Biogas from Fresh Spring and Summer Grass: Effect of the Harvesting Period
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Site and Field Operations
2.2. Lab Scale Anaerobic Digestion System and Experimental Setup
2.3. Input and Output Analysis
- dC = variation of the concentration of the monitored gas in the time interval (mg·m−3)
- dt = time interval (h)
- V = inner volume of the static chamber (m3);
- A = base area of the chamber, and hence, the emitting area (m2);
- M = mass of the sample (g)
3. Results and Discussion
3.1. Characteristics of the Feedstocks
3.2. Biogas Quality
3.3. Biogas Production and Yield
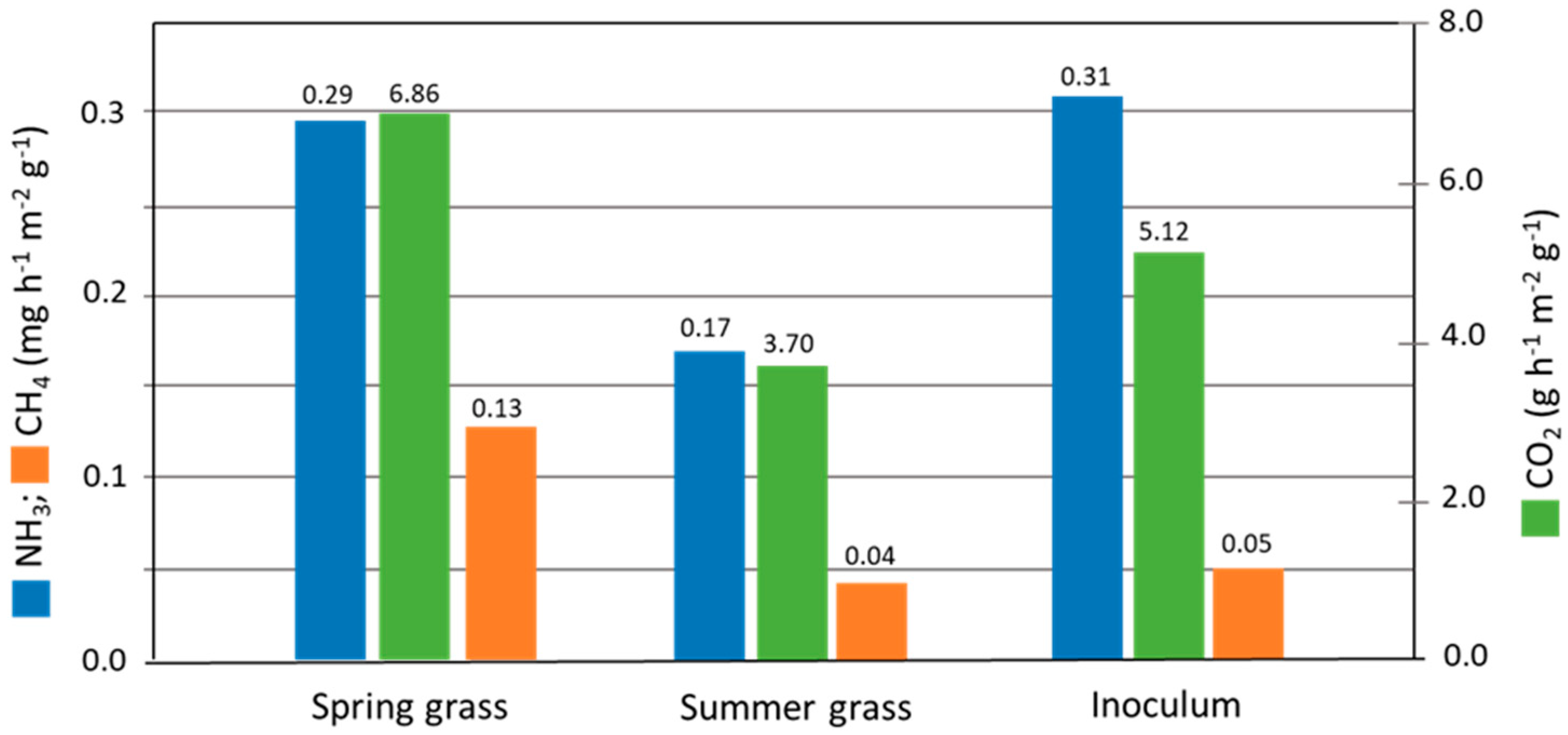
3.4. Gaseous Emissions
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Diaz, L.F.; Chiumenti, A.; Savage, G.M.; Eggerth, L.L. Managing the Organic fraction of Municipal Solid Waste. BioCycle 2006, 47, 50–54. [Google Scholar]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Machmüller, A.; Hopfner-Sixt, K.; Bodiroza, V.; Hrbek, R.; Friedel, J.; Pötsch, E.; Wagentristl, H.; et al. Methane production through anaerobic digestion of various energy crops grown in sustainable crop rotations. Bioresour. Technol. 2007, 98, 3204–3212. [Google Scholar] [CrossRef] [PubMed]
- Appels, L.; Lauwers, L.; Degrève, J.; Helsen, L.; Lievens, B.; Willems, K.; Van Impe, J.; Dewil, R. Anaerobic digestion in global bio-energy production: Potential and research challenges. Renew. Sustain. Energy Rev. 2011, 15, 4295–4301. [Google Scholar] [CrossRef]
- Coppolecchia, D.; Gardoni, D.; Baldini, C.; Borgonovo, F.; Guarino, M. The influence on biogas production of three slurry-handling systems in dairy farms. J. Agric. Eng. 2015, 46, 30–35. [Google Scholar] [CrossRef]
- Pezzuolo, A.; Dumont, B.; Sartori, L.; Marinello, F.; Migliorati, M.D.A.; Basso, B. Evaluating the impact of soil conservation measures on soil organic carbon at the farm scale. Comput. Electron. Agric. 2017, 135, 175–182. [Google Scholar] [CrossRef]
- Oviedo-Ocaña, E.R.; Dominguez, I.; Torres-Lozada, P.; Marmolejo-Rebellón, L.F.; Komilis, D.; Sanchez, A. A qualitative model to evaluate biowaste composting management systems using causal diagrams: A case study in Colombia. J. Clean. Prod. 2016, 133, 201–211. [Google Scholar] [CrossRef]
- Gerin, P.A.; Vliegen, F.; Jossart, J.M. Energy and CO2 balance of maize and grass as energy crops for anaerobic digestion. Bioresour. Technol. 2008, 99, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Pöschl, M.; Ward, S.; Owende, P. Evaluation of energy efficiency of various biogas production and utilization pathways. Appl. Energy 2010, 87, 3305–3321. [Google Scholar] [CrossRef]
- Da Borso, F.; Chiumenti, A.; Sigura, M.; Pezzuolo, A. Influence of automatic feeding systems on design and management of dairy farms. J. Agric. Eng. 2017, 48, 48–52. [Google Scholar] [CrossRef]
- Chiumenti, R.; Chiumenti, A.; da Borso, F.; Limina, S.; Landa, A. Anaerobic Digestion of Swine Manure in Conventional and Hybrid Pilot Scale Plants: Performance and Gaseous Emissions Reduction. In Proceedings of the International Syposium (ASABE 2009), Reno, NV, USA, 21–24 June 2009. [Google Scholar]
