A Pressurized Vitiated Co-Flow Burner and Its Preliminary Application for a Methane Lifted Flame
Abstract
:1. Introduction
2. Experimental Setup and Procedure
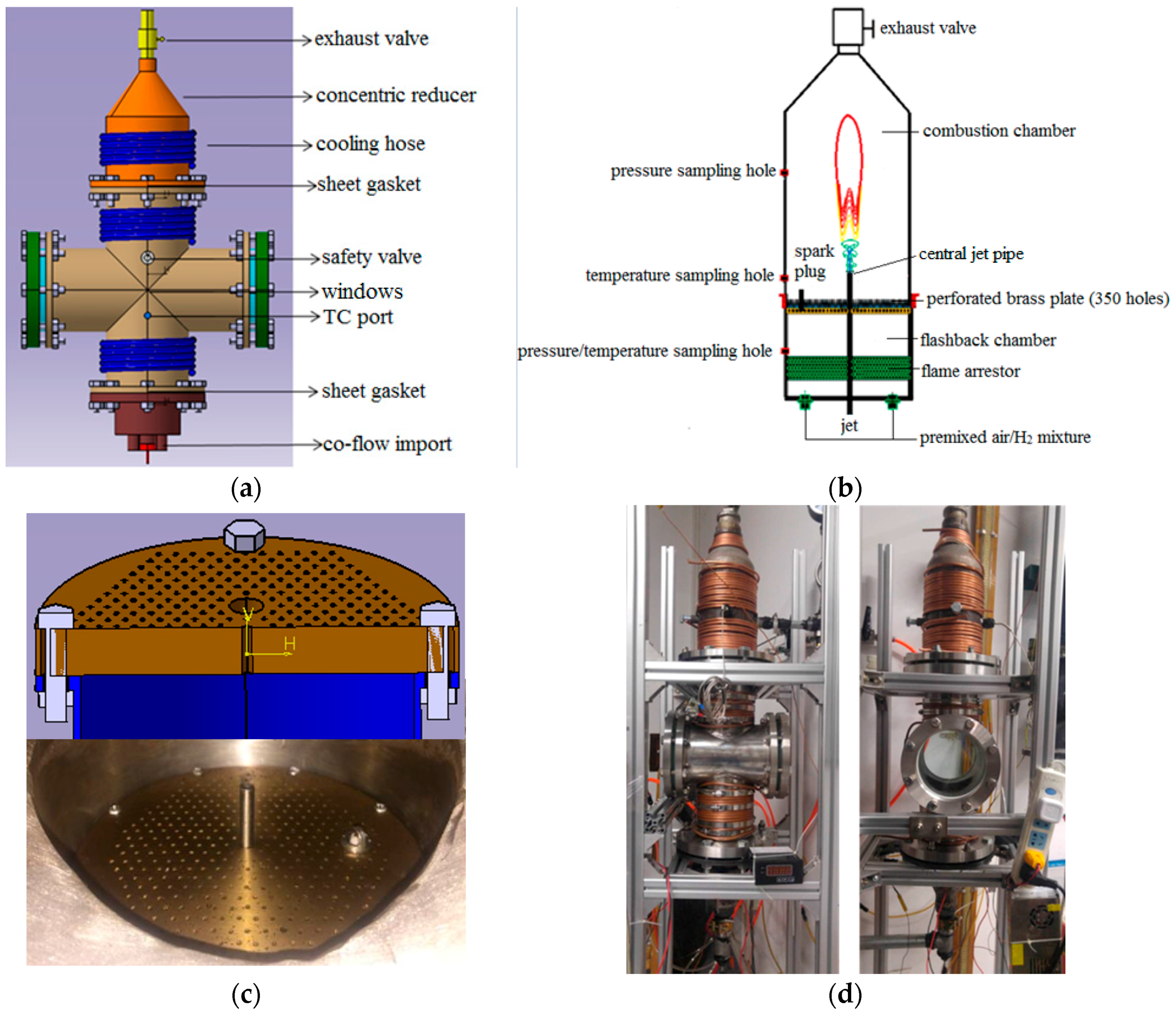
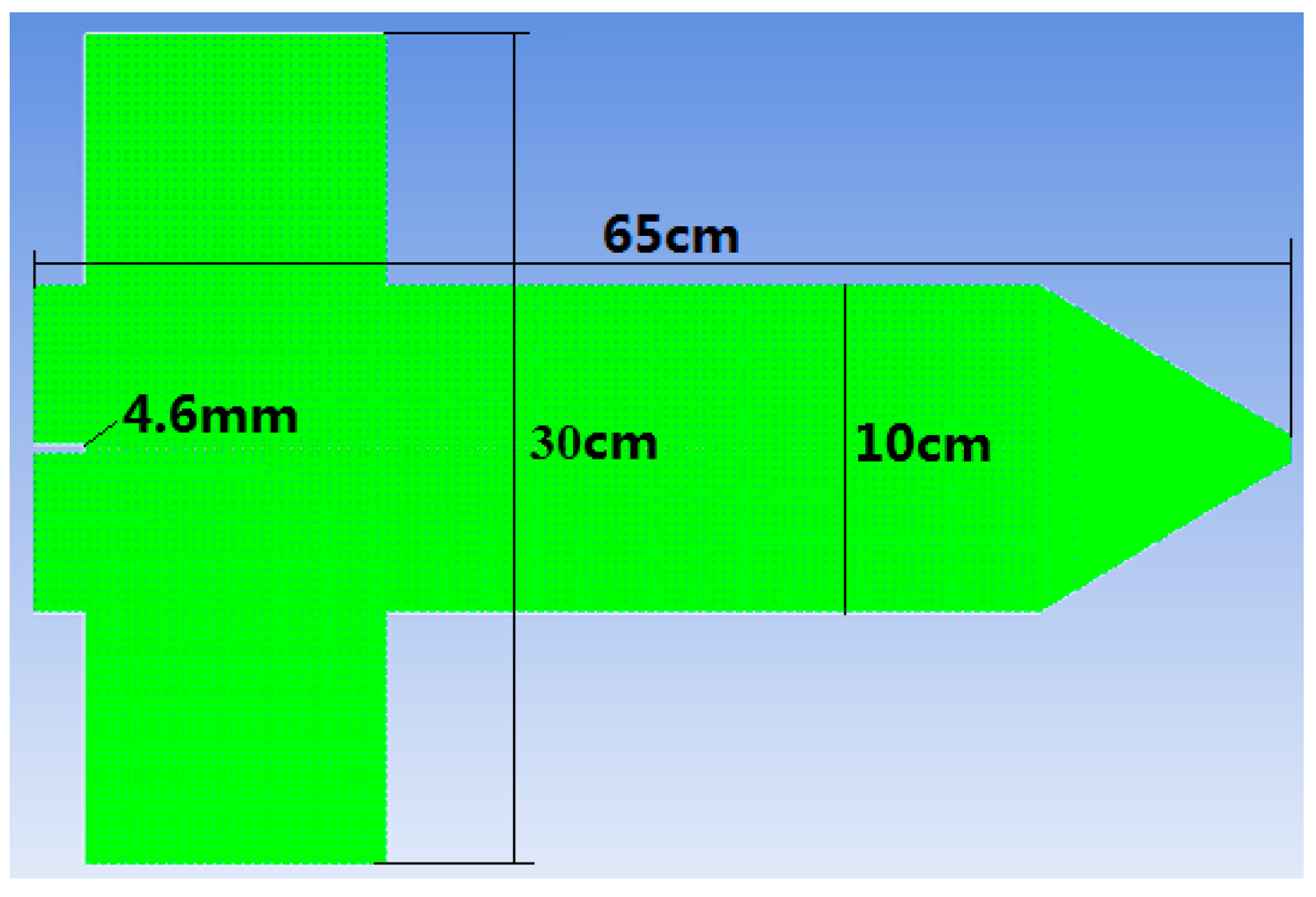
2.1. Structure of PVCB
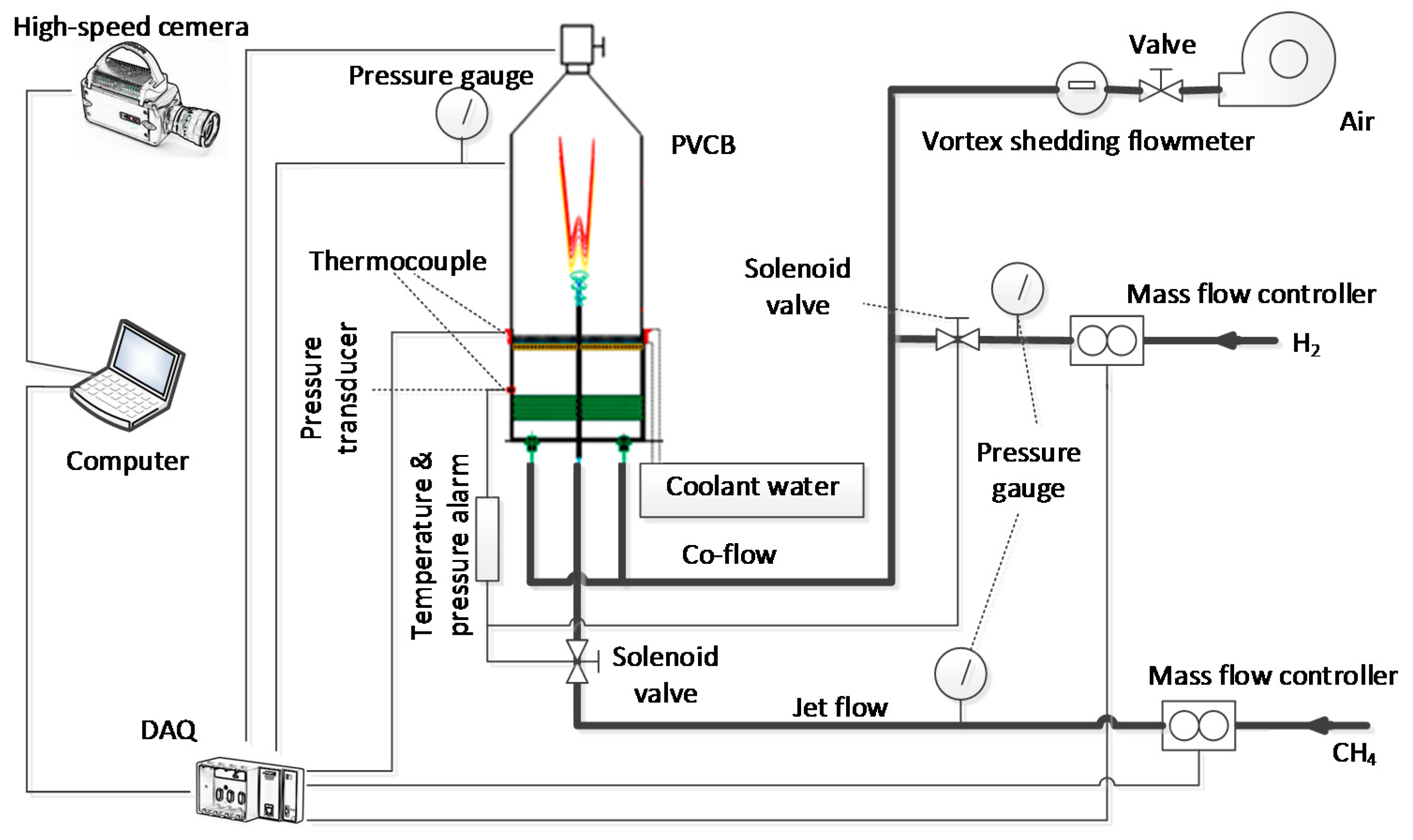
