Electrospun Core-Shell Nanofiber as Separator for Lithium-Ion Batteries with High Performance and Improved Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.3. Characterization
2.4. Electrochemical Characterization
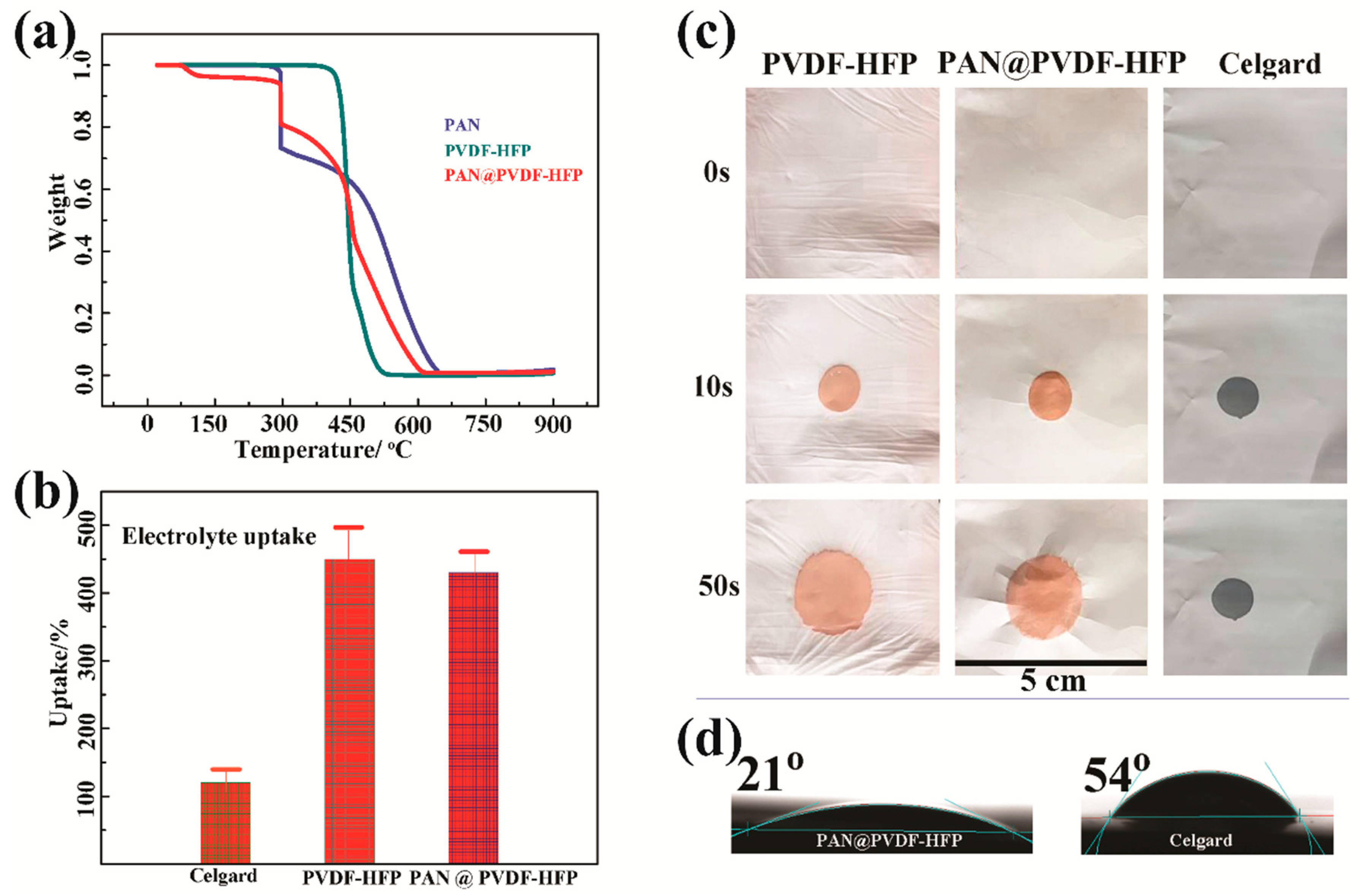
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Miao, Y.; Hynan, P.; Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Recent Progress in Advanced Materials for Lithium Ion Batteries. Materials 2013, 6, 156–183. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Lin, D.C.; Zhao, J.; Lu, Z.D.; Liu, Y.Y.; Liu, C.; Lu, Y.Y.; Wang, H.T.; Yan, K.; Tao, X.Y.; et al. Composite lithium metal anode by melt infusion of lithium into a 3D conducting scaffold with lithiophilic coating. PNAS 2016, 113, 2862–2867. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, Y.H.; Wang, L.; Tian, G.Y.; He, X.M. The application of self-assembled hierarchical structures in lithium-ion batteries. Prog. Chem. 2018, 30, 1761–1769. [Google Scholar]
- Liang, Z.; Yan, K.; Zhou, G.; Pei, A.; Zhao, J.; Sun, Y.; Xie, J.; Li, Y.; Shi, F.; Liu, Y.; et al. Composite lithium electrode with mesoscale skeleton via simple mechanical deformation. Sci. Adv. 2019, 5, eaau5655. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kang, Y.Q.; Jin, Y.H.; Wang, L.; Tian, G.Y.; He, X.M. Silicon-Based and -Related Materials for Lithium-Ion Batteries. Prog. Chem. 2019, 31, 613–630. [Google Scholar] [CrossRef]
- Liang, Z.; Tao, X.; Cui, Y. Black TiO2 Nanomaterials for Lithium–Sulfur Batteries. Black TiO2 Nanomater. Energy Appl. 2017, 275–304. [Google Scholar] [CrossRef]
- Ji, W.X.; Wang, F.; Liu, D.T.; Qian, J.F.; Cao, Y.L.; Chen, Z.X.; Yang, H.X.; Ai, X.P. Building thermally stable Li-ion batteries using a temperature-responsive cathode. J. Mater. Chem. 2016, 4, 11239–11246. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.Y.; Lin, D.C.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef]
- Lee, K.T.; Jeong, S.; Cho, J. Roles of surface chemistry on safety and electrochemistry in lithium ion batteries. Acc. Chem. Res. 2013, 46, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, D.B.; Wu, J.; Dong, L.Y.; Zhu, Y.J.; Hu, X.L. Flexible, high-wettability and fire-resistant separators based on hydroxyapatite nanowires for advanced lithium-ion batteries. Adv. Mater. 2017, 29, 1703548. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.N.; Ouyang, M.G.; Liu, X.; Lu, L.G.; Xia, Y.; He, X.M. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X.W. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, M.Y.; Lin, C.E.; Zhu, B.K. Progress in polymeric separators for lithium ion batteries. RSC Adv. 2015, 5, 89848–89860. [Google Scholar] [CrossRef]