- Dinuccio, E.; Balsari, P.; Gioelli, F.; Menardo, S. Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses. Bioresour. Technol. 2010, 101, 3780–3783. [Google Scholar] [CrossRef] [PubMed]
- Valenti, F.; Porto, S.M.; Cascone, G.; Arcidiacono, C. Potential biogas production from agricultural by-products in Sicily. A case study of citrus pulp and olive pomace. J. Agric. Eng. 2017, 48, 196–202. [Google Scholar] [CrossRef]
- Eggerth, L.L.; Diaz, L.F.; Chang, M.T.F.; Iseppi, L. Marketing of composts. Waste Manag. 2007, 8, 325–355. [Google Scholar]
- Boscaro, D.; Pezzuolo, A.; Grigolato, S.; Cavalli, R.; Marinello, F.; Sartori, L. Preliminary analysis on mowing and harvesting grass along riverbanks for the supply of anaerobic digestion plants in north-eastern Italy. J. Agric. Eng. 2015, 46, 100–104. [Google Scholar] [CrossRef]
- Blokhina, Y.N.; Prochnow, A.; Plöchl, M.; Luckhaus, C.; Heiermann, M. Concepts and profitability of biogas production from landscape management grass. Bioresour. Technol. 2011, 102, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Zhong, J.; Hansen, J. Anaerobic digestion of dairy processing waste, algae, and grass in pilot and full scale. Trans. ASABE 2014, 57, 609–614. [Google Scholar]
- Boscaro, D.; Pezzuolo, A.; Sartori, L.; Marinello, F.; Mattioli, A.; Bolzonella, D.; Grigolato, S. Evaluation of the energy and greenhouse gases impacts of grass harvested on riverbanks for feeding anaerobic digestion plants. J. Clean. Prod. 2018, 172, 4099–4109. [Google Scholar] [CrossRef]
- Mattioli, A.; Boscaro, D.; Dalla Venezia, F.; Santacroce, F.C.; Pezzuolo, A.; Sartori, L.; Bolzonella, D. Biogas from residual grass: A territorial approach for sustainable bioenergy production. Waste Biomass. Valoriz. 2017, 8, 2747–2756. [Google Scholar] [CrossRef]
- Colantoni, A.; Delfanti, L.; Recanatesi, F.; Tolli, M.; Lord, R. Land use planning for utilizing biomass residues in Tuscia Romana (central Italy): Preliminary results of a multi criteria analysis to create an agro-energy district. Land Use Policy. 2016, 50, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Bonzini, S.; Verro, R.; Otto, S.; Lazzaro, L.; Finizio, A.; Zanin, G.; Vighi, M. Experimental validation of a geographical information systems-based procedure for predicting pesticide exposure in surface water. Environ. Sci. Technol. 2006, 40, 7561–7569. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, S.; Prosdocimi, M.; Tarolli, P.; Borin, M. Assessment of Energy Potential from Wetland Plants along the Minor Channel Network on an Agricultural Floodplain. Environ. Sci. Pollut. Res. 2014, 22, 2479–2490. [Google Scholar] [CrossRef] [PubMed]
- Hensgen, F.; Richter, F.; Wachendorf, M. Integrated generation of solid fuel and biogas from green cut material from landscape conservation and private households. Bioresour. Technol. 2011, 102, 10441–10450. [Google Scholar] [CrossRef] [PubMed]