2.2. Configuration of the PVCB System
- (1)
- The background pressure of the PVCB is controllable.
- (2)
- The co-flow does not directly flow into an open space. Therefore, the temperature in the valid region is more stable, and the valid region is extended
3. Results and Discussion
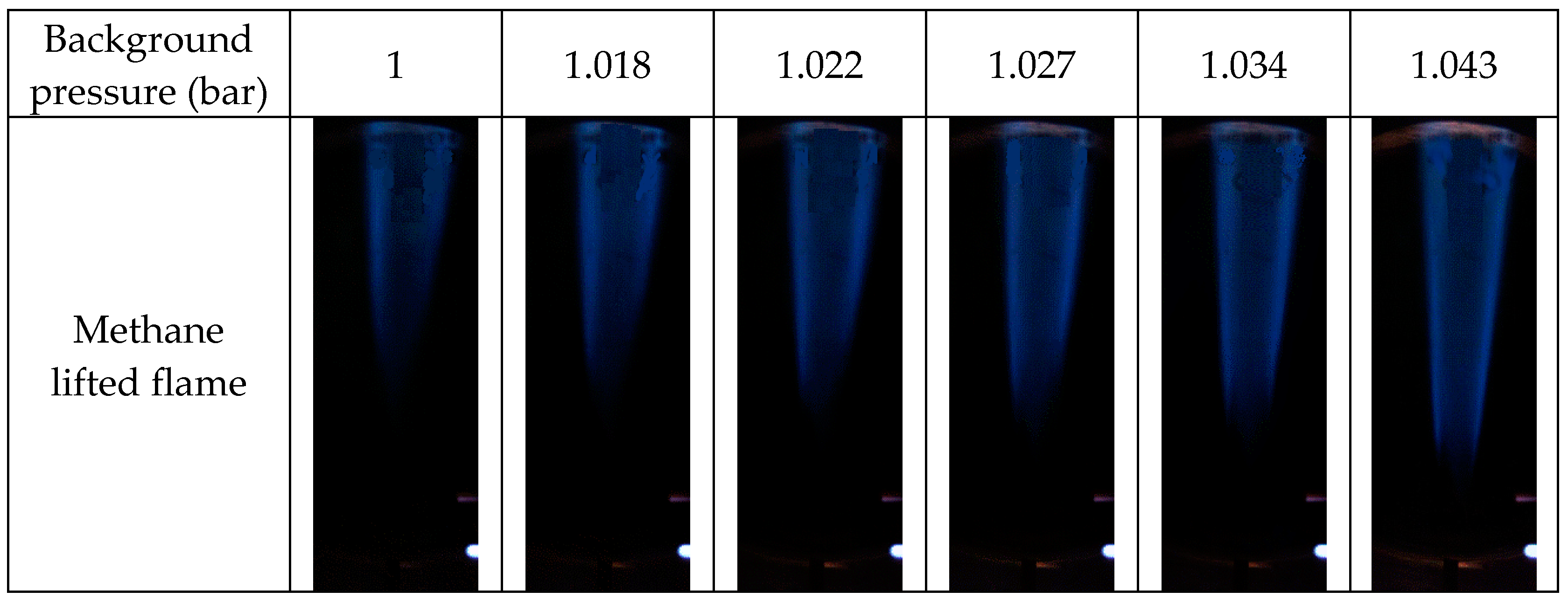
