Carbon Dioxide Capture from Flue Gas Using Tri-Sodium Phosphate as an Effective Sorbent
Abstract
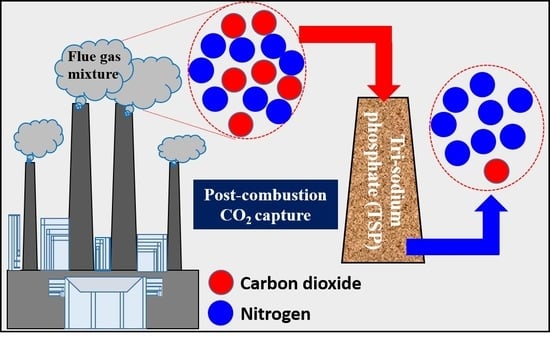
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Materials
2.3. Apparatus and Procedure
2.4. Regeneration Process
2.5. The Calculation for the Amount of Gas Consumed
2.6. Calculation of Rate of Gas Consumption
2.7. The Calculation for CO2 Capture Efficiency (η)
3. Results and Discussion
3.1. Material Characterization Analysis
3.2. Sorption Reaction Mechanism
3.3. Gas Uptake Kinetics
3.3.1. Effect of Temperature on the Gas Uptake Kinetics
3.3.2. Effect of Gas Pressure on the CO2 Uptake Kinetics
3.4. Gas Phase Analysis
3.5. Effect of CO2 Concentration on Gas Uptake
3.6. Comparison of CO2 Uptake with the Solid Adsorbent Available in the Literature
3.7. Gas Uptake Kinetics in The Regenerated TSP
3.8. Scalability and Conceptual Prototype
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jorgenson, A.; Longhofer, W.; Grant, D. Disproportionality in Power Plants’ Carbon Emissions: A Cross-National Study. Sci. Rep. 2016, 6, 28661. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Albo, J.; Luis, P.; Irabien, A. Carbon Dioxide Capture from Flue Gases Using a Cross-Flow Membrane Contactor and the Ionic Liquid 1-Ethyl-3-methylimidazolium Ethylsulfate. Ind. Eng. Chem. Res. 2010, 49, 11045–11051. [Google Scholar] [CrossRef]
- Drage, T.C.; Blackman, J.M.; Pevida, C.; Snape, C.E. Evaluation of Activated Carbon Adsorbents for CO2 Capture in Gasification. Energy Fuels 2009, 23, 2790–2796. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Meyer, L.A. AR5 Synthesis Report: Climate Change 2014; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Le Quéré, C.; Peters, G.P.; Andres, R.J.; Andrew, R.M.; Boden, T.A.; Ciais, P.; Friedlingstein, P.; Houghton, R.A.; Marland, G.; Moriarty, R.; et al. Global carbon budget 2013. Earth Syst. Sci. Data 2014, 6, 235–263. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.M.K.; Curcic, I.; Gabriel, J.; Tsang, S.C.E. Recent Advances in CO2 Capture and Utilization. ChemSusChem 2008, 1, 893–899. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Singh, D.; Croiset, E.; Douglas, P.L.; Douglas, M.A. Techno-economic study of CO2 capture from an existing coal-fired power plant: MEA scrubbing vs. O2/CO2 recycle combustion. Energy Convers. Manag. 2003, 44, 3073–3091. [Google Scholar] [CrossRef]
- Gouedard, C.; Picq, D.; Launay, F.; Carrette, P.L. Amine degradation in CO2 capture. I. A review. Int. J. Greenh. Gas. Con. 2012, 10, 244–270. [Google Scholar] [CrossRef]
- Ding, Y.; Alpay, E. Equilibria and kinetics of CO2 adsorption on hydrotalcite adsorbent. Chem. Eng. Sci. 2000, 55, 3461–3474. [Google Scholar] [CrossRef]
- Mérel, J.; Clausse, M.; Meunier, F. Carbon dioxide capture by indirect thermal swing adsorption using 13X zeolite. Environ. Prog. Sustain. 2006, 25, 327–333. [Google Scholar] [CrossRef]
- Na, B.K.; Koo, K.K.; Eum, H.M.; Lee, H.; Song, H.K. CO2 recovery from flue gas by PSA process using activated carbon. Korean J. Chem. Eng. 2001, 18, 220–227. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef]
- Mason, J.A.; Sumida, K.; Herm, Z.R.; Krishna, R.; Long, J.R. Evaluating metal–organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 2011, 4, 3030–3040. [Google Scholar] [CrossRef]
- Olivier, M.G.; Jadot, R. Adsorption of Light Hydrocarbons and Carbon Dioxide on Silica Gel. J. Chem. Eng. Data 1997, 42, 230–233. [Google Scholar] [CrossRef]