- Tsapekos, P.; Kougias, P.G.; Egelund, H.; Larsen, U.; Pedersen, J.; Trénel, P.; Angelidaki, I. Mechanical pretreatment at harvesting increases the bioenergy output from marginal land grasses. Renew. Energy. 2017, 111, 914–921. [Google Scholar] [CrossRef]
- Bishop, G.C.; Burns, R.T.; Shepherd, T.A.; Moody, L.B.; Gooch, C.A.; Spajic, R.; Pronto, J. Evaluation of laboratory biochemical methane potentials as a predictor of anaerobic dairy manure digester biogas and methane production. In Proceedings of the American Society of Agricultural and Biological Engineers Annual International Meeting, Reno, NV, USA, 21–24 June 2009; pp. 5254–5265. [Google Scholar]
- Safferman, S.I.; Kirk, D.M.; Faivor, L.L.; Haan, W.W. Anaerobic digestion processes. In Bioremediation and Sustainability: Research and Applications; Wiley: Hoboken, NJ, USA, 2012; pp. 103–136. [Google Scholar]
- Ahn, H.K.; Smith, M.C.; Konrad, S.L.; White, J.W. Evaluation of biogas production potential by dry anaerobic digestion of switchgrass-animal manure mixtures. Appl. Biochem. Biotechnol. 2010, 160, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Lehtomäki, A.; Huttunen, S.; Lehtinen, T.M.; Rintala, J.A. Anaerobic digestion of grass silage in batch leach bed processes for methane production. Bioresour. Technol. 2008, 99, 3267–3278. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wu, G.; Lawlor, P.G.; Frost, J.P.; Zhan, X. Methane production from anaerobic co-digestion of the separated solid fraction of pig manure with dried grass silage. Bioresour. Technol. 2012, 104, 289–297. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21th ed.; APHA: Washington, DC, USA, 2005; pp. 5–41. [Google Scholar]
- Chiumenti, A. Complete nitrification-denitrification of swine manure in a full-scale, non-conventional composting system. Waste Manag. 2015, 46, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Chiumenti, A.; da Borso, F.; Pezzuolo, A.; Sartori, L.; Chiumenti, R. Ammonia and greenhouse gas emissions from slatted dairy barn floors cleaned by robotic scrapers. Res. Agric. Eng. 2018, 64, 26–33. [Google Scholar] [Green Version]
- Frison, N.; Chiumenti, A.; Katsou, E.; Malamis, S.; Bolzonella, D.; Fatone, F. Mitigating off-gas emissions in the biological nitrogen removal via nitrite process treating anaerobic effluents. J. Clean. Prod. 2015, 93, 126–133. [Google Scholar] [CrossRef]
- Chiumenti, A.; Boscaro, D.; da Borso, F.; Sartori, L.; Pezzuolo, A. Anaerobic digestion of grass: Effect of the harvesting period on biogas yield. In Proceedings of the ASABE Annual International Meeting 2017, Spokane, WA, USA, 16–19 July 2017. [Google Scholar]
- Baldini, M.; da Borso, F.; Ferfuia, C.; Danuso, F. Ensilage suitability and bio-methane yield of Arundo donax and Miscanthus giganteus. J. Ind. Crops Prod. 2017, 95, 264–275. [Google Scholar] [CrossRef]
- Jantrania, A.R.; White, R.K. High-solids anaerobic fermentation of poultry manure. In Proceedings of the Fifth International Symposium on Agricultural Waste, St. Joseph, MI, USA, 16–17 December 1985; pp. 73–80. [Google Scholar]
