Experimental Investigation of the Use of Waste Mineral Oils as a Fuel with Organic-Based Mn Additive
Abstract
1. Introduction
2. Materials and Method
2.1. Waste Mineral Oil
2.2. Organic-Based Mn Additive and Fuel
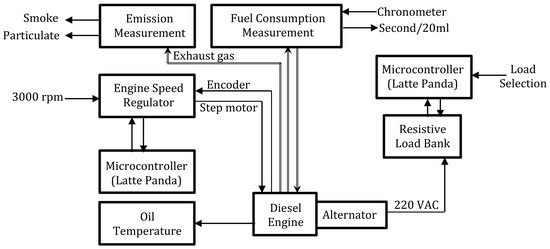
2.3. Experimental Setup
2.4. Error Analysis and Uncertainties
3. Results and Discussion
3.1. Some Properties of the Obtained Test Fuel
3.2. Effect of Mn Additive on Stable Working Temperature
3.3. Effect of Mn Additive on Specific Fuel Consumption
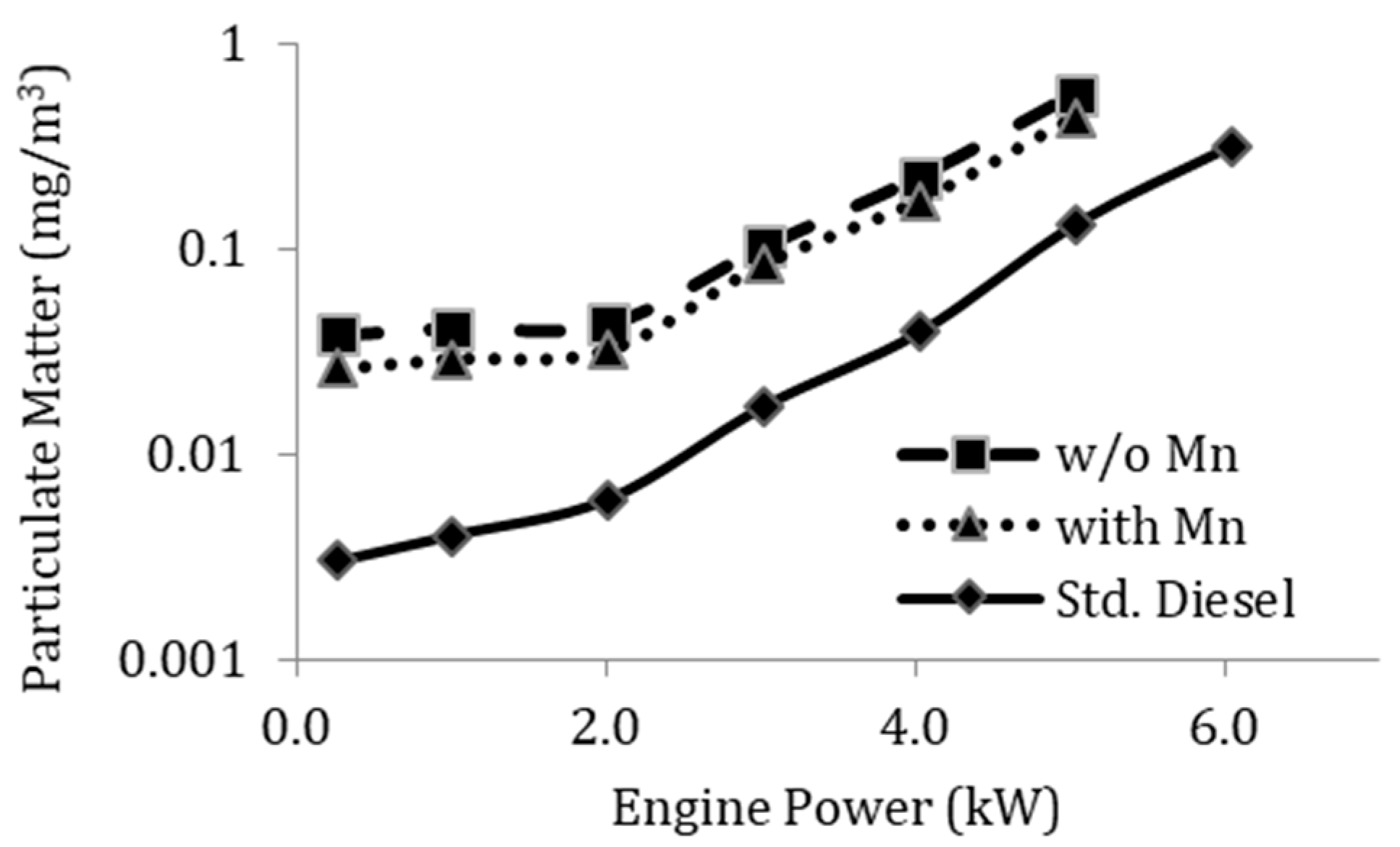
3.4. Effect of Mn Additive on Exhaust Emission
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Knothe, G.; Krahl, J.; Van Gerpen, J. The Biodiesel Handbook, 2nd ed.; Elsevier: England, UK, 2010; pp. 5–96. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Widayat, W.; Duma, A. Ultrasound Assısted in Situ Esterification of Rubber Seeds Oil for Biodiesel Production (Research Note). Int. J. Eng. Trans. C 2016, 29, 1635–1641. [Google Scholar] [CrossRef]
- Watson, K.S.; Meierhoefer, C.H. Use or disposal of by-products and spent material from the vegetable oil processing industry in the U.S. J. Am. Oil Chem. Soc. 1976, 53, 437–442. [Google Scholar] [CrossRef]
- Hassani, M.; Amini, G.; Najafpour, D.; Rabie, M. A two-step Catalytic Production of Biodiesel from waste cooking oil. Int. J. Eng. Trans. C 2013, 26, 563–570. [Google Scholar] [CrossRef]
- Samavati, M.; Martin, A.; Santarelli, M.; Nemanova, V. Synthetic Diesel Production as a Form of Renewable Energy Storage. Energies 2018, 11, 1223. [Google Scholar] [CrossRef]
- Hamilton, J.; Negnevitsky, M.; Wang, X. Economics of Renewable Energy Integration and Energy Storage via Low Load Diesel Application. Energies 2018, 11, 1080. [Google Scholar] [CrossRef]
- Rowhani, A.; Rainey, T.J. Scrap Tyre Management Pathways and Their Use as a Fuel—A Review. Energies 2016, 9, 888. [Google Scholar] [CrossRef]
- Rosa, R.N. The Role of Synthetic Fuels for a Carbon Neutral Economy. C 2017, 3, 11. [Google Scholar] [CrossRef]
- Trongkaew, P.; Utistham, T.; Reubroycharoen, P.; Hinchiranan, N. Photocatalytic Desulfurization of Waste Tire Pyrolysis Oil. Energies 2011, 4, 1880–1896. [Google Scholar] [CrossRef]
- Lam, S.S.; Russell, A.D.; Chase, H.A. Microwave pyrolysis, a novel process for recycling waste automotive engine oil. Energy 2010, 35, 2985–2991. [Google Scholar] [CrossRef]