- Zhu, X.M.; Jiang, X.Y.; Ai, X.P.; Yang, H.X.; Cao, Y.L. A highly thermostable ceramic-grafted microporous polyethylene separator for safer lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 24119–24126. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.Z.; Wang, Y.R.; Yu, T.; Chen, H.; Zhao, Z.B.; Guan, S.Y. Polyimide binder by combining with polyimide separator for enhancing the electrochemical performance of lithium ion batteries. Electrochim. Acta 2016, 216, 1–7. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.B.; Wu, C.L.M.; Liao, C.B.; Li, L. Tannic-acid-coated polypropylene membrane as a separator for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 16003–16010. [Google Scholar] [CrossRef]
- Wu, D.Z.; Deng, L.; Sun, Y.; Teh, K.S.; Shi, C.; Tan, Q.L.; Zhao, J.B.; Sun, D.H.; Lin, L.W. A high-safety PVDF/Al2O3 composite separator for Li-ion batteries via tip-induced electrospinning and dip-coating. RSC Adv. 2017, 7, 24410–24416. [Google Scholar] [CrossRef]
- Liang, N.Q.; Fang, J.H.; Guo, X.X. A simple approach for preparation of porous polybenzimidazole membranes as a promising separator for lithium ion batteries. J. Mater. Chem. A 2017, 5, 15087–15095. [Google Scholar] [CrossRef]
- Pankaj, A.P.; Zhang, Z.M. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar]
- Pi, J.K.; Wu, G.P.; Yang, H.C.; Arges, C.G.; Xu, Z.K. Separators with biomineralized zirconia coatings for enhanced thermo- and electro-performance of lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 21971–21978. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhang, P.; Shi, C.; Chen, L.; Dai, J.; Zhao, J. The functional separator coated with core–shell structured silica–poly (methyl methacrylate) sub-microspheres for lithium-ion batteries. J. Membr. Sci. 2015, 474, 148–155. [Google Scholar] [CrossRef]
- Sun, G.; Sun, L.; Xie, H.; Liu, J. Electrospinning of Nanofibers for Energy Applications. Nanomaterials 2016, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Liu, Z.H.; Kong, Q.S.; Zhang, C.J.; Pang, S.P.; Yue, L.P.; Wang, X.J.; Yao, J.H.; Cui, G.L. Renewable and superior thermal-resistant cellulose-based composite nonwoven as lithium-ion battery separator. ACS Appl. Mater. Interfaces 2013, 5, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.H.; Shi, C.; Li, C.; Shen, X.; Peng, L.Q.; Wu, D.Z.; Sun, D.H.; Zhang, P.; Zhao, J.B. A rational design of separator with substantially enhanced thermal features for lithium-ion batteries by the polydopamine-ceramic composite modification of polyolefin membranes. Energy Environ. Sci. 2016, 9, 3252–3261. [Google Scholar] [CrossRef]
- Gong, S.; Jeon, H.; Lee, H.; Ryou, M.H.; Lee, Y.M. Effects of an integrated separator/electrode assembly on enhanced thermal stability and rate capability of lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 17814–17821. [Google Scholar] [CrossRef]
- Croce, F.; Focarete, M.L.; Hassoun, J.; Meschini, I.; Scrosati, B. A safe, high-rate and high-energy polymer lithium-ion battery based on gelled membranes prepared by electrospinning. Energy Environ. Sci. 2011, 4, 921–927. [Google Scholar] [CrossRef]