- Chiumenti, A.; da Borso, F.; Limina, S. Dry anaerobic digestion of cow manure and agricultural products in a full-scale plant: Efficiency and comparison with wet fermentation. Waste Manag. 2018, 71, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Lossie, U.; Pütz, P. Targeted Control of Biogas Plants with the Help of FOS/TAC; Practice Report Hach-Lange; Hach: Loveland, CO, USA, 2015. [Google Scholar]
- Mulbry, W.; Selmer, K.; Lansing, S. Effect of liquid surface area on hydrogen sulfide oxidation during micro-aeration in dairy manure digesters. PLoS ONE 2017, 12, 0185738. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Samani, Z.; Hanson, A.; Smith, G. Energy recovery from grass using two-phase anaerobic digestion. Waste Manag. 2002, 22, 1–5. [Google Scholar] [CrossRef]
- Nizami, A.S.; Orozco, A.; Groom, E.; Dieterich, B.; Murphy, J.D. How much gas can we get from grass? Appl. Energy 2012, 92, 783–790. [Google Scholar] [CrossRef]







| Feedstock | TS (%) | VS (%TS) | pH | Redox (mV) | Acidity/Alkalinity | TKN (g·kg−1) | (g·kg−1) | Lignin (%TS) | Cellulose (%TS) | Hemicellulose (%TS) |
|---|---|---|---|---|---|---|---|---|---|---|
| Spring Grass | 32.43 ± 2.27 | 79.21 ± 0.03 | n.d | n.d. | n.d. | 7.65 ± 1.29 | n.d. | 3.93 ± 0.43 | 21.24 ± 1.35 | 23.07 ± 3.32 |
| Inoculum | 7.85 ± 0.09 | 80.80 ± 0.05 | 8.2 ± 0.1 | −400 ± 10 | 0.24 ± 0.02 | 4.19 ± 0.41 | 2.92 ± 0.35 | 12.54 ± 0.69 | 19.62 ± 1.21 | 18.14 ± 1.20 |
| Input Mix * | 8.90 | 68.41 | n.d | n.d. | n.d. | 3.90 | 2.21 | 11.63 | 19.79 | 18.66 |
| Digestate | 6.28 ± 0.09 | 71.79 ± 0.66 | 7.9 ± 0.1 | −391 ± 16 | 0.15 ± 0.03 | 3.90 ± 0.03 | 2.29 ± 0.06 | 11.69 ± 0.34 | 12.71 ± 0.26 | 13.57 ± 0.46 |
| Summer Grass | 33.30 ± 4.31 | 90.26 ± 1.23 | n.d | n.d. | n.d. | 10.22 ± 2.69 | n.d. | 8.65 ± 4.25 | 34.72 ± 4.96 | 28.12 ± 1.22 |
| Inoculum | 7.28 ± 0.10 | 78.45 ± 0.06 | 7.8 ± 0.1 | −395 ± 8 | 0.18 ± 0.03 | 3.94 ± 0.28 | 3.07 ± 0.27 | 11.32 ± 0.90 | 18.35 ± 0.99 | 16.22 ± 1.22 |
| Input Mix * | 8.55 | 67.64 | n.d | n.d. | n.d. | 3.84 | 2.22 | 11.01 | 20.24 | 17.59 |
| Digestate | 6.06 ± 0.28 | 76.09 ± 1.00 | 7.8 ± 0.1 | −455 ± 7 | 0.19 ± 0.02 | 3.83 ± 0.87 | 2.53 ± 0.25 | 12.46 ± 0.57 | 12.91 ± 0.90 | 11.73 ± 1.22 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiumenti, A.; Boscaro, D.; Da Borso, F.; Sartori, L.; Pezzuolo, A. Biogas from Fresh Spring and Summer Grass: Effect of the Harvesting Period. Energies 2018, 11, 1466. https://doi.org/10.3390/en11061466
Chiumenti A, Boscaro D, Da Borso F, Sartori L, Pezzuolo A. Biogas from Fresh Spring and Summer Grass: Effect of the Harvesting Period. Energies. 2018; 11(6):1466. https://doi.org/10.3390/en11061466
Chicago/Turabian StyleChiumenti, Alessandro, Davide Boscaro, Francesco Da Borso, Luigi Sartori, and Andrea Pezzuolo. 2018. "Biogas from Fresh Spring and Summer Grass: Effect of the Harvesting Period" Energies 11, no. 6: 1466. https://doi.org/10.3390/en11061466