3.1. Flame Structure
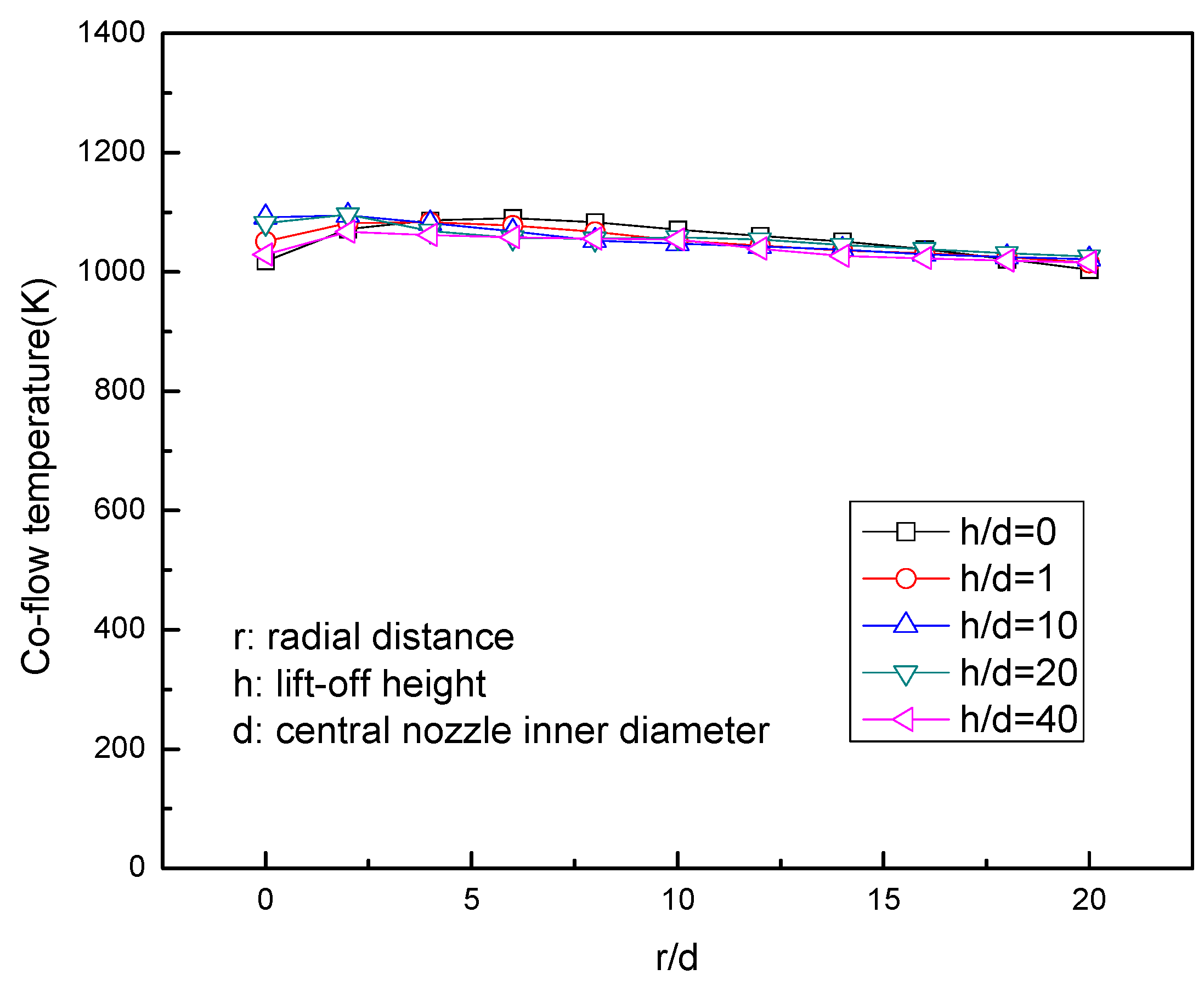
3.2. Temperature Distribution
3.3. Calculated Velocity and Temperature Distribution
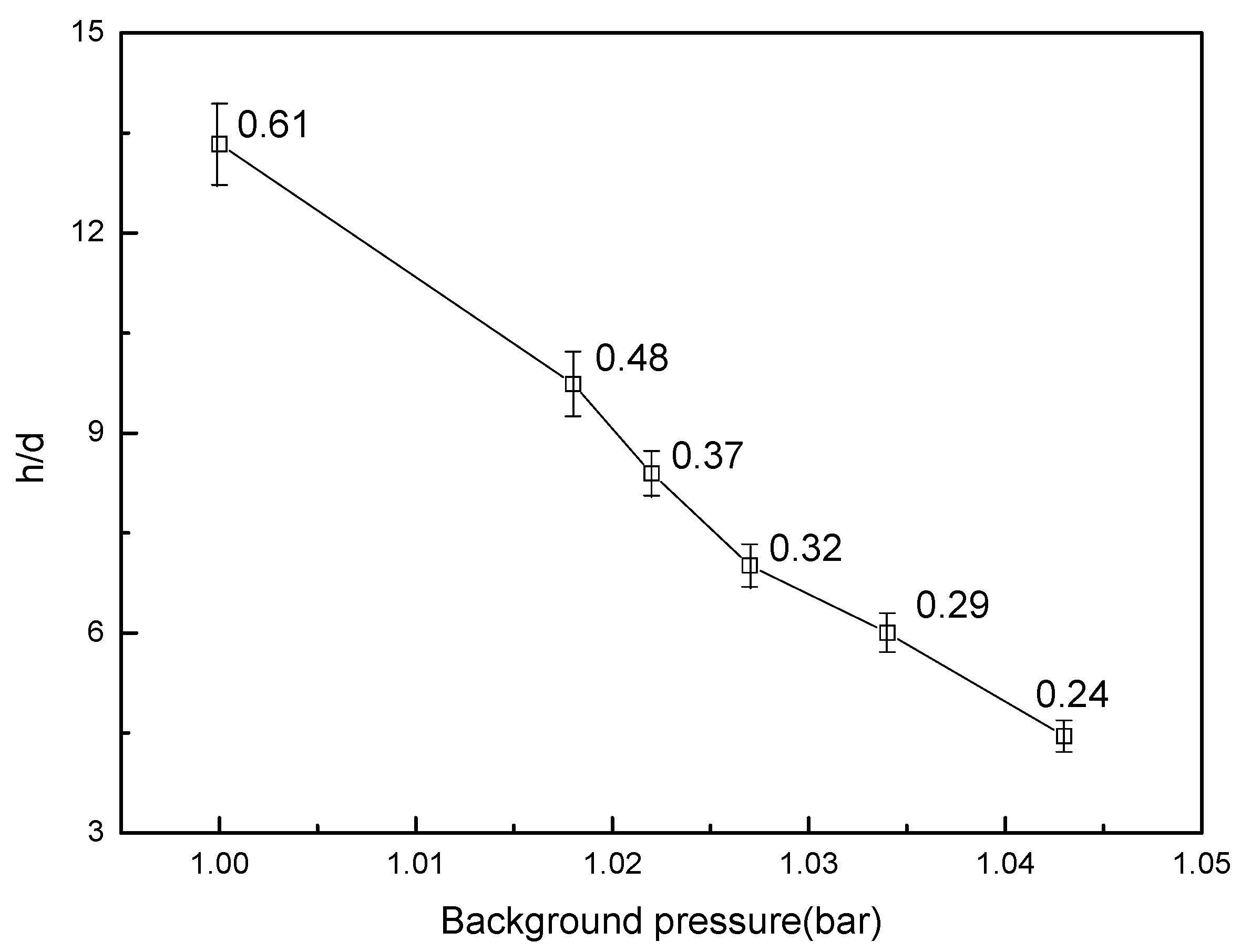
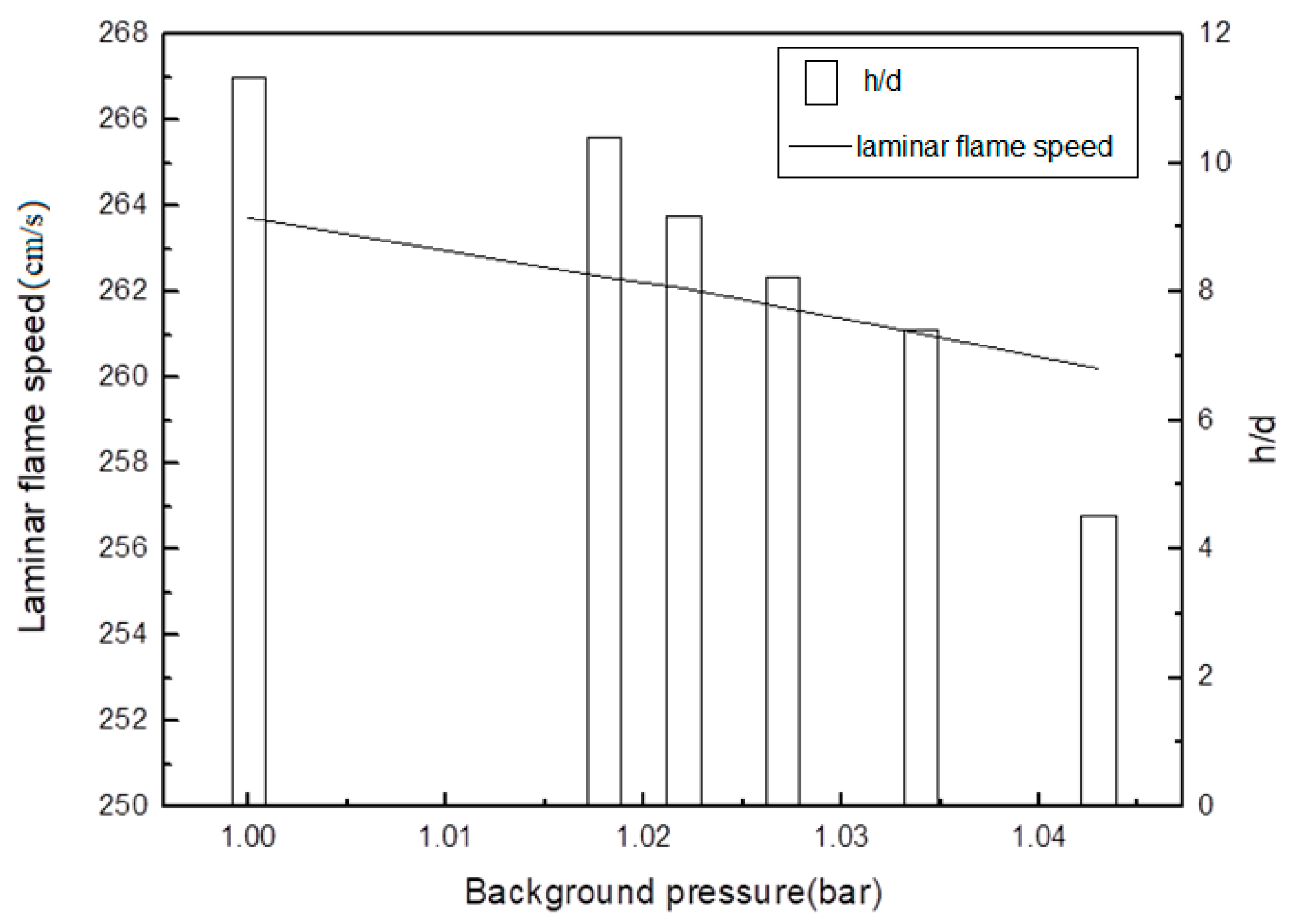
3.4. Flame Stabilization
4. Conclusions
- (1)
- A burner for studying turbulent combustion and flame stabilization was built and presented. The burner has a controllable background pressure (1–1.5 bar).
- (2)
- The temperature in the ROI is more stable (300–1300 K), and the ROI is extended, which provides more accurate boundary conditions.
- (3)
- A preliminary application for a methane lifted flame was tested in the PVCB; the experimental results indicate that a lifted flame is significantly influenced by background pressure. The lift-off height decreased to 40% when the background pressure increased by 4.3%. With the increase in background pressure, the standard deviation of the lift-off height decreased, which indicates that the lift-off height is more stable at a higher background pressure.
- (4)
- The comparison of the experimental results and the Chemkin simulation results shows that autoignition delay dominates the stabilization mechanism of the lifted flame in the PVCB, which agrees with the work of Jangi et al. [12].
