lines in Vehicles" Energies 11, no. 5: 1099. https://doi.org/10.3390/en11051099





