Characterization and Analysis of Malaysian Macroalgae Biomass as Potential Feedstock for Bio-Oil Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Feedstocks
- i.
- Caulerpa lentillifera is one of the species used for food production. Hence, it will cause slight competition in the food industry. This species is one of the major species that has been grown and produced in Sabah.
- ii.
- Gracilaria coronopifolia is another established species which is categorised under red algae species. It is a tough species that can withstand various condition.
- iii.
- Chaetomorpha linum is a general species which can also be cultivated in a reef tank. This species is normally used in aquariums and has not been used for food or another pharmateutical benefit. Chaetomorpha have a considerably high growth rate. The cultivation of Chaetomorpha species also has the potential to give a continuous supply without competing with the other industry of seaweed.
2.2. Elemental Analysis and Calorific Value Determination
2.3. Thermogravimetric Analysis (TGA) of Algal Biomass
2.4. Kinetic Model
2.5. Spectroscopic Analysis by Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Demineralization/Ash Content Determination by Proximate Analysis
3. Results and Discussion
3.1. Physiochemical Properties of Algae Biomass
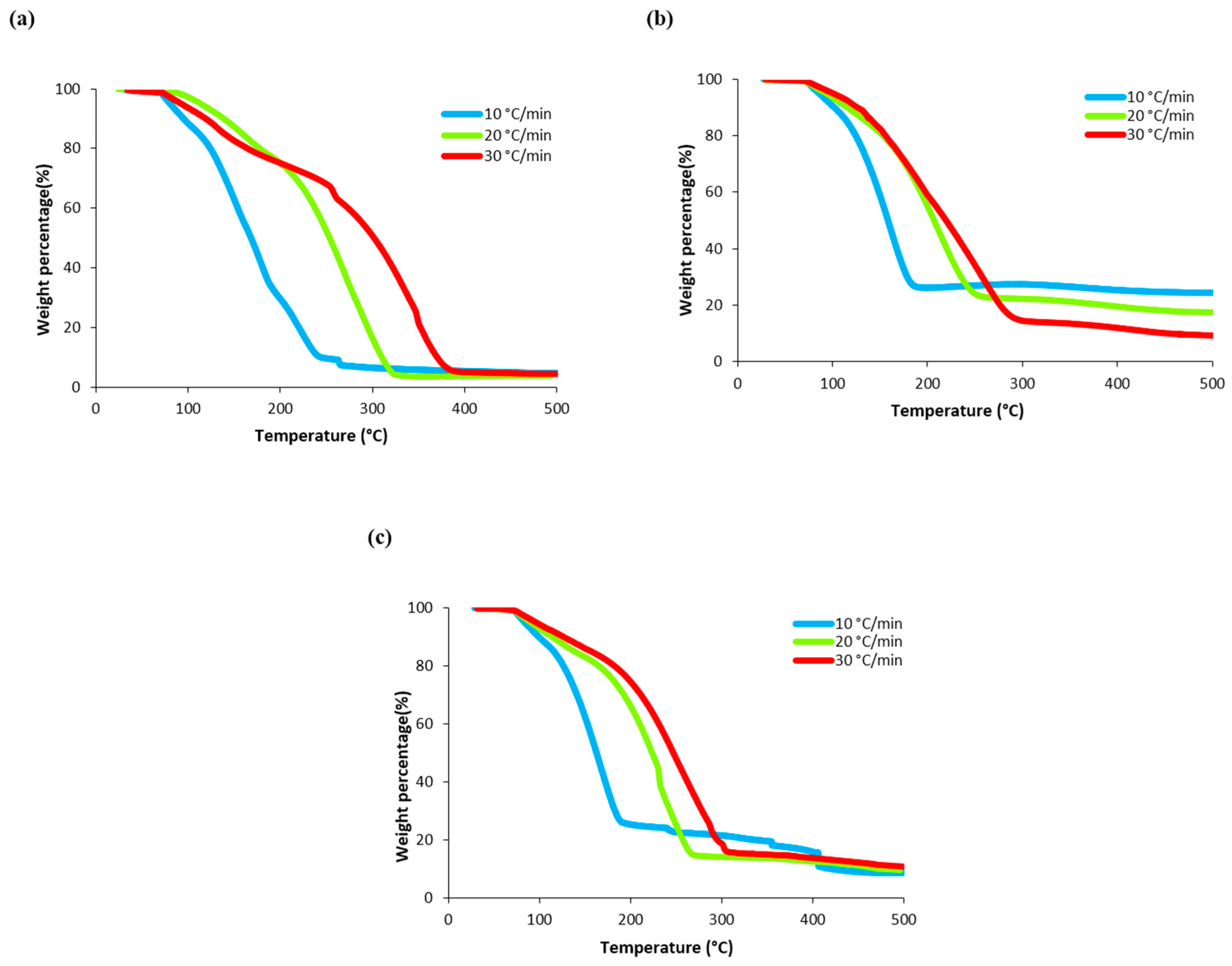
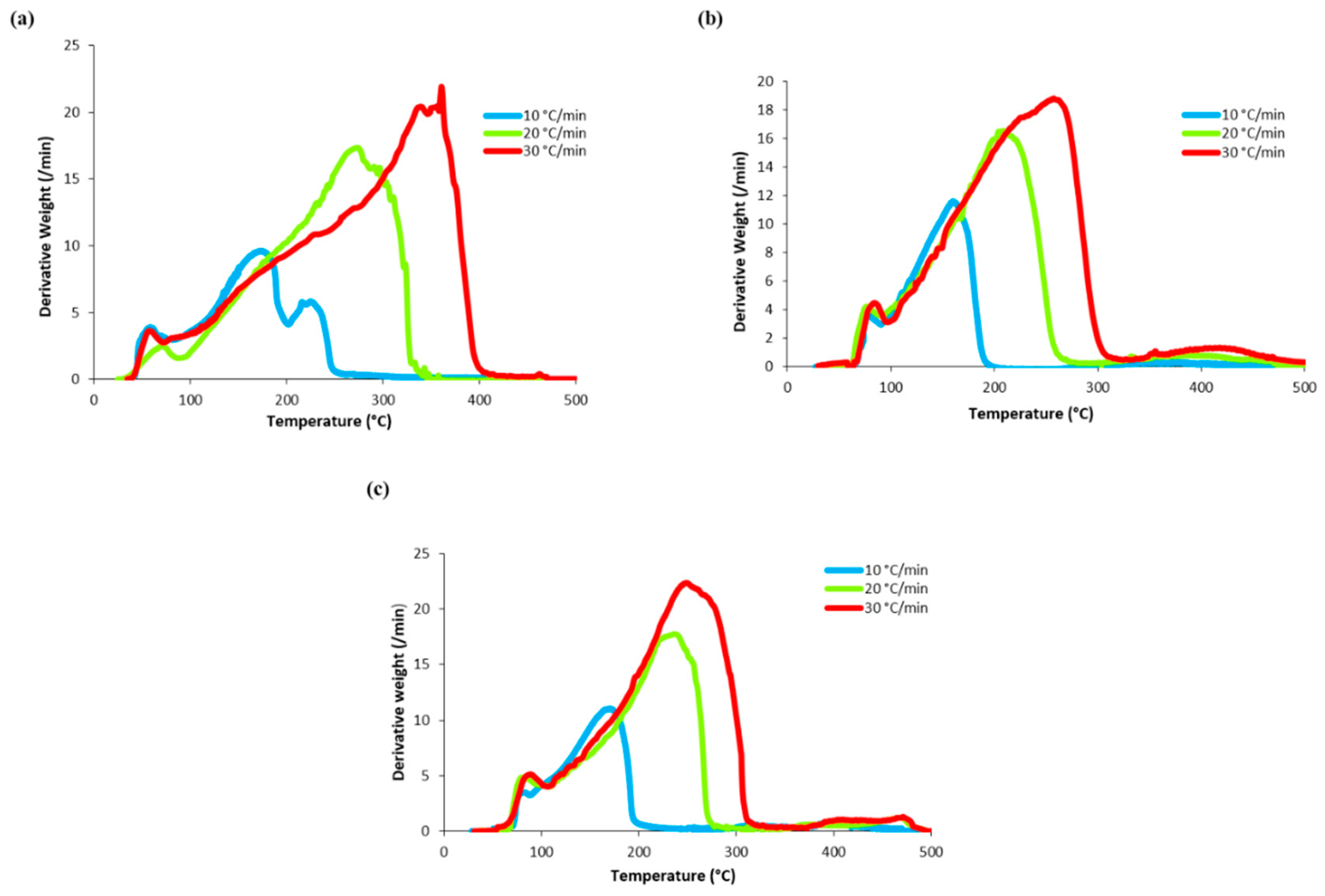
3.2. Thermogravimetric Analysis (TGA) of Algae Biomass
3.3. Kinetic Analysis of Algal Biomass
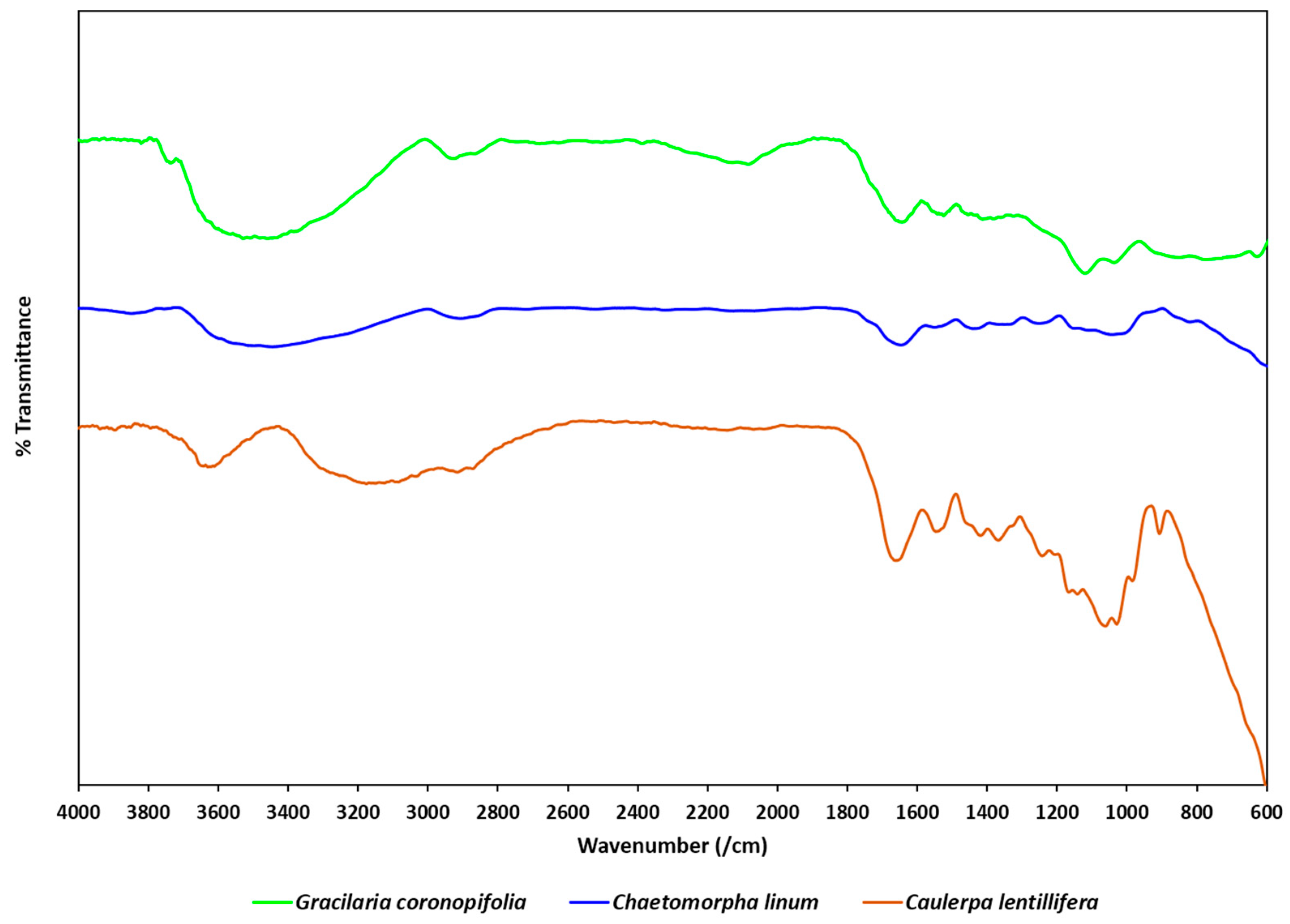
3.4. Spectroscopic Analysis by FTIR
3.5. Demineralization and Ash Content Determination by Proximate Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USEPA. Guidelines for Water Reuse; U.S. Agency for International Development: Washington, DC, USA, 2012; p. 643.
- Latif, A.N.I.S.; Ong, M.Y.; Nomanbhay, S. Hydrothermal liquefaction of Malaysian’s algal biomass for high-quality bio-oil production. Eng. Life Sci. 2019, 246–269. [Google Scholar] [CrossRef]
- Nhat, H.P.; Ngo, H.H.; Guo, W.S.; Chang, S.W.; Nguyen, D.D.; Nguyen, P.D.; Bui, X.T.; Zhang, X.B.; Guo, J.B. Can algae-based technologies be an affordable green process for biofuel production and wastewater remediation? Bioresour. Technol. 2018, 256, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Ipina, A.A.; Urrutia, L.M.; Urrutia, L.D.; Portilla, A.D. Thermal oxidative decomposition estimation combining TGA and DSC as optimization targets for PMMA. J. Phys. Conf. Ser. 2018, 1107, 032011. [Google Scholar] [CrossRef]
- Edreis, E.M.A.; Yao, H. Kinetic thermal behaviour and evaluation of physical structure of sugar cane bagasse char during non-isothermal steam gasification. J. Mater. Res. Technol. 2016, 5, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Saffe, A.; Fernandez, A.; Mazza, G.; Rodriguez, R. Prediction of regional agro-industrial wastes characteristics by thermogravimetric analysis to obtain bