ZnS/SiO2 Passivation Layer for High-Performance of TiO2/CuInS2 Quantum Dot Sensitized Solar Cells
Abstract
1. Introduction
2. Results and Discussion
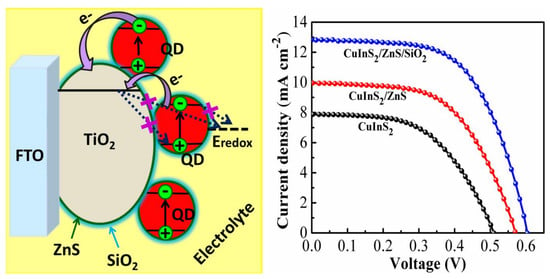
2.1. Morphological Characterization
2.2. Electrochemical Characterization
3. Materials and Methods
3.1. Preparation of TiO2 Electrodes
3.2. Deposition of CuInS2 QDs on TiO2 Electrodes
3.3. Deposition of ZnS, ZnS/SiO2 Passivation Layers on TiO2/CuInS2 Electrodes
3.4. QDSSC Fabrication
3.5. Characterizations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, H.J.; Wang, M.; Chen, P.; Gamelin, D.R.; Zakeeruddin, S.M.; Grätzel, M.; Nazeeruddin, M.K. Efficient CdSe Quantum Dot-Sensitized Solar Cells Prepared by an Improved Successive Ionic Layer Adsorption and Reaction Process. Nano Lett. 2009, 9, 4221–4227. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Jia, J.; Lin, Y.; Zhou, X. Enhanced performance of CdTe quantum dot sensitized solar cell via anion exchanges. J. Power Sources 2015, 277, 215–221. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Haritha, M.V.; Kim, S.K.; Kim, H.J. A strategy to improve the energy conversion efficiency and stability of quantum dot-sensitized solar cells using manganese-doped cadmium sulfide quantum dots. Dalton Trans. 2015, 44, 630–638. [Google Scholar] [CrossRef] [PubMed]
- González-Pedro, V.; Sima, C.; Marzari, G.; Boix, P.P.; Giménez, S.; Shen, Q.; Dittrich, T.; Seró, I.M. High performance PbS Quantum Dot Sensitized Solar Cells exceeding 4% efficiency: the role of metal precursors in the electron injection and charge separation. Phys. Chem. Chem. Phys. 2013, 15, 13835–13843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, J.; Church, C.P.; Miller, E.M.; Luther, J.M.; Klimov, V.I.; Beard, M.C. PbSe Quantum Dot Solar Cells with More than 6% Efficiency Fabricated in Ambient Atmosphere. Nano Lett. 2014, 14, 6010–6015. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V. Quantum Dot Solar Cells. Semiconductor Nanocrystals as Light Harvesters. J. Phys. Chem. C 2008, 112, 18737–18753. [Google Scholar] [CrossRef]
- Kongkanand, A.; Tvrdy, K.; Takechi, K.; Kuno, M.; Kamat, P.V. Quantum Dot Solar Cells. Tuning Photoresponse through Size and Shape Control of CdSe−TiO2 Architecture. J. Am. Chem. Soc. 2008, 130, 4007–4015. [Google Scholar] [CrossRef] [PubMed]
- Booth, M.; Brown, A.P.; Evans, S.D.; Critchley, K. Determining the Concentration of CuInS2 Quantum Dots from the Size-Dependent Molar Extinction Coefficient. Chem. Mater. 2012, 24, 2064–2070. [Google Scholar] [CrossRef]
- Leach, A.D.P.; Macdonald, J.E. Optoelectronic Properties of CuInS2 Nanocrystals and Their Origin. J. Phys. Chem. Lett. 2016, 7, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Li, T.L.; Lee, Y.L.; Teng, H. CuInS2 quantum dots coated with CdS as high-performance sensitizers for TiO2 electrodes in photoelectrochemical cells. J. Mater. Chem. 2011, 21, 5089–5098. [Google Scholar] [CrossRef]
- Bang, J.H.; Kamat, P.V. Quantum Dot Sensitized Solar Cells. A Tale of Two Semiconductor Nanocrystals: CdSe and CdTe. ACS Nano 2009, 3, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wan, Q.; Luan, C.; Mei, F.; Zhao, Q.; An, P.; Liang, Z.; Xu, G.; Zapien, J.A. Fabrication of CuInS2-Sensitized Solar Cells via an Improved SILAR Process and Its Interface Electron Recombination. ACS Appl. Mater. Interfaces 2013, 5, 10605–10613. [Google Scholar] [CrossRef] [PubMed]
- Kontos, A.G.; Likodimos, V.; Vassalou, E.; Kapogianni, I.; Raptis, Y.S.; Raptis, C.; Falaras, P. Nanostructured titania films sensitized by quantum dot chalcogenides. Nanoscale Res. Lett. 2011, 6, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Bang, J.; Park, J.; Kim, S.; Park, S.M. Multilayered semiconductor (CdS/CdSe/ZnS)-sensitized TiO2 mesoporous solar cells: All prepared by successive ionic layer adsorption and reaction processes. Chem. Mater. 2010, 22, 5636–5643. [Google Scholar] [CrossRef]
