Adapting the MgO-CO2 Working Pair for Thermochemical Energy Storage by Doping with Salts: Effect of the (LiK)NO3 Content †
Abstract
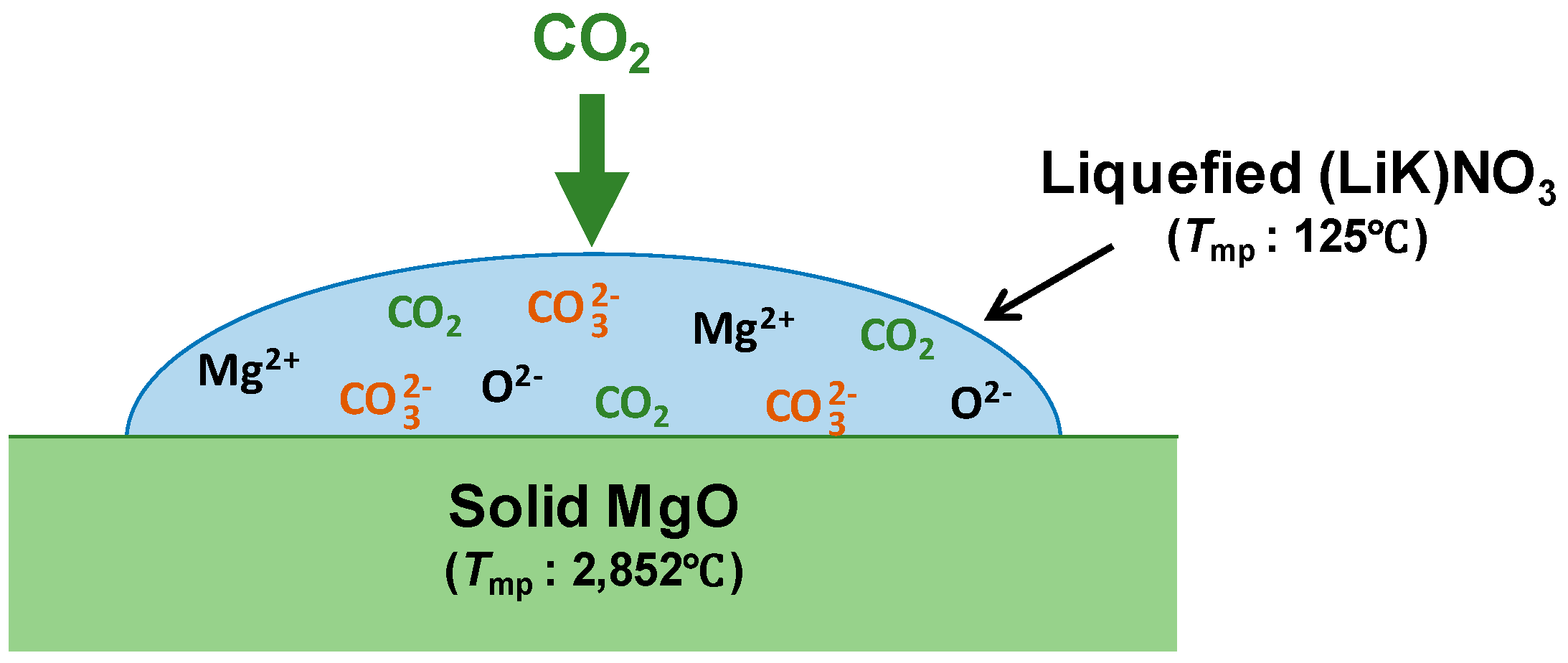
:1. Introduction
2. Experimental
2.1. Materials Preparation
2.2. Materials Characterization
3. Results and Discussion
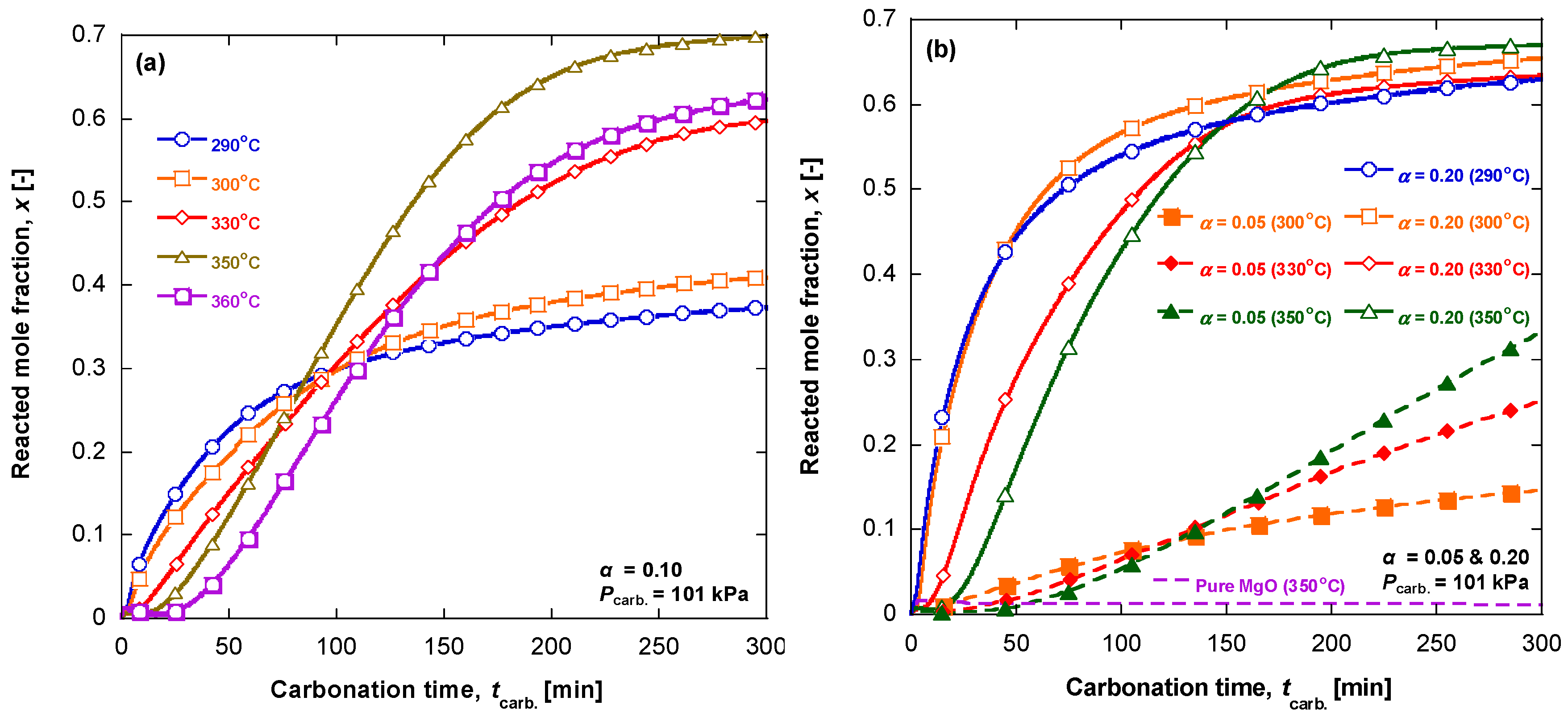
3.1. Carbonation Kinetics for Various Salt Contents
3.2. Carbonation Kinetics under Various Reaction Temperatures
- -
- the temperature effect on the CO2 and MgO solubilities in the melt. It is known that the CO2 solubility in a KNO3 melt passes a maximum at 375 °C and reduces at the higher temperature, whereas the CO2 solubility in a LiNO3 melt gradually increases with temperature [24];
- -
- the dependence of the CO2, Mg2+ and O2− diffusivities on temperature, as their diffusion can be responsible for very slow approaching the equilibrium.