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Vanquickenborne, L.; Van Tiggelen, A. The stabilization mechanism of lifted diffusion flames. Combust. Flame 1966, 10, 59–69. [Google Scholar] [CrossRef]
- Schefer, R.W.; Goix, P.J. Mechanism of flame stabilization in turbulent, lifted-jet flames. Combust. Flame 1998, 112, 559–574. [Google Scholar] [CrossRef]
- Navarro-Martinez, S.; Kronenburg, A. Flame stabilization mechanisms in lifted flames. Flow Turbul. Combust. 2011, 87, 377–406. [Google Scholar] [CrossRef]
- Cabra, R.; Myhrvold, T.; Chen, J.Y.; Dibble, R.W.; Karpetis, A.N.; Barlow, R.S. Simultaneous laser Raman-Rayleigh-LIF measurements and numerical modeling results of a lifted turbulent H2/N2 jet flame in a vitiated coflow. Proc. Combust. Inst. 2002, 29, 1881–1888. [Google Scholar] [CrossRef]
- Gordon, R.L.; Masri, A.R.; Bilger, R.W. Further characterisation of lifted hydrogen and methane flames issuing into a vitiated coflow. In Proceedings of the 5th Asia-Pacific Conference on Combustion, Adelaide, Australia, 17–20 July 2005. [Google Scholar]
- North, A.J.; Dibble, R.W. Liftoff heights of lifted N2-in-H2 jet flames issuing into a vitiated co-flow measured using Schlieren imaging. In Proceedings of the 7th US National Technical Meeting of the Combustion Institute, Georgia Institute of Technology, Atlanta, GA, USA, 20–23 March 2011. [Google Scholar]
- Johannessen, B.; North, A.; Dibble, R.; Løvås, T. Experimental studies of autoignition events in unsteady hydrogen–air flames. Combust. Flame 2015, 162, 3210–3219. [Google Scholar] [CrossRef]
- Wu, Z.; Starner, S.H.; Bilger, R.W. Lift-off heights of turbulent H2/N2 jet flames in a vitiated co-flow. In Proceedings of the 2003 Australian Symposium on Combustion and the 8th Australian Flame Days, Melbourne, Australia, 25–27 June 2003. [Google Scholar]
- Wu, Z.; Masri, A.R.; Bilger, R.W. An experimental investigation of the turbulence structure of a lifted H2/N2 jet flame in a vitiated coflow. Flow Turbul. Combust. 2006, 76, 61–81. [Google Scholar] [CrossRef]
- Gordon, R.L.; Masri, A.R.; Mastorakos, E. Simultaneous Rayleigh temperature, OH- and CH2O-LIF imaging of methane jets in a vitiated coflow. Combust. Flame 2008, 155, 181–195. [Google Scholar] [CrossRef]
- Gordon, R.L.; Masri, A.R.; Mastorakos, E. Heat release rate as represented by [OH] × [CHO] and its role in autoignition. Combust. Theory Model. 2009, 13, 645–670. [Google Scholar] [CrossRef]
- Jangi, M.; Zhao, X.; Haworth, D.C.; Bai, X.-S. Stabilization and liftoff length of a non-premixed methane/air jet flame discharging into a high-temperature environment: An accelerated transported PDF method. Combust. Flame 2015, 162, 408–419. [Google Scholar] [CrossRef]
- Cabra, R.; Chen, J.Y.; Dibble, R.W.; Karpetis, A.N.; Barlow, R.S. Lifted methane–air jet flames in a vitiated co-flow. Combust. Flame 2005, 143, 491–506. [Google Scholar] [CrossRef]
- Upatnieks, A.; Driscoll, J.F.; Rasmussen, C.C.; Ceccio, S.L. Liftoff of turbulent jet flames—assessment of edge flame and other concepts using cinema-PIV. Combust. Flame 2004, 138, 259–272. [Google Scholar] [CrossRef]
- Boulanger, J.; Vervisch, L.; Reveillon, J.; Ghosal, S. Effects of heat release in laminar diffusion flames lifted on round jets. Combust. Flame 2003, 134, 355–368. [Google Scholar] [CrossRef]
- Mastorakos, E. Ignition of turbulent non-premixed flames. Prog. Energy Combust. Sci. 2009, 35, 57–97. [Google Scholar] [CrossRef]
- Masri, A.R.; Cao, R.; Pope, S.B.; Goldin, G. PDF calculations of turbulent lifted flames of H2/N2 fuel issuing into a vitiated co-flow. Combust. Theory Model. 2004, 8, 1–22. [Google Scholar] [CrossRef]
- Cao, P.R.; Pope, S.B.; Masri, A.R. Turbulent lifted flames in a vitiated co-flow investigated using joint PDF calculations. Combust. Flame 2005, 142, 438–453. [Google Scholar] [CrossRef]
- Chen, J.H.; Choudhary, A.; De Supinski, B.; DeVries, M.; Hawkes, E.R.; Klasky, S.; Liao, W.K.; Ma, K.L.; Mellor-Crummey, J.; Podhorszki, N.; et al. Terascale direct numerical simulations of turbulent combustion using S3D. Comput. Sci. Discov. 2009, 2, 015001. [Google Scholar] [CrossRef]
- Yoo, C.S.; Sankaran, R.; Chen, J.H. Three-dimensional direct numerical simulation of a turbulent lifted hydrogen jet flame in heated coflow: Flame stabilization and structure. J. Fluid Mech. 2009, 640, 453–481. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, J.; Li, L.G. Study on characteristics of controllable active thermo-atmosphere of a vitiated co-flow combustor. Chin. Sci. Bull. 2005, 50, 704–707. [Google Scholar] [CrossRef]
- Wu, Z.; Bao, T.; Zhang, Q.; Yan, S.; Deng, J. Experimental study on spray combustion characteristics of gasoline–diesel blended fuel in a controllable active thermo-atmosphere. Fuel 2014, 135, 374–379. [Google Scholar] [CrossRef]
- North, A.; Magar, M.; Chen, J.Y.; Dibble, R.; Gruber, A. Effect of pressure, environment temperature, jet velocity and nitrogen dilution on the liftoff characteristics of a N2-in-H2 jet flame in a vitiated co-flow. Eurasian Chem.-Technol. J. 2014, 16, 141–148. [Google Scholar] [CrossRef]
- North, A.J. Experimental Investigations of Partially Premixed Hydrogen Combustion in Gas Turbine Environments. Ph.D. Thesis, The University of the California, Berkeley, CA, USA, 2013. [Google Scholar]
- Dunn, M.J.; Bilger, R.W.; Masri, A.R. Experimental characterisation of a vitiated co-flow piloted premixed burner. In Proceedings of the 5th Asia-Pacific Conference on Combustion, Adelaide, Australia, 17–20 July 2005; pp. 417–420. [Google Scholar]
- Kalghatgi, G.T. Lift-off Heights and Visible Lengths of Vertical Turbulent Jet Diffusion Flames in Still Air. Combust. Sci. Technol. 1984, 41, 17–29. [Google Scholar]


| Equivalence Ratio | H2 | O2 | N2 | Temperature (K) | Density (kg·m−3) | Velocity (m·s−1) |
|---|---|---|---|---|---|---|
| Mol Ratio | ||||||
| 0.05 | 0.10 | 1 | 3.76 | 470.1744 | 0.740884 | 1.404891 |
| 0.1 | 0.20 | 1 | 3.76 | 631.5275 | 0.54671 | 1.911782 |
| 0.15 | 0.30 | 1 | 3.76 | 783.4902 | 0.436818 | 2.402531 |
| 0.2 | 0.40 | 1 | 3.76 | 926.2674 | 0.366292 | 2.876672 |
| 0.25 | 0.50 | 1 | 3.76 | 1061.13 | 0.317006 | 3.337119 |
| 0.3 | 0.60 | 1 | 3.76 | 1189.406 | 0.280428 | 3.787171 |
| 0.35 | 0.70 | 1 | 3.76 | 1311.71 | 0.252155 | 4.228033 |
| Equivalence Ratio | O2 | H2O | N2 | OH |
|---|---|---|---|---|
| Mol Ratio | ||||
| 0.05 | 1.98 × 10−1 | 2.08 × 10−2 | 7.82 × 10−1 | 0.00 |
| 0.1 | 1.85 × 10−1 | 4.12 × 10−2 | 7.74 × 10−1 | 0.00 |
| 0.15 | 1.73 × 10−1 | 6.11 × 10−2 | 7.66 × 10−1 | 0.00 |
| 0.2 | 1.61 × 10−1 | 8.06 × 10−2 | 7.58 × 10−1 | 2.67 × 10−8 |
| 0.25 | 1.50 × 10−1 | 9.98 × 10−2 | 7.50 × 10−1 | 4.12 × 10−7 |
| 0.3 | 1.38 × 10−1 | 1.19 × 10−1 | 7.43 × 10−1 | 3.14 × 10−6 |
| 0.35 | 1.27 × 10−1 | 1.37 × 10−1 | 7.36 × 10−1 | 1.50 × 10−5 |
| Dimension | 2D |
| Cells | 45763 |
| Nodes | 46642 |
| Near-wall | Standard wall functions |
| Co-flow inlet | Mass-flow-inlet |
| Outlet | Pressure outlet |
| Viscous model | Realizable k–ε |
| Solver type | Density-based Steady Axisymmetric |
| Solution methods | Implicit AUSM (Advection Upstream Splitting Method) |
| Local time step | Courant number = 0.5 |
| Co-flow temperature (K) | 1133 |
| Equivalence ratio | 0.32 |
| Co-flow mass flow rate (m³·h−1) | 33 |
| Jet fuel flow rate (m·s−1) | 37.6 |
| Jet fuel | Methane |
| Background pressure before injection (bar) | 1.0, 1.01, 1.015, 1.020, 1.025, 1.030 |
| Background pressure after injection (bar) | 1.0, 1.018, 1.022, 1.027, 1.034, 1.043 |
| Exposure time (ms) | 30 |
| Frame rate (frames/s) | 30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Q.; Wu, Z.; Zhang, Q.; Xie, W.; Li, L.; Deng, J. A Pressurized Vitiated Co-Flow Burner and Its Preliminary Application for a Methane Lifted Flame. Energies 2018, 11, 1402. https://doi.org/10.3390/en11061402
Qin Q, Wu Z, Zhang Q, Xie W, Li L, Deng J. A Pressurized Vitiated Co-Flow Burner and Its Preliminary Application for a Methane Lifted Flame. Energies. 2018; 11(6):1402. https://doi.org/10.3390/en11061402
Chicago/Turabian StyleQin, Qiushi, Zhijun Wu, Qing Zhang, Wei Xie, Liguang Li, and Jun Deng. 2018. "A Pressurized Vitiated Co-Flow Burner and Its Preliminary Application for a Methane Lifted Flame" Energies 11, no. 6: 1402. https://doi.org/10.3390/en11061402




