SDC-Infiltrated Microporous Silver Membrane with Superior Resistance to Thermal Agglomeration for Cathode-Supported Solid Oxide Fuel Cells
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of SDC Precursor Solution and SDC Infiltration Process
2.2. Fabrication of SDC-Infiltrated Silver Cathode-Supported Cell
2.3. Electrochemical Characterization
3. Result and Discussion
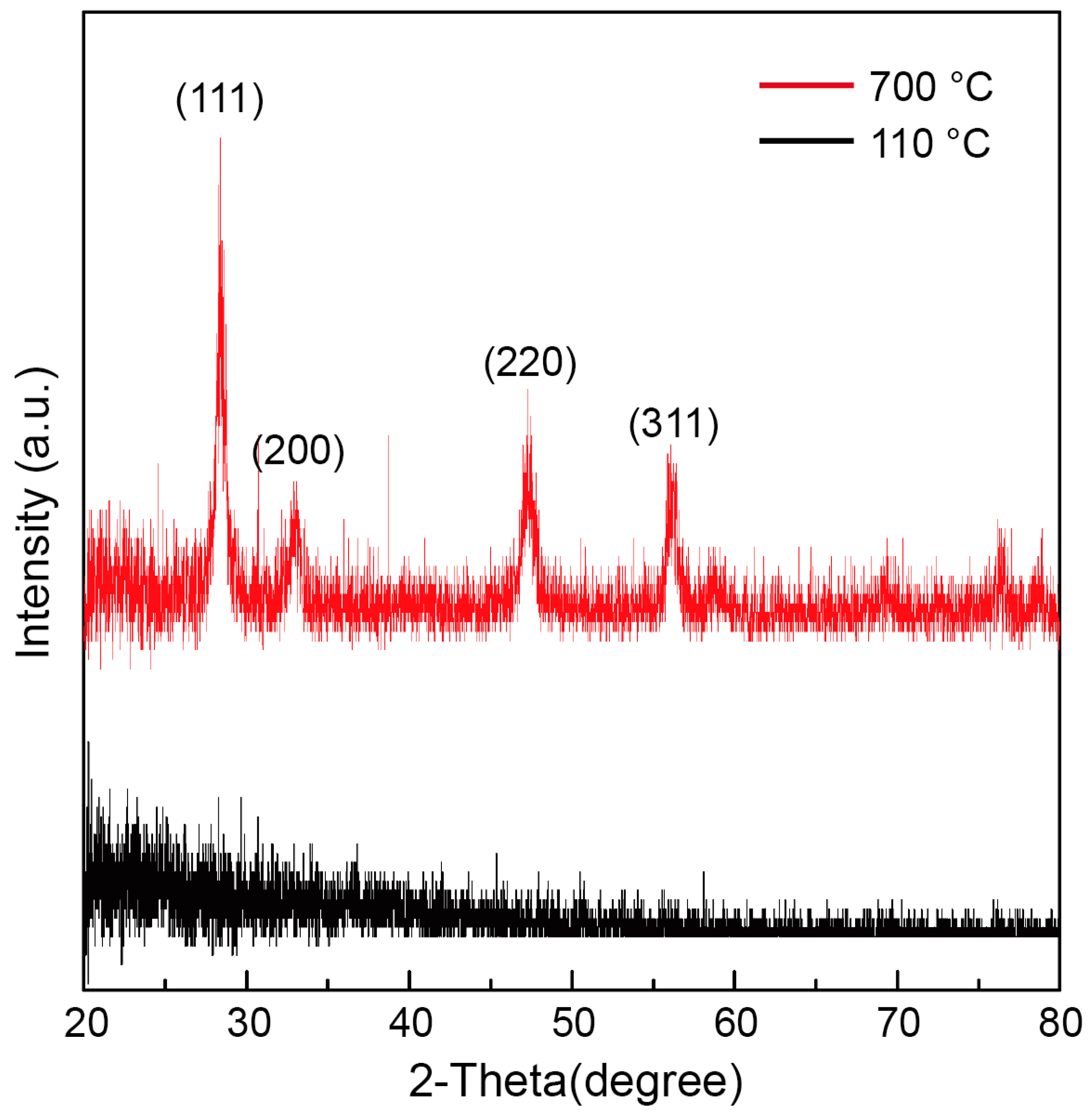
3.1. Crystallinity of SDC Precursor Solution
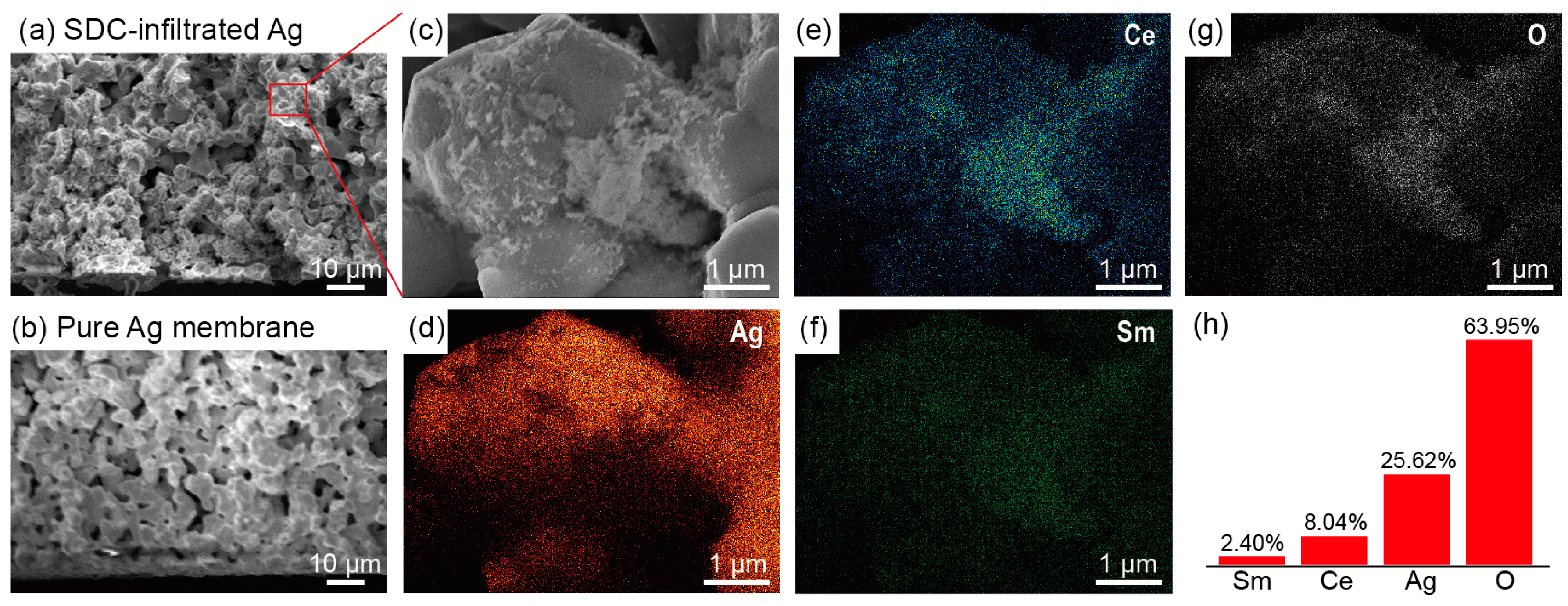
3.2. Morphology and Element Distribution of SDC-Infiltrated Silver Membrane
3.3. Thermo-Morphological Stability of SDC-Infiltrated Silver Membrane
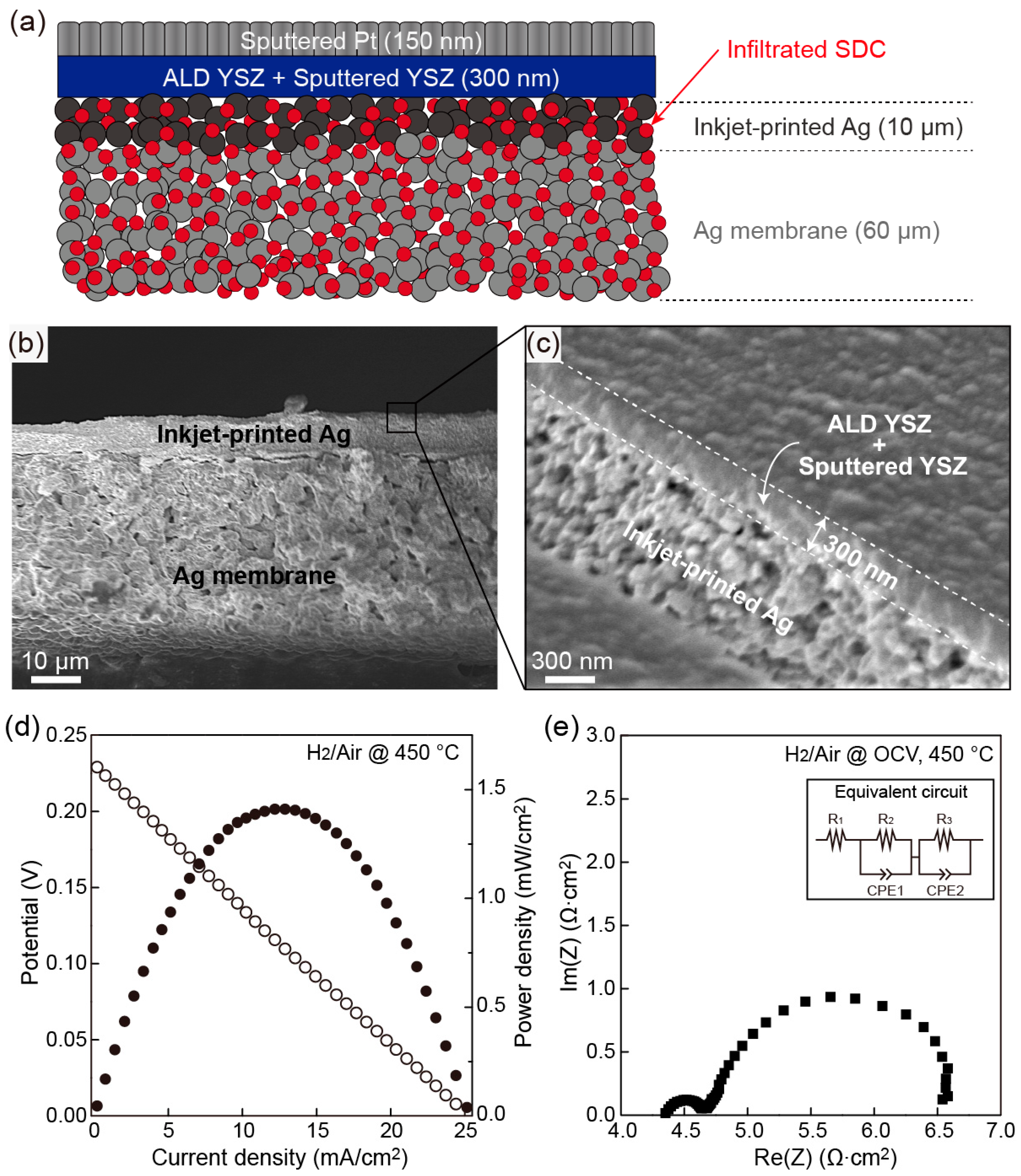
3.4. Electrochemical Characterization of SDC-Infiltrated Silver Cathode-Supported Cell
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Su, P.C.; Chao, C.C.; Shim, J.H.; Fasching, R.; Prinz, F.B. Solid oxide fuel cell with corrugated thin film electrolyte. Nano Lett. 2008, 8, 2289–2292. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Su, P.C.; Park, Y.I.; Saito, Y.; Prinz, F.B. Thin-Film Solid Oxide Fuel Cells on Porous Nickel Substrates with Multistage Nanohole Array. J. Electrochem. Soc. 2006, 153, A554. [Google Scholar] [CrossRef]
- Rey-Mermet, S.; Muralt, P. Solid oxide fuel cell membranes supported by nickel grid anode. Solid State Ion. 2008, 179, 1497–1500. [Google Scholar] [CrossRef]
- Moon, Y.J.; Kang, H.; Kang, K.; Moon, S.J.; Hwang, J.Y. Effect of Thickness on Surface Morphology of Silver Nanoparticle Layer During Furnace Sintering. J. Electron. Mater. 2015, 44, 1192–1199. [Google Scholar] [CrossRef]
