Synergistic Enhancement of Ternary Poly(3,4-ethylenedioxythiophene)/Graphene Oxide/Manganese Oxide Composite as a Symmetrical Electrode for Supercapacitors
Abstract
:1. Introduction
2. Results
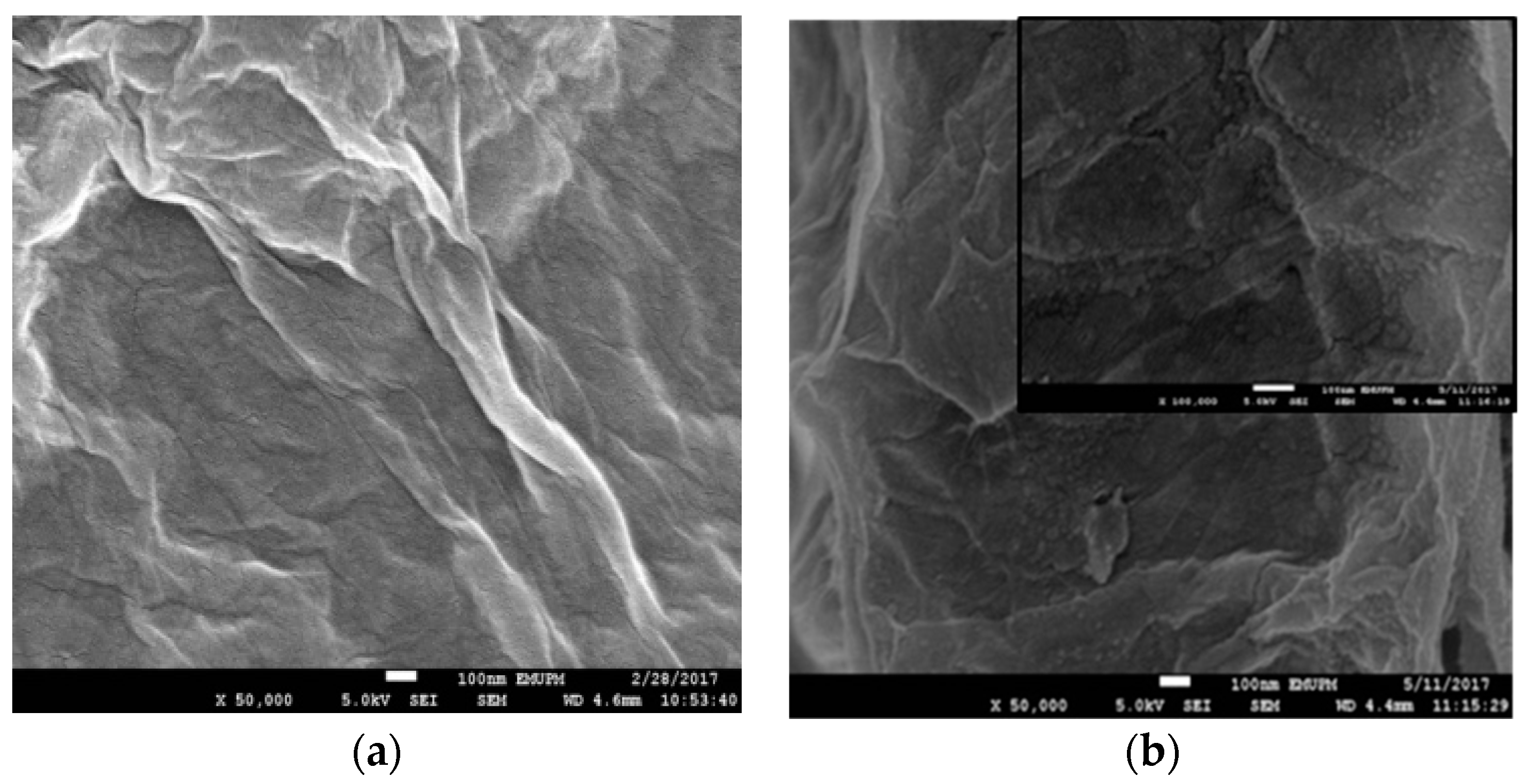
2.1. Morphological and Structural Analysis
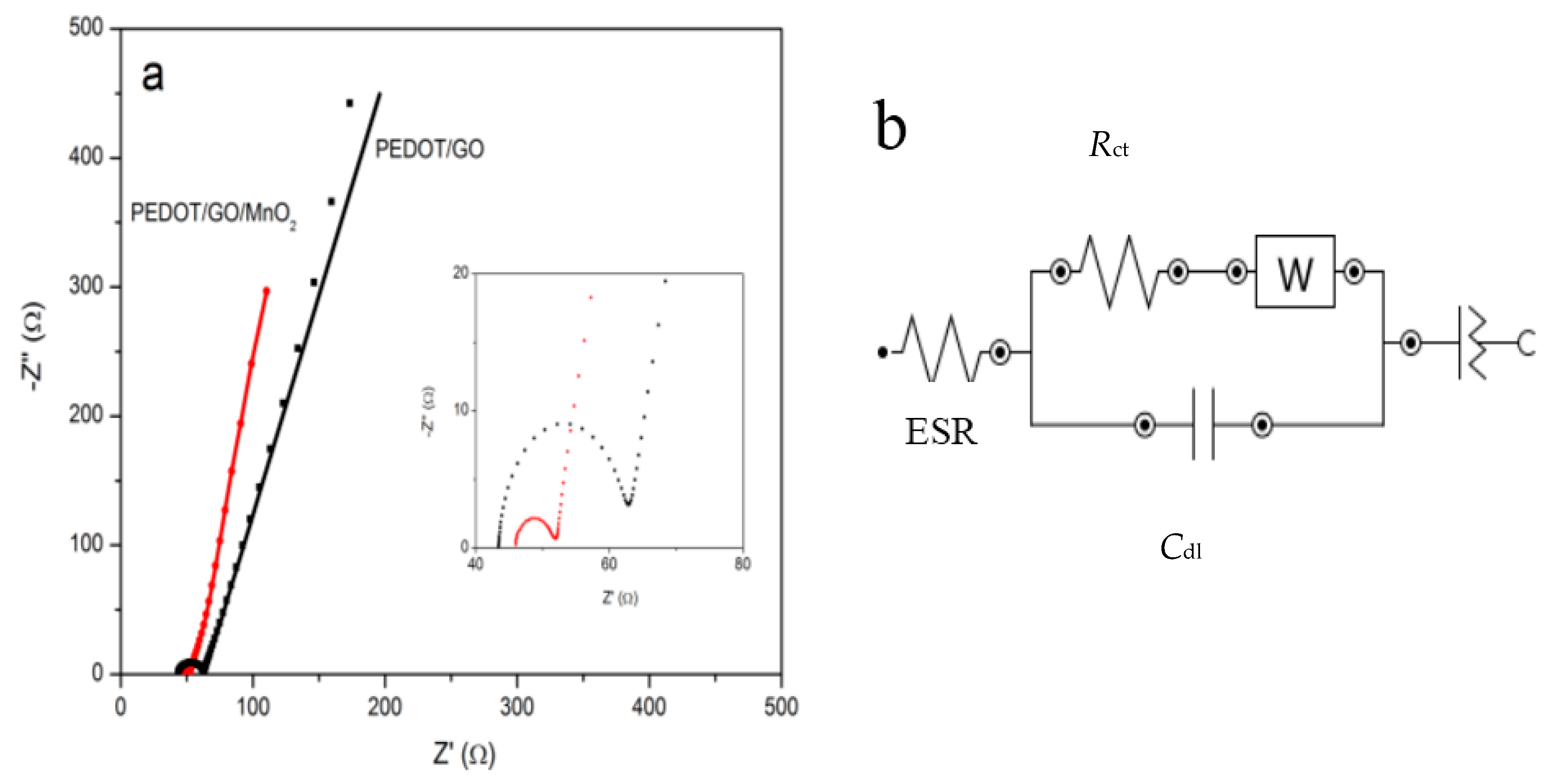
2.2. Electrochemical Measurements
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of PEDOT/GO/MnO2 Composite
3.3. Assembly and Characterization of Symmetric Electrodes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raut, S.S.; Sankapal, B.R. Comparative studies on MWCNTs, Fe2O3 and Fe2O3/MWCNTs thin films towards supercapacitor application. New J. Chem. 2016, 40, 2619–2627. [Google Scholar] [CrossRef]
- Chang, J.K.; Lee, M.T.; Huang, C.H.; Tsai, W.T. Physicochemical properties and electrochemical behavior of binary manganese-cobalt oxide electrodes for supercapacitor applications. Mater. Chem. Phys. 2008, 108, 124–131. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Wang, T.; Shao, J.; Wang, D.; Yang, Y.-W. Mesoporous transition metal oxides for supercapacitors. Nanomaterials 2015, 5, 1667–1689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xu, J.; Zhu, X.; Lu, L.; Duan, X.; Hu, D.; Dong, L.; Sun, H.; Gao, Y.; Wu, Y. Poly(3,4-ethylenedioxythiophene) nanorods grown on graphene oxide sheets as electrochemical sensing platform for rutin. J. Electroanal. Chem. 2015, 739, 66–72. [Google Scholar] [CrossRef]
- Cha, I.; Ji, E.; Seok, H.; Kim, J.-H.; Ho, Y.; Song, C. Facile electrochemical synthesis of polydopamine-incorporated graphene oxide/PEDOT hybrid thin films for pseudocapacitive behaviors. Synth. Met. 2014, 195, 162–166. [Google Scholar] [CrossRef]
- Soojeong, L.; Suk Cho, M.; Hyuck, L.; Jae-Do, N.; Youngkwan, L. A facile synthetic route for well defined multilayer films of graphene and PEDOT via an electrochemical method. J. Mater. Chem. 2012, 22, 1899–1903. [Google Scholar]