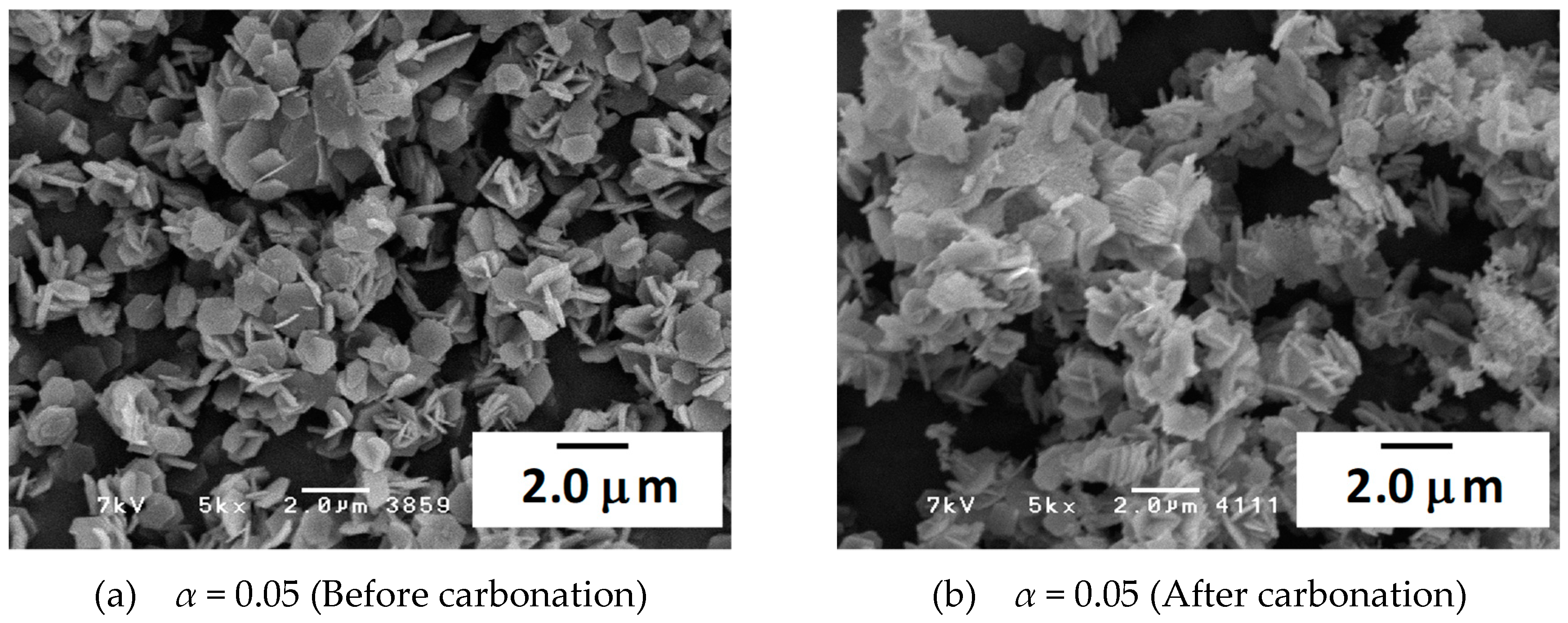
3.3. Characterization of the Materials by SEM
3.4. Evaluation of the Specific Useful Heat
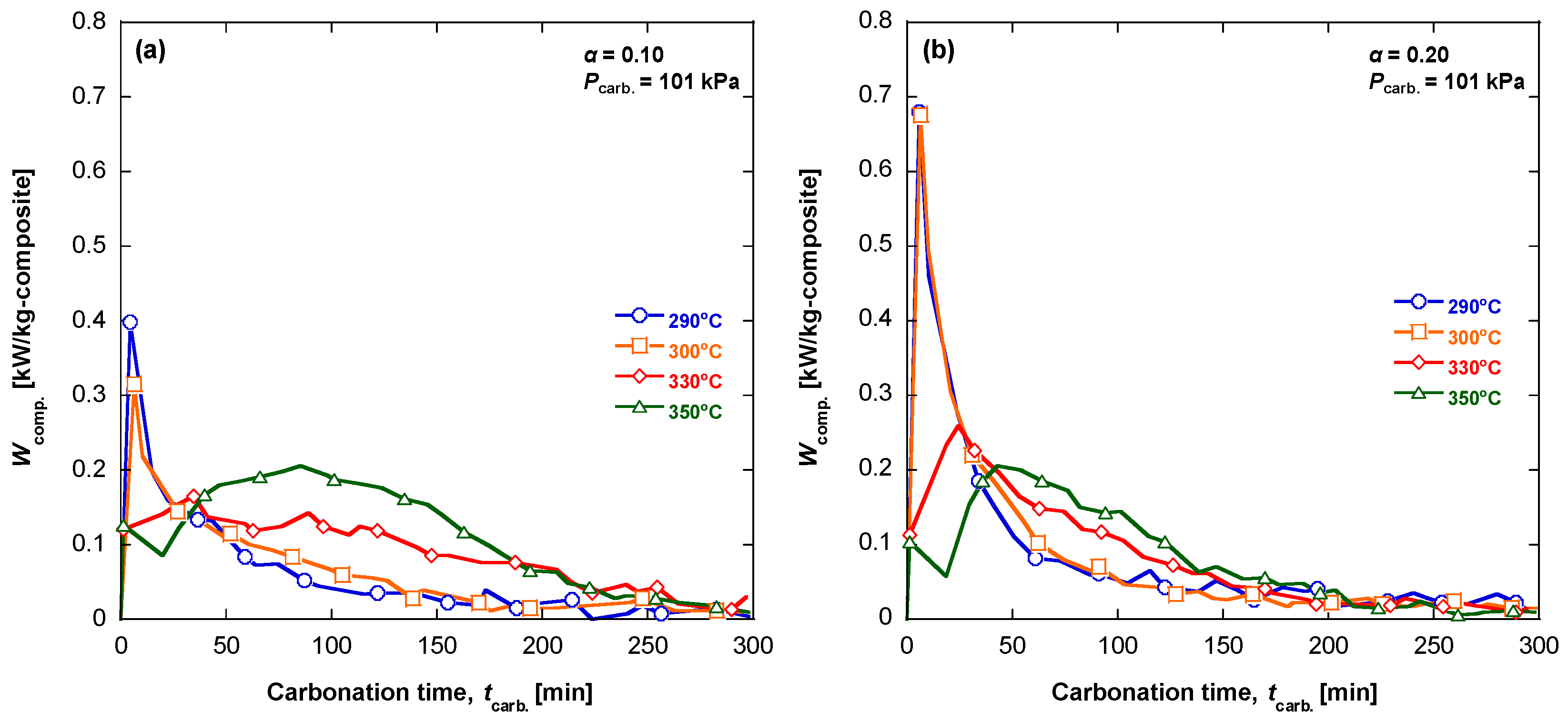
3.5. Evaluation of the Specific Power of Heat Release
4. Material Durability on Cyclic Experiment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| TCES | thermochemical energy storage |
| TG | thermogravimetric |
| SEM | scanning electron microscopy |
| n | amount of a chemical compound [mol] |
| n0 | initial amount of MgO in a composite [mol] |
| Mcomp. | molar mass of a composite [g/mol] |
| Qcomp. | specific useful heat [kJ/kg-composite] |
| Tcarb. | carbonation temperature [°C] |
| Teq | equilibrium transition temperature [°C] |
| Tmp | melting point [°C] |
| Wcomp. | heat output [kW/kg] |
| X | reacted mole fraction, [-] |
| A | molar fraction of salt in a composite [-] |
| reaction enthalpy [kJ/mol] |
References
- Zsembinszki, G.; Sole, A.; Barreneche, C.; Prieto, C.; Fernández, A.I.; Cabeza, L.F. Review of reactors with potential use in thermochemical energy storage in concentrated solar power plants. Energies 2018, 11, 2358. [Google Scholar] [CrossRef]
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1-Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Kalaiselvam, S.; Parameshwaran, R.; Kalaiselvam, S.; Parameshwaran, R. Thermal Energy Storage Technologies for Sustainability, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2014; pp. 127–144. ISBN 978-0124172913. [Google Scholar]
- Lovegrove, K.; Luzzi, A.; Soldiani, I.; Kreetz, H. Developing ammonia based thermochemical energy storage for dish power plants. Sol. Energy 2004, 76, 331–337. [Google Scholar] [CrossRef]
- Shkatulov, A.I.; Aristov, Y. Thermochemical Energy Storage using LiNO3-Doped Mg(OH)2: A Dehydration Study. Energy Technol. 2018, 6, 1844–1851. [Google Scholar] [CrossRef]