- Simrick, N.J.; Kilner, J.A.; Atkinson, A. Thermal stability of silver thin films on zirconia substrates. Thin Solid Films 2012, 520, 2855–2867. [Google Scholar] [CrossRef]
- West, M.; Manthiram, A. Synthesis of 3-dimensional silver networks and their application in solid oxide fuel cells. Int. J. Hydrogen Energy 2015, 40, 4234–4240. [Google Scholar] [CrossRef]
- Lee, Y.H.; Cho, G.Y.; Chang, I.; Ji, S.; Kim, Y.B.; Cha, S.W. Platinum-based nanocomposite electrodes for low-temperature solid oxide fuel cells with extended lifetime. J. Power Sources 2016, 307, 289–296. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, M.; Neoh, K.C.; Jang, D.Y.; Kim, H.J.; Shin, J.M.; Kim, G.T.; Shim, J.H. High-Performance Silver Cathode Surface Treated with Scandia-Stabilized Zirconia Nanoparticles for Intermediate Temperature Solid Oxide Fuel Cells. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Li, Y.K.; Choi, H.J.; Kim, H.K.; Chean, N.K.; Kim, M.; Koo, J.; Jeong, H.J.; Jang, D.Y.; Shim, J.H. Nanoporous silver cathodes surface-treated by atomic layer deposition of Y:ZrO2 for high-performance low-temperature solid oxide fuel cells. J. Power Sources 2015, 295, 175–181. [Google Scholar] [CrossRef]
- Neoh, K.C.; Han, G.D.; Kim, M.; Kim, J.W.; Choi, H.J.; Park, S.W.; Shim, J.H. Nanoporous silver cathode surface treated by atomic layer deposition of CeOx for low-temperature solid oxide fuel cells. Nanotechnology 2016, 27. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.; Ji, S.; Park, J.; Lee, M.H.; Cha, S.W. Ultrathin YSZ Coating on Pt Cathode for High Thermal Stability and Enhanced Oxygen Reduction Reaction Activity. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Liu, K.-Y.; Fan, L.; Yu, C.-C.; Su, P.-C. Thermal stability and performance enhancement of nano-porous platinum cathode in solid oxide fuel cells by nanoscale ZrO2 capping. Electrochem. Commun. 2015, 56, 65–69. [Google Scholar] [CrossRef]