- Zhu, X.M.; Jiang, X.Y.; Ai, X.P.; Yang, H.X.; Cao, Y.L. TiO2 ceramic-grafted polyethylene separators for enhanced thermostability and electrochemical performance of lithium-ion batteries. J. Membr. Sci. 2016, 504, 97–103. [Google Scholar] [CrossRef]
- Kang, S.M.; Ryou, M.H.; Choi, J.C.; Lee, H. Mussel- and diatom-inspired silica coating on separators yields improved power and safety in Li-ion batteries. Chem. Mater. 2012, 24, 3481–3485. [Google Scholar] [CrossRef]
- Liu, M.H.; Zhang, P.P.; Gou, L.T.; Hou, Z.Y.; Huang, B. Enhancement on the thermostability and wettability of lithium-ion batteries separator via surface chemical modification. Mater. Lett. 2017, 208, 98–101. [Google Scholar] [CrossRef]
- Cho, T.H.; Tanaka, M.; Onishi, H.; Kondo, Y.; Nakamura, T.; Yamazaki, H.; Tanase, S.; Sakai, T. Battery performances and thermal stability of polyacrylonitrile nano-fiber-based nonwoven separators for Li-ion battery. J. Power Sources 2008, 181, 155–160. [Google Scholar] [CrossRef]
- Cao, L.; An, P.; Xu, Z.; Huang, J. Performance evaluation of electrospun polyimide non-woven separators for high power lithium-ion batteries. J. Electroanal. Chem. 2016, 767, 34–39. [Google Scholar] [CrossRef]
- Bansal, D.; Meyer, B.; Salomon, M. Gelled membranes for Li and Li-ion batteries prepared by electrospinning. J. Power Sources 2008, 178, 848–851. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.Q.; Han, Y.; Zhang, L.; Li, S.S.; Tang, B.; Xu, S.M.; Xu, Z.H. Ether modified poly(ether ether ketone) nonwoven membrane with excellent wettability and stability as a lithium ion battery separator. J. Power Sources 2018, 378, 176–183. [Google Scholar] [CrossRef]
- Costa, C.M.; Maria, M.; Silva, M.M.; Lanceros-Méndez, S. Battery separators based on vinylidene fluoride (VDF) polymers and copolymers for lithium ion battery applications. RSC Adv. 2013, 3, 11404–11417. [Google Scholar] [CrossRef]
- Xiao, K.; Zhai, Y.Y.; Yu, J.Y.; Ding, B. Nanonet-structured poly (m-phenylene isophthalamide)-polyurethane membranes with enhanced thermostability and wettability for high power lithium ion batteries. RSC Adv. 2015, 5, 55478–55485. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Zhao, Y.; Li, Y. Electrospun Core-Shell Nanofiber as Separator for Lithium-Ion Batteries with High Performance and Improved Safety. Energies 2019, 12, 3391. https://doi.org/10.3390/en12173391
Liang Z, Zhao Y, Li Y. Electrospun Core-Shell Nanofiber as Separator for Lithium-Ion Batteries with High Performance and Improved Safety. Energies. 2019; 12(17):3391. https://doi.org/10.3390/en12173391
Chicago/Turabian StyleLiang, Zheng, Yun Zhao, and Yanxi Li. 2019. "Electrospun Core-Shell Nanofiber as Separator for Lithium-Ion Batteries with High Performance and Improved Safety" Energies 12, no. 17: 3391. https://doi.org/10.3390/en12173391
APA StyleLiang, Z., Zhao, Y., & Li, Y. (2019). Electrospun Core-Shell Nanofiber as Separator for Lithium-Ion Batteries with High Performance and Improved Safety. Energies, 12(17), 3391. https://doi.org/10.3390/en12173391