- Shkatulov, A.I.; Aristov, Y. Calcium hydroxide doped by KNO3 as a promising candidate for thermochemical storage of solar heat. RSC Adv. 2017, 7, 42929–42939. [Google Scholar] [CrossRef]
- Lutz, M.; Bhouri, M.; Linder, M.; Bürger, I. Adiabatic magnesium hydride system for hydrogen storage based on thermochemical heat storage: Numerical analysis of the dehydrogenation. Appl. Energy 2019, 236, 1034–1048. [Google Scholar] [CrossRef]
- Wentworth, W.E.; Chen, E. Simple thermal decomposition reactions for storage of solar thermal energy. Solar Energy 1976, 18, 205–214. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Gonzalez-Aguilar, J.; Romero, M.; Coronado, J.M. Solar energy on demand: A review on high temperature thermochemical heat storage systems and materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Flamant, G. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715. [Google Scholar] [CrossRef]
- Pan, Z.H.; Zhao, C.Y. Gas–solid thermochemical heat storage reactors for high-temperature applications. Energy 2017, 130, 155–173. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef] [Green Version]
- L’vov, B.V.; Ugolkov, V.L. Kinetics of free-surface decomposition of magnesium, strontium and barium carbonates analyzed thermogravimetrically by the third-law method. Thermochim. Acta 2004, 409, 13–18. [Google Scholar] [CrossRef]
- Bhatta, L.K.G.; Subramanyam, S.; Chengala, M.D.; Olivera, S.; Venkatesh, K. Progress in hydrotalcite like compounds and metal-based oxides for CO2 capture: A review. J. Clean. Prod. 2015, 103, 171–196. [Google Scholar] [CrossRef]
- Shkatulov, A.I.; Kim, S.T.; Miura, H.; Kato, Y.; Aristov, Y.I. Adapting the MgO-CO2 working pair for thermochemical energy storage by doping with salts. Energy Convers. Manag. 2019, 185, 473–481. [Google Scholar] [CrossRef]