- Jiang, S.P. Nanoscale and nano-structured electrodes of solid oxide fuel cells by infiltration: Advances and challenges. Int. J. Hydrogen Energy 2012, 37, 449–470. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Xia, C.R.; Chen, F.L. Nano-structured composite cathodes for intermediate-temperature solid oxide fuel cells via an infiltration/impregnation technique. Electrochim. Acta 2010, 55, 3595–3605. [Google Scholar] [CrossRef]
- Ding, D.; Li, X.X.; Lai, S.Y.; Gerdes, K.; Liu, M.L. Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 2014, 7, 552–575. [Google Scholar] [CrossRef]
- Huang, H.; Nakamura, M.; Su, P.C.; Fasching, R.; Saito, Y.; Prinz, F.B. High-performance ultrathin solid oxide fuel cells for low-temperature operation. J. Electrochem. Soc. 2007, 154, B20–B24. [Google Scholar] [CrossRef]
- Beckel, D.; Bieberle-Hutter, A.; Harvey, A.; Infortuna, A.; Muecke, U.P.; Prestat, M.; Rupp, J.L.M.; Gauckler, L.J. Thin films for micro solid oxide fuel cells. J. Power Sources 2007, 173, 325–345. [Google Scholar] [CrossRef]
- Takagi, Y.; Lai, B.-K.; Kerman, K.; Ramanathan, S. Low temperature thin film solid oxide fuel cells with nanoporous ruthenium anodes for direct methane operation. Energy Environ. Sci. 2011, 4, 3473–3478. [Google Scholar] [CrossRef]
- Garbayo, I.; Pla, D.; Morata, A.; Fonseca, L.; Sabate, N.; Tarancon, A. Full ceramic micro solid oxide fuel cells: Towards more reliable MEMS power generators operating at high temperatures. Energy Environ. Sci. 2014, 7, 3617–3629. [Google Scholar] [CrossRef]