- Tang, P.; Han, L.; Zhang, L. Facile Synthesis of Graphite/PEDOT/MnO2 Composites on Commercial Supercapacitor Separator Membranes as Flexible and High-Performance Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2014, 6, 10506–10515. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Liu, Y.; Li, Y.; Zhuo, R.; Wu, Z.; Ren, P.; Li, S.; Wang, J.; Yan, P.; Geng, Z. Synthesis and electrochemical properties of MnO2/rGO/PEDOT:PSS ternary composite electrode material for supercapacitors. Mater. Lett. 2014, 127, 53–55. [Google Scholar] [CrossRef]
- Si, P.; Ding, S.; Lou, X.-W.; Kim, D.-H. An electrochemically formed three-dimensional structure of polypyrrole/graphene nanoplatelets for high-performance supercapacitors. RSC Adv. 2011, 1, 1271–1278. [Google Scholar] [CrossRef]
- Mcallister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-alonso, O.M.; Milius, D.L.; Car, R.; Prud, R.K.; et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. J. Am. Chem. Soc. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Nabilah Azman, N.H.; Lim, H.N.; Sulaiman, Y. Effect of electropolymerization potential on the preparation of PEDOT/graphene oxide hybrid material for supercapacitor application. Electrochim. Acta 2016, 188, 785–792. [Google Scholar] [CrossRef]
- Eeu, Y.C.; Lim, H.N.; Lim, Y.S.; Zakarya, S.A.; Huang, N.M. Electrodeposition of polypyrrole/reduced graphene oxide/iron oxide nanocomposite as supercapacitor electrode material. J. Nanomater. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Yu, D.; Yao, J.; Qiu, L.; Wang, Y.; Zhang, X.; Feng, Y.; Wang, H. In situ growth of Co3O4 nanoparticles on a-MnO2 nanotubes: A new hybrid for high-performance supercapacitors. J. Mater. Chem. A 2014, 2, 8465–8471. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, B.; Liu, G.; Dong, Y.; Cui, X.; Peng, X.; Zou, A.; Li, D. Micropore-dominant Vanadium and Iron Co-doped MnO2 hybrid film electrodes for high-performance supercapacitors. J. Electrochem. Soc. 2016, 163, A2725–A2732. [Google Scholar] [CrossRef]