- Chang, J.Y.; Su, L.F.; Li, C.H.; Chang, C.C.; Lin, J.M. Efficient “green” quantum dot-sensitized solar cells based on Cu2S–CuInS2–ZnSe architecture. Chem. Commun. 2012, 48, 4848–4850. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yuan, S.; Fan, J.; Hou, Z.; Zhou, W.; Du, Z.; Wu, S. CuInS2 quantum dot-sensitized TiO2 nanorod array photoelectrodes: Synthesis and performance optimization. Nanoscale Res. Lett. 2012, 7, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Zhou, X.; Qiu, Z.; Liu, C.; Chen, J.; Yue, W.; Wang, M.; Bi, H. Reduced graphene oxide-supported aggregates of CuInS2 quantum dots as an effective hybrid electron acceptor for polymer-based solar cells. Carbon 2016, 96, 532–540. [Google Scholar] [CrossRef]
- Han, M.; Jia, J.; Yu, L.; Yi, G. Fabrication and photoelectrochemical characteristics of CuInS2 and PbS quantum dot co-sensitized TiO2 nanorod photoelectrodes. RSC Adv. 2015, 5, 51493–51500. [Google Scholar] [CrossRef]
- Diguna, L.J.; Qing, S.; Kobayashi, J.; Toyoda, T. High efficiency of CdSe quantum-dot-sensitized TiO2 inverse opal solar cells. Appl. Phys. Lett. 2007, 91, 023116–023118. [Google Scholar] [CrossRef]
- De la Fuente, M.S.; Sánchez, R.S.; González-Pedro, V.; Boix, P.P.; Mhaisalkar, S.G.; Rincón, M.E.; Bisquert, J.; Mora-Seró, I. Effect of Organic and Inorganic Passivation in Quantum-Dot-Sensitized Solar Cells. J. Phys. Chem. Lett. 2013, 4, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Gopi, C.V.V.M.; Haritha, M.V.; Kim, S.K.; Kim, H.J. Improved photovoltaic performance and stability of quantum dot sensitized solar cells using Mn–ZnSe shell structure with enhanced light absorption and recombination control. Nanoscale 2015, 7, 12552–12563. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.W.; Gopi, C.V.V.M.; Kim, S.K.; Rao, S.S.; Jeong, M.S. Improved performance of quantum dot-sensitized solar cells adopting a highly efficient cobalt sulfide/nickel sulfide composite thin film counter electrode. J. Power Sources 2014, 268, 163–170. [Google Scholar] [CrossRef]
- Liu, Z.; Miyauchi, M.; Uemura, Y.; Cui, Y.; Hara, K.; Zhao, Z.; Sunahara, K.; Furube, A. Enhancing the performance of quantum dots sensitized solar cell by SiO2 surface coating. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, D.J.; Rao, S.S.; Savariraj, A.D.; Kyoung, K.S.; Son, M.K.; Gopi, C.V.V.M.; Prabakar, K. Highly efficient solution processed nanorice structured NiS counterelectrode for quantum dot sensitized solar cell. Electrochim. Acta 2014, 127, 427–432. [Google Scholar] [CrossRef]
- Kim, H.J.; Sik, L.M.; Gopi, C.V.V.M.; Haritha, M.V.; Rao, S.S.; Kim, S.K. Cost-effective and morphology controllable PVP based highly efficient CuS counter electrodes for high-efficiency quantum dot-sensitized solar cells. Dalton Trans. 2015, 44, 11340–11351. [Google Scholar] [CrossRef] [PubMed]







| Cell | Voc (V) | Jsc (mA cm−2) | FF | η% | RS (Ω) | RCE (Ω) | Rct (Ω) |
|---|---|---|---|---|---|---|---|
| CuInS2 | 0.509 | 7.87 | 0.537 | 2.15 | 9.34 | 0.84 | 30.65 |
| CuInS2/ZnS | 0.569 | 9.95 | 0.571 | 3.23 | 10.03 | 1.03 | 23.95 |
| CuInS2/ZnS/SiO2 | 0.603 | 12.83 | 0.598 | 4.63 | 10.24 | 1.16 | 55.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Bae, J.-H.; Seo, H.; Shiratani, M.; Venkata Veera Muralee Gopi, C. ZnS/SiO2 Passivation Layer for High-Performance of TiO2/CuInS2 Quantum Dot Sensitized Solar Cells. Energies 2018, 11, 1931. https://doi.org/10.3390/en11081931
Kim H-J, Bae J-H, Seo H, Shiratani M, Venkata Veera Muralee Gopi C. ZnS/SiO2 Passivation Layer for High-Performance of TiO2/CuInS2 Quantum Dot Sensitized Solar Cells. Energies. 2018; 11(8):1931. https://doi.org/10.3390/en11081931
Chicago/Turabian StyleKim, Hee-Je, Jin-Ho Bae, Hyunwoong Seo, Masaharu Shiratani, and Chandu Venkata Veera Muralee Gopi. 2018. "ZnS/SiO2 Passivation Layer for High-Performance of TiO2/CuInS2 Quantum Dot Sensitized Solar Cells" Energies 11, no. 8: 1931. https://doi.org/10.3390/en11081931
APA StyleKim, H.-J., Bae, J.-H., Seo, H., Shiratani, M., & Venkata Veera Muralee Gopi, C. (2018). ZnS/SiO2 Passivation Layer for High-Performance of TiO2/CuInS2 Quantum Dot Sensitized Solar Cells. Energies, 11(8), 1931. https://doi.org/10.3390/en11081931