- Baek, J.D.; Yoon, Y.-J.; Lee, W.; Su, P.-C. A circular membrane for nano thin film micro solid oxide fuel cells with enhanced mechanical stability. Energy Environ. Sci. 2015, 8, 3374–3380. [Google Scholar] [CrossRef]
- Bernay, C.; Ringuede, A.; Colomban, P.; Lincot, D.; Cassir, M. Yttria-doped zirconia thin films deposited by atomic layer deposition ALD: A structural, morphological and electrical characterisation. J. Phys. Chem. Solids 2003, 64, 1761–1770. [Google Scholar] [CrossRef]
- Shim, J.H.; Chao, C.C.; Huang, H.; Prinz, F.B. Atomic layer deposition of yttria-stabilized zirconia for solid oxide fuel cells. Chem. Mater. 2007, 19, 3850–3854. [Google Scholar] [CrossRef]
- Heiroth, S.; Lippert, T.; Wokaun, A.; Döbeli, M.; Rupp, J.L.M.; Scherrer, B.; Gauckler, L.J. Yttria-stabilized zirconia thin films by pulsed laser deposition: Microstructural and compositional control. J. Eur. Ceram. Soc. 2010, 30, 489–495. [Google Scholar] [CrossRef]
- Pergolesi, D.; Fabbri, E.; D’Epifanio, A.; Di Bartolomeo, E.; Tebano, A.; Sanna, S.; Licoccia, S.; Balestrino, G.; Traversa, E. High proton conduction in grain-boundary-free yttrium-doped barium zirconate films grown by pulsed laser deposition. Nat. Mater. 2010, 9, 846–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerman, K.; Lai, B.K.; Ramanathan, S. Free standing oxide alloy electrolytes for low temperature thin film solid oxide fuel cells. J. Power Sources 2012, 202, 120–125. [Google Scholar] [CrossRef]
- Kerman, K.; Lai, B.K.; Ramanathan, S. Pt/Y0.16Zr0.84O1.92/Pt thin film solid oxide fuel cells: Electrode microstructure and stability considerations. J. Power Sources 2011, 196, 2608–2614. [Google Scholar] [CrossRef]