- Wang, M.; Yao, L.; Wang, J.; Zhang, Z.; Qiao, W.; Long, D.; Ling, L. Adsorption and regeneration study of polyethylenimine-impregnated millimeter-sized mesoporous carbon spheres for post-combustion CO2 capture. Appl. Energy 2016, 168, 282–290. [Google Scholar] [CrossRef]
- Zhou, C.; He, K.; Lv, W.; Chen, Y.; Tang, S.; Liu, C.; Yue, H.; Liang, B. Energy and Economic Analysis for Post-combustion CO2 Capture using Amine-Functionalized Adsorbents in a Temperature Vacuum Swing Process. Energy Fuels 2019, 33, 1774–1784. [Google Scholar] [CrossRef]
- Sakpal, A.K.T.; Kamble, S.; Kumar, R. Carbon dioxide capture using amine functionalized silica gel. Indian J. Chem. A 2012, 51, 1214–1222. [Google Scholar]
- Zeman, F.S.; Lackner, K.S. Capturing carbon dioxide directly from the atmosphere. World Resour. Rev. 2004, 16, 157–172. [Google Scholar]
- Wright, A.B.; Lackner, K.S. Removal of Carbon Dioxide from Air. U.S. Patent 20060051274A1, 9 March 2006. [Google Scholar]
- Snell, F.D. Trisodium Phosphate—Its Manufacture and Use. Ind. Eng. Chem. 1931, 23, 470–474. [Google Scholar] [CrossRef]
- Balsora, H.K.; Mondal, M.K. Solubility of CO2 in aqueous TSP. Fluid Phase Equilibria 2012, 328, 21–24. [Google Scholar] [CrossRef]
- Balsora, H.K.; Mondal, M.K. Solubility of CO2 in an Aqueous Blend of Diethanolamine and Trisodium Phosphate. J. Chem. Eng. Data 2011, 56, 4691–4695. [Google Scholar] [CrossRef]
- Kumar, A.; Sakpal, T.; Linga, P.; Kumar, R. Impact of Fly Ash Impurity on the Hydrate-Based Gas Separation Process for Carbon Dioxide Capture from a Flue Gas Mixture. Ind. Eng. Chem. Res. 2014, 53, 9849–9859. [Google Scholar] [CrossRef]
- Smith, J.M. Introduction to Chemical Engineering Thermodynamics; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Miller, F.A.; Wilkins, C.H. Infrared Spectra and Characteristic Frequencies of Inorganic Ions. Anal. Chem. 1952, 24, 1253–1294. [Google Scholar] [CrossRef]
- Belton, P.S.; Clarke, T.A.; Meyrick, D. A novel reaction between carbon dioxide and trisodium orthophosphate dodecahydrate shown by photoelectron spectroscopy and X-ray diffraction. J. Inorg. Nucl. Chem. 1981, 43, 614–615. [Google Scholar] [CrossRef]
- Kamarudin, K.S.N. Structural and gas adsorption characteristics of zeolite adsorbents. Ph.D Thesis, University Technology Malaysia, Johor, Malaysia, 2006. [Google Scholar]
- Song, H.-J.; Lee, S.; Maken, S.; Park, J.-J.; Park, J.-W. Solubilities of carbon dioxide in aqueous solutions of sodium glycinate. Fluid Phase Equilibria 2006, 246, 1–5. [Google Scholar] [CrossRef]
- Lee, S.; Filburn, T.P.; Gray, M.; Park, J.-W.; Song, H.-J. Screening Test of Solid Amine Sorbents for CO2 Capture. Ind. Eng. Chem. Res. 2008, 47, 7419–7423. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel Polyethylenimine-Modified Mesoporous Molecular Sieve of MCM-41 Type as High-Capacity Adsorbent for CO2 Capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Jenab, M.H.; Vahidi, M.; Mehrabi, M. Solubility of Carbon Dioxide in Aqueous Mixtures of DIPA+MDEA and DIPA+PZ Solutions. J. Chin. Chem. Soc. 2006, 53, 283–286. [Google Scholar] [CrossRef]
- Singh, P.; Brilman, D.W.F.; Groeneveld, M.J. Evaluation of CO2 solubility in potential aqueous amine-based solvents at low CO2 partial pressure. Int. J. Greenh. Gas Control 2011, 5, 61–68. [Google Scholar] [CrossRef]