- Wei, C.; Pang, H.; Zhang, B.; Lu, Q.; Liang, S.; Gao, F. Two-Dimensional β-MnO2 Nanowire Network with Enhanced Electrochemical Capacitance. Sci. Rep. 2013, 3, 2193. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lei, W.; Yao, T.; Xia, X.; Huang, W.; Hao, Q.; Wang, X. One-pot synthesis of graphene/SnO2/PEDOT ternary electrode material for supercapacitors. Electrochim. Acta 2013, 108, 118–126. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Lim, Y.S.; Tan, Y.P.; Lim, H.N.; Huang, N.M.; Tan, W.T.; Yarmo, M.A.; Yin, C.-Y. Potentiostatically deposited polypyrrole/graphene decorated nano-manganese oxide ternary film for supercapacitors. Ceram. Int. 2014, 40, 3855–3864. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef] [PubMed]
- Sankar, K.V.; Selvan, R.K. The ternary MnFe2O4/graphene/polyaniline hybrid composite as negative electrode for supercapacitors. J. Power Sources 2015, 275, 399–407. [Google Scholar] [CrossRef]
- Choi, H.-J.; Jung, S.-M.; Seo, J.-M.; Chang, D.W.; Dai, L.; Baek, J.-B. Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 2012, 1, 534–551. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, M.; Wang, S.; Zhang, X.; Huan, L.; Diao, G. Preparation of sandwich-like ternary hierarchical nanosheets manganese dioxide/polyaniline/reduced graphene oxide as electrode material for supercapacitor. Chem. Eng. J. 2016, 304, 29–38. [Google Scholar] [CrossRef]
- Pan, C.; Gu, H.; Dong, L. Synthesis and electrochemical performance of polyaniline @MnO2/graphene ternary composites for electrochemical supercapacitors. J. Power Sources 2016, 303, 175–181. [Google Scholar] [CrossRef]
- Han, G.; Liu, Y.; Kan, E.; Tang, J.; Zhang, L.; Wang, H.; Tang, W. Sandwich-structured MnO2/polypyrrole/reduced graphene oxide hybrid composites for high-performance supercapacitors. RSC Adv. 2014, 4, 9898–9904. [Google Scholar] [CrossRef]
- Ramli, N.I.T.; Abdul Rashid, S.; Sulaiman, Y.; Mamat, M.S.; Mohd Zobir, S.A.; Krishnan, S. Physicochemical and electrochemical properties of carbon nanotube/graphite nanofiber hybrid nanocomposites for supercapacitor. J. Power Sources 2016, 328, 195–202. [Google Scholar] [CrossRef]