- Procházka, P.; Hönig, V. Economic Analysis of Diesel-Fuel Replacement by Crude Palm Oil in Indonesian Power Plants. Energies 2018, 11, 504. [Google Scholar]
- Mitchell, H. Legislation and regulation of waste oil disposal. Environ. Policy Law 1977, 3, 27. [Google Scholar] [CrossRef]
- UNEP United Nations Environment Programme; Tamunaidu, P.; Bhatia, S.; Nasim, M.N.; Pervez, M.S.; Yarasu, R.B.; Aguirre Ortega, B. Waste oils as alternative fuel for diesel engine: A review. Stand. Test Methods Pet. Prod. Lubr. 1998, 5, 30–43. [Google Scholar] [CrossRef]
- Arpa, O.; Yumrutas, R.; Demirbas, A. Production of diesel-like fuel from waste engine oil by pyrolitic distillation. Appl. Energy 2010, 87, 122–127. [Google Scholar] [CrossRef]
- European Parliament and of the Council. Directive 2008/98/EC. Off. J. Eur. Union 2008, L312, 3–30. [Google Scholar]
- Audibert, F. Waste Engine Oils; Elsevier: New York, NY, USA, 2006. [Google Scholar]
- Balat, M. Diesel-like fuel obtained by catalytic pyrolysis of waste engine oil. Energy Explor. Exploit. 2008, 26, 197–208. [Google Scholar] [CrossRef]
- Balat, M.; Demirbas, M.F.; Balat, M. Pyrolysis of waste engine oil in the presence of wood ash. Energy Sources Part A Recovery Util. Environ. Effects 2009, 31, 1494–1499. [Google Scholar] [CrossRef]
- Demirbas, A. Gasoline-like fuel from waste engine oil via catalytic pyrolysis. Energy Sources Part A Recovery Util. Environ. Effects 2008, 30, 1433–1441. [Google Scholar] [CrossRef]
- Kannan, M.; Saravanan, C.G. Behaviour of zeolite 4a in the extraction process of the diesel like fuel obtained from waste engine oil. J. Eng. Sci. Technol. 2015, 10, 1553–1559. [Google Scholar]
- Maceiras, R.; Alfonsín, V.; Morales, F.J. Recycling of waste engine oil for diesel production. Waste Manag. 2017, 60, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, B.; Zachariah, Z.T. Production of fuel from waste engine oil and study of performance and emission characteristics in a diesel engine. Int. J. ChemTech Res. 2016, 9, 474–481. [Google Scholar]
- Zandi-Atashbar, N.; Ensafi, A.A.; Ahoor, A.H. Nano-CeO2/SiO2 as an efficient catalytic conversion of waste engine oil into liquid fuel. J. Clean. Prod. 2017, 166, 1010–1019. [Google Scholar] [CrossRef]
- Aburas, H.; Bafail, A.; Demirbas, A. The pyrolizing of waste lubricating oil (WLO) into diesel fuel over a supported calcium oxide additive. Petrol. Sci. Technol. 2015, 33, 226–236. [Google Scholar] [CrossRef]
- Petder. Waste Engıne Oıl Management Project. Available online: http://www.petder.org.tr/ (accessed on 19 February 2018).
- Ori-Jesu, A.; Ori-Jesu, M. Biodegradation of mineral oils—A review. Afr. J. Biotechnol. 2009, 8, 915–920. [Google Scholar]
- Fingas, M. Handbook of Oil Spill Science and Technology; John Wiley & Sons.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Çaynak, S.; Gürü, M.; Biçer, A.; Keskin, A.; Içingür, Y. Biodiesel production from pomace oil and improvement of its properties with synthetic manganese additive. Fuel 2009, 88, 534–538. [Google Scholar] [CrossRef]
- Gürü, M.; Artukoǧlu, B.D.; Keskin, A.; Koca, A. Biodiesel production from waste animal fat and improvement of its characteristics by synthesized nickel and magnesium additive. Energy Convers. Manag. 2009, 50, 498–502. [Google Scholar] [CrossRef]
- Gürü, M.; Karakaya, U.; Altıparmak, D.; Alıcılar, A. Improvement of Diesel fuel properties by using additives. Energy Convers. Manag. 2002, 43, 1021–1025. [Google Scholar] [CrossRef]
- Gürü, M.; Koca, A.; Can, Ö.; Çinar, C.; Şahin, F. Biodiesel production from waste chicken fat based sources and evaluation with Mg based additive in a diesel engine. Renew. Energy 2010, 35, 637–643. [Google Scholar] [CrossRef]
- Keskin, A.; Gürü, M.; Altıparmak, D. Influence of metallic based fuel additives on performance and exhaust emissions of diesel engine. Energy Convers. Manag. 2011, 52, 60–65. [Google Scholar] [CrossRef]
- Keskin, A.; Gürü, M.; Altiparmak, D. Biodiesel production from tall oil with synthesized Mn and Ni based additives: Effects of the additives on fuel consumption and emissions. Fuel 2007, 86, 1139–1143. [Google Scholar] [CrossRef]
- Keskin, A.; Gürü, M.; Altiparmak, D. Influence of tall oil biodiesel with Mg and Mo based fuel additives on diesel engine performance and emission. Bioresour. Technol. 2008, 99, 6434–6438. [Google Scholar] [CrossRef] [PubMed]
- Specifications of the Diesel Engine Generator. Available online: http://www.bobig.cn/HTML/Product/Engine/BD186FA_FAE.html (accessed on 19 February 2018).
- Holman, J.P. Experimental Methods for Engineers; McGraw Hill Press: New York, NY, USA, 2001. [Google Scholar]
- Lapuerta, M.; Rodríguez-Fernández, J.; Fernández-Rodríguez, D.; Patiño-Camino, R. Modeling viscosity of butanol and ethanol blends with diesel and biodiesel fuels. Fuel 2017, 199, 332–338. [Google Scholar] [CrossRef]
- EN 590. Available online: http://www.envirochem.hu/www.envirochem.hu/documents/EN_590_2009_hhV05.pdf (accessed on 19 February 2018).
- Jakhrani, A.Q.; Othman, A.-K.; Rigit, A.R.H.; Samo, S.R. Estimation of carbon footprints from diesel generator emissions. In Proceedings of the 2012 International Conference on Green and Ubiquitous Technology, Miami, FL, USA, 7–8 July 2012; pp. 78–81. [Google Scholar]
- Geng, P.; Cao, E.; Tan, Q.; Wei, L. Effects of alternative fuels on the combustion characteristics and emission products from diesel engines: A review. Renew. Sustain. Energy Rev. 2017, 71, 523–534. [Google Scholar] [CrossRef]
- NguyenThi, T.X.; Bazile, J.-P.; Bessières, D. Density Measurements of Waste Cooking Oil Biodiesel and Diesel Blends over Extended Pressure and Temperature Ranges. Energies 2018, 11, 1212. [Google Scholar] [CrossRef]
- Yusop, A.F.; Mamat, R.; Yusaf, T.; Najafi, G.; Yasin, M.H.M.; Khathri, A.M. Analysis of Particulate Matter (PM) Emissions in Diesel Engines Using Palm Oil Biodiesel Blended with Diesel Fuel. Energies 2018, 11, 1039. [Google Scholar] [CrossRef]
- Da Silva Araújo, F.D.; do Nascimento Cavalcante, A.; Sousa, M.D.B.; de Moura, C.V.R.; Chaves, M.H.; Aued-Pimentel, S.; Fernandes Caruso, M.S.; Tozetto, L.J.; Kaline Morais Chaves, S. Biodiesel Production from Bombacopsis glabra Oil by Methyl Transesterification Method. Energies 2017, 10, 1360. [Google Scholar] [CrossRef]







| Specification | Explanation |
|---|---|
| Generator model | P-7500 DE |
| Engine model | 186-FAE |
| Alternator type | Monophase |
| Max alternator power | 5 kW |
| Contunious alternator power | 4.5 kW |
| Alternator speed | 3000 rpm (50 Hz) |
| Alternator mechanical efficiency | 0.8 |
| Mean fuel consumption | 2.23 L/h |
| Max engine power | 6.4 kW@3000 rpm |
| Contunions engine power | 5.7 kW@3000 rpm |
| Cooling system | Air cooled |
| Intake system | Natural aspirated |
| Stroke × Diameter | 86 mm × 72 mm |
| Compression ratio | 19:1 |
| Stroke volume | 418 cm3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdalyan, B.; Orman, R.Ç. Experimental Investigation of the Use of Waste Mineral Oils as a Fuel with Organic-Based Mn Additive. Energies 2018, 11, 1512. https://doi.org/10.3390/en11061512
Özdalyan B, Orman RÇ. Experimental Investigation of the Use of Waste Mineral Oils as a Fuel with Organic-Based Mn Additive. Energies. 2018; 11(6):1512. https://doi.org/10.3390/en11061512
Chicago/Turabian StyleÖzdalyan, Bülent, and Recep Ç. Orman. 2018. "Experimental Investigation of the Use of Waste Mineral Oils as a Fuel with Organic-Based Mn Additive" Energies 11, no. 6: 1512. https://doi.org/10.3390/en11061512
APA StyleÖzdalyan, B., & Orman, R. Ç. (2018). Experimental Investigation of the Use of Waste Mineral Oils as a Fuel with Organic-Based Mn Additive. Energies, 11(6), 1512. https://doi.org/10.3390/en11061512