- Ishitobi, H.; Uruma, K.; Takeuchi, M.; Ryu, J.; Kato, Y. Dehydration and hydration behavior of metal-salt-modified materials for chemical heat pumps. Appl. Therm. Eng. 2013, 50, 1639–1644. [Google Scholar] [CrossRef]
- Shkatulov, A.I.; Krieger, T.; Zaikovskiy, V.; Chesalov, Y.; Aristov, Y.I. Doping magnesium hydroxide with sodium nitrate: A new approach to tune the dehydration reactivity of heat-storage materials. ACS Appl. Mater. Interfaces 2014, 6, 19966–19977. [Google Scholar] [CrossRef] [PubMed]
- Shkatulov, A.I.; Aristov, Y. Modification of magnesium and calcium hydroxides with salts: An efficient way to advanced materials for storage of middle-temperature heat. Energy 2015, 85, 667–676. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. First-principle study of CaO/Ca(OH)2 thermochemical energy storage system by Li or Mg cation doping. Chem. Eng. Sci. 2014, 117, 293–300. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. Thermodynamic and kinetic study of dehydration process of CaO/Ca(OH)2 thermochemical heat storage system with Li doping. Chem. Eng. Sci. 2015, 138, 86–92. [Google Scholar] [CrossRef]
- Gao, W.; Zhou, T.; Gao, Y.; Louis, B.; O’Hare, D.; Wang, Q. Molten salts-modified MgO-based adsorbents for intermediate-temperature CO2 capture: A review. J. Energy Chem. 2017, 26, 830–838. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, K.; Gao, Y. The phase diagram of LiNO3-KNO3. Thermochim. Acta 2002, 385, 81–84. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.S.; Li, W.Z.; Rohatgi, A.; Duan, Y.; Singh, P.; Li, L.; King, D.L. Phase transfer catalyzed fast CO2 absorption by MgO-based absorbents with high cycling capacity. Adv. Mater. Interfaces 2014, 1, 1400030. [Google Scholar] [CrossRef]
- Sada, E.; Katoh, S.; Yoshii, H.; Takemoto, I.; Shiomi, N. Solubility of carbon dioxide in molten alkali halides and nitrates and their binary mixtures. J. Chem. Eng. Data 1981, 26, 279–281. [Google Scholar] [CrossRef]