- Tontiwachwuthikul, P.; Meisen, A.; Lim, C.J. Solubility of carbon dioxide in 2-amino-2-methyl-1-propanol solutions. J. Chem. Eng. Data 1991, 36, 130–133. [Google Scholar] [CrossRef]
- Huang, H.Y.; Yang, R.T.; Chinn, D.; Munson, C.L. Amine-Grafted MCM-48 and Silica Xerogel as Superior Sorbents for Acidic Gas Removal from Natural Gas. Ind. Eng. Chem. Res. 2003, 42, 2427–2433. [Google Scholar] [CrossRef]
- Arenillas, A.; Smith, K.M.; Drage, T.C.; Snape, C.E. CO2 capture using some fly ash-derived carbon materials. Fuel 2005, 84, 2204–2210. [Google Scholar] [CrossRef]
- Harlick, P.J.E.; Sayari, A. Applications of Pore-Expanded Mesoporous Silica 5. Triamine Grafted Material with Exceptional CO2 Dynamic and Equilibrium Adsorption Performance. Ind. Eng. Chem. Res. 2007, 46, 446–458. [Google Scholar] [CrossRef]
- Ma, X.; Wang, X.; Song, C. “Molecular Basket” Sorbents for Separation of CO2 and H2S from Various Gas Streams. J. Am. Chem. Soc. 2009, 131, 5777–5783. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Marand, E.; Ida, J.; Guliants, V.V. Polysulfone and Mesoporous Molecular Sieve MCM-48 Mixed Matrix Membranes for Gas Separation. Chem. Mater. 2006, 18, 1149–1155. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, X.; Wang, D.; Song, C.; Wang, Y. Development of silica-gel-supported polyethylenimine sorbents for CO2 capture from flue gas. AIChE J. 2012, 58, 2495–2502. [Google Scholar] [CrossRef]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Pérez, E.S. Amino functionalized mesostructured SBA-15 silica for CO2 capture: Exploring the relation between the adsorption capacity and the distribution of amino groups by TEM. Microporous Mesoporous Mater. 2012, 158, 309–317. [Google Scholar] [CrossRef]
- McDonald, T.M.; Lee, W.R.; Mason, J.A.; Wiers, B.M.; Hong, C.S.; Long, J.R. Capture of Carbon Dioxide from Air and Flue Gas in the Alkylamine-Appended Metal–Organic Framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 2012, 134, 167056–167065. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B. Carbon-Based Adsorbents for Post-combustion CO2 Capture: A Critical Review. Environ. Sci. Technol. 2016, 501, 47276–47289. [Google Scholar]
- Patel, H.A.; Byun, J.; Yavuz, C.T. Carbon Dioxide Capture Adsorbents: Chemistry and Methods. ChemSusChem 2017, 10, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Oschatz, M.; Antonietti, M. A search for selectivity to enable CO2 capture with porous adsorbents. Energy Environ. Sci. 2018, 11, 57–70. [Google Scholar] [CrossRef]
- Deng, M.; Park, H.G. Spacer-Assisted Amine-Coiled Carbon Nanotubes for CO2 Capture. Langmuir 2019, 35, 4453–4459. [Google Scholar] [CrossRef] [PubMed]










| Exp. No. | Experimental Pressure, Pexp (MPa) | Composition (%) | Experimental Temperature (Texp) | Gas Uptake (mg of CO2/g of Sorbent) | Std. Dev. | |
|---|---|---|---|---|---|---|
| CO2 | N2 | |||||
| 1a | 3.5 | 100 | 0 | 15 | 68 | 2.64 |
| 1b | 3.5 | 100 | 0 | 15 | 64 | |
| 1c | 3.5 | 100 | 0 | 15 | 63 | |
| 2a | 3.5 | 100 | 0 | 30 | 198 | 4.72 |
| 2b | 3.5 | 100 | 0 | 30 | 189 | |
| 2c | 3.5 | 100 | 0 | 30 | 191 | |
| 3a | 3.5 | 100 | 0 | 50 | 120 | 2.08 |
| 3b | 3.5 | 100 | 0 | 50 | 117 | |
| 3c | 3.5 | 100 | 0 | 50 | 116 | |
| 4a | 2.0 | 100 | 0 | 30 | 189 | 1.52 |
| 4b | 2.0 | 100 | 0 | 30 | 192 | |
| 4c | 2.0 | 100 | 0 | 30 | 191 | |
| 5a | 5.0 | 100 | 0 | 30 | 196 | 4.04 |
| 5b | 5.0 | 100 | 0 | 30 | 193 | |
| 5c | 5.0 | 100 | 0 | 30 | 188 | |
| 6a | 3.5 | 85 | 15 | 30 | 181 | 2.0 |