- Zhou, H.; Zhai, H.-J.; Han, G. Superior performance of highly flexible solid-state supercapacitor based on the ternary composites of graphene oxide supported poly(3,4-ethylenedioxythiophene)-carbon nanotubes. J. Power Sources 2016, 323, 125–133. [Google Scholar] [CrossRef]
- Shabani Shayeh, J.; Ehsani, A.; Ganjali, M.R.; Norouzi, P.; Jaleh, B. Conductive polymer/reduced graphene oxide/Au nano particles as efficient composite materials in electrochemical supercapacitors. Appl. Surf. Sci. 2015, 353, 594–599. [Google Scholar] [CrossRef]
- Zhou, H.; Han, G.; Xiao, Y.; Chang, Y.; Zhai, H.-J. Facile preparation of polypyrrole/graphene oxide nanocomposites with large areal capacitance using electrochemical codeposition for supercapacitors. J. Power Sources 2014, 263, 259–267. [Google Scholar] [CrossRef]
- Wang, W.; Hao, Q.; Lei, W.; Xia, X.; Wang, X. Graphene/SnO2/polypyrrole ternary nanocomposites as supercapacitor electrode materials. RSC Adv. 2012, 2, 10268–10274. [Google Scholar] [CrossRef]
- Prasankumar, T.; Irthaza Aazem, V.S.; Raghavan, P.; Prem Ananth, K.; Biradar, S.; Ilangovan, R.; Jose, S. Microwave assisted synthesis of 3D network of Mn/Zn bimetallic oxide-high performance electrodes for supercapacitors. J. Alloys Compd. 2017, 695, 2835–2843. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azman, N.H.N.; Lim, H.N.; Mamat, M.S.; Sulaiman, Y. Synergistic Enhancement of Ternary Poly(3,4-ethylenedioxythiophene)/Graphene Oxide/Manganese Oxide Composite as a Symmetrical Electrode for Supercapacitors. Energies 2018, 11, 1510. https://doi.org/10.3390/en11061510
Azman NHN, Lim HN, Mamat MS, Sulaiman Y. Synergistic Enhancement of Ternary Poly(3,4-ethylenedioxythiophene)/Graphene Oxide/Manganese Oxide Composite as a Symmetrical Electrode for Supercapacitors. Energies. 2018; 11(6):1510. https://doi.org/10.3390/en11061510
Chicago/Turabian StyleAzman, Nur Hawa Nabilah, Hong Ngee Lim, Md Shuhazlly Mamat, and Yusran Sulaiman. 2018. "Synergistic Enhancement of Ternary Poly(3,4-ethylenedioxythiophene)/Graphene Oxide/Manganese Oxide Composite as a Symmetrical Electrode for Supercapacitors" Energies 11, no. 6: 1510. https://doi.org/10.3390/en11061510