- Harada, T.; Hatton, T.A. Colloidal Nanoclusters of MgO Coated with Alkali Metal Nitrates/Nitrites for Rapid, High Capacity CO2 Capture at Moderate Temperature. Chem. Mater. 2015, 27, 8153–8161. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, J.; Zhang, Y.; Gao, W.; Harada, T.; Huang, L.; Hatton, T.A.; Wang, Q. Alkali Nitrates Molten Salt Modified Commercial MgO for Intermediate-Temperature CO2 Capture: Optimization of the Li/Na/K Ratio. Ind. Eng. Chem. Res. 2017, 56, 1509–1517. [Google Scholar] [CrossRef]
- Kim, S.T.; Ryu, J.; Kato, Y. The optimization of mixing ratio of expanded graphite mixed chemical heat storage material for magnesium oxide/water chemical heat pump. Appl. Therm. Eng. 2014, 66, 274–281. [Google Scholar] [CrossRef]
- Myagmarjav, O.; Ryu, J.; Kato, Y. Waste heat recovery from iron production by using magnesium oxide/water chemical heat pump as thermal energy storage. ISIJ Int. 2015, 55, 464–472. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.T.; Miura, H.; Takasu, H.; Kato, Y.; Shkatulov, A.; Aristov, Y. Adapting the MgO-CO2 Working Pair for Thermochemical Energy Storage by Doping with Salts: Effect of the (LiK)NO3 Content. Energies 2019, 12, 2262. https://doi.org/10.3390/en12122262
Kim ST, Miura H, Takasu H, Kato Y, Shkatulov A, Aristov Y. Adapting the MgO-CO2 Working Pair for Thermochemical Energy Storage by Doping with Salts: Effect of the (LiK)NO3 Content. Energies. 2019; 12(12):2262. https://doi.org/10.3390/en12122262
Chicago/Turabian StyleKim, Seon Tae, Haruka Miura, Hiroki Takasu, Yukitaka Kato, Alexandr Shkatulov, and Yuri Aristov. 2019. "Adapting the MgO-CO2 Working Pair for Thermochemical Energy Storage by Doping with Salts: Effect of the (LiK)NO3 Content" Energies 12, no. 12: 2262. https://doi.org/10.3390/en12122262