| 6b | 3.5 | 85 | 15 | 30 | 177 | |
| 6c | 3.5 | 85 | 15 | 30 | 179 | |
| 7a | 3.5 | 16.1 | 83.9 | 30 | 140 | 2.88 |
| 7b | 3.5 | 16.1 | 83.9 | 30 | 140 | |
| 7c | 3.5 | 16.1 | 83.9 | 30 | 135 | |
| * 8a | 3.5 | 100 | 0 | 30 | 169 | - |
| * 8b | 3.5 | 100 | 0 | 30 | 162 | |
| # 9a | 3.5 | 100 | 0 | 30 | 145 | - |
| # 9b | 3.5 | 100 | 0 | 30 | 139 | |
| Parameter | 1st Stage | 2nd Stage | 3rd Stage | 4th Stage | 5th Stage |
|---|---|---|---|---|---|
| Feed gas (CO2) composition mol fraction | 0.161 | 0.161 | 0.161 | 0.161 | 0.161 |
| Gas phase composition (CO2) at the end of the experiment mol fraction | 0.0116 | 0.0116 | 0.0117 | 0.077 | 0.156 |
| CO2 capture efficiency (η)% | 92.79 | 92.79 | 92.73 | 52.17 | 3.10 |
| Materials | Experimental Conditions | Gas Uptake (mg of CO2/g of Sorbent) | Reference | |
|---|---|---|---|---|
| CO2 (mol%) | Temperature (°C) | |||
| Xerogel | 100 | 25 | 49.3 | Huang et al., 2003 [36] |
| MCM-48 | 5 | 25 | 50.2 | Huang et al., 2003 [36] |
| Fly ash-derived carbon materials | 15 | 75 | 45 | Arenillas et al., 2005 [37] |
| MCM-41 | 5 | 25 | 42.7 | Harlick et al., 2006 [38] |
| MCM-41x | 5 | 25 | 47.5 | Harlick et al., 2006 [38] |
| PE-MCM-41 d | 5 | 25 | 68.2 | Harlick et al., 2006 [38] |
| MBS-1 a | 14.9 | 75 | 89.2 | Ma et al., 2009 [39] |
| MBS-2 | 14.9 | 75 | 140.0 | Ma et al., 2009 [39] |
| MCM-41 c | 100 | 25 | 82.3 | Kamarudin, 2009 [29] |
| MCM-41 | 100 | 50 | 22.5 | Kamarudin, 2009 [29] |
| SBA-15 | 100 | 50 | 33.8 | Kamarudin, 2009 [29] |
| MCM-48 | 100 | 25 | 35.2 | Kim et al., 2009 [40] |
| MCM-48 | 100 | 25 | 17.6 | Kim et al., 2009 [40] |
| Silica Gel | 15.1 | 75 | 138.0 | Zhang et al., 2012 [41] |
| SBA-15 b | 23 | 45 | 78.5 | Sanz et al., 2012 [42] |
| SBA-15 | 18 | 45 | 80.3 | Sanz et al., 2012 [42] |
| M2(dobpdc)-Modified MOF-74 | 15 | 40 | 138.2 | McDonald et al., 2012 [43] |
| Activated carbon | 17 | 15–25 | 107–142 | Samanta et al., 2012 [8] |
| Purified SWNT | 17 | 35 | 190 | Samanta et al., 2012 [8] |
| Zeolite 13X | 17 | 20–25 | 115.7–205 | Samanta et al., 2012 [8] |
| molecular sieve 13X | 17 | 20–25 | 95–158 | Samanta et al., 2012 [8] |
| K2CO3 e | 15 | 100 | 92.4 | Samanta et al., 2012 [8] |
| Na2CO3 f | 10 | 50–70 | 114 | Samanta et al., 2012 [8] |
| MOC Composites g | 100 | 25 | 58–71 | Creamer and Gao, 2016 [44] |
| CTS-GO-15 h | 100 | 25 | 174 | Creamer and Gao, 2016 [44] |
| Hollow fibres i | 10 | 35 | 25 | Patel et al., 2017 [45] |
| SIFSIX-2-Cu-I j | 90 | 25 | 238 | Oschatz and Antonietti, 2018 [46] |
| PEI-purine-CNT | 100 | 50 | 170.0 | Deng et al., 2019 [47] |
| PEI–CNT | 100 | 50 | 170.0 | Deng et al., 2019 [47] |
| TSP | 16.1 | 30 | 140.0 | Present study |
| TSP | 85 | 30 | 181.0 | Present study |
| TSP | 100 | 30 | 198.0 | Present study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakpal, T.; Kumar, A.; Aman, Z.M.; Kumar, R. Carbon Dioxide Capture from Flue Gas Using Tri-Sodium Phosphate as an Effective Sorbent. Energies 2019, 12, 2889. https://doi.org/10.3390/en12152889
Sakpal T, Kumar A, Aman ZM, Kumar R. Carbon Dioxide Capture from Flue Gas Using Tri-Sodium Phosphate as an Effective Sorbent. Energies. 2019; 12(15):2889. https://doi.org/10.3390/en12152889
Chicago/Turabian StyleSakpal, Tushar, Asheesh Kumar, Zachary M. Aman, and Rajnish Kumar. 2019. "Carbon Dioxide Capture from Flue Gas Using Tri-Sodium Phosphate as an Effective Sorbent" Energies 12, no. 15: 2889. https://doi.org/10.3390/en12152889