- Tschope, A.; Birringer, R. Grain size dependence of electrical conductivity in polycrystalline cerium oxide. J. Electroceram. 2001, 7, 169–177. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Ran, R.; Chen, Y.; Shao, Z.; Liu, M. Promotion of Oxygen Reduction by Exsolved Silver Nanoparticles on a Perovskite Scaffold for Low-Temperature Solid Oxide Fuel Cells. Nano Lett. 2016, 16, 512–518. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-H.; Baek, J.D.; Fan, L.; Wiria, F.E.; Su, P.-C.; Lee, S.H. SDC-Infiltrated Microporous Silver Membrane with Superior Resistance to Thermal Agglomeration for Cathode-Supported Solid Oxide Fuel Cells. Energies 2018, 11, 2181. https://doi.org/10.3390/en11092181
Lee T-H, Baek JD, Fan L, Wiria FE, Su P-C, Lee SH. SDC-Infiltrated Microporous Silver Membrane with Superior Resistance to Thermal Agglomeration for Cathode-Supported Solid Oxide Fuel Cells. Energies. 2018; 11(9):2181. https://doi.org/10.3390/en11092181
Chicago/Turabian StyleLee, Tsung-Han, Jong Dae Baek, Liangdong Fan, Florencia Edith Wiria, Pei-Chen Su, and Seong Hyuk Lee. 2018. "SDC-Infiltrated Microporous Silver Membrane with Superior Resistance to Thermal Agglomeration for Cathode-Supported Solid Oxide Fuel Cells" Energies 11, no. 9: 2181. https://doi.org/10.3390/en11092181
APA StyleLee, T.-H., Baek, J. D., Fan, L., Wiria, F. E., Su, P.-C., & Lee, S. H. (2018). SDC-Infiltrated Microporous Silver Membrane with Superior Resistance to Thermal Agglomeration for Cathode-Supported Solid Oxide Fuel Cells. Energies, 11(9), 2181. https://doi.org/10.3390/en11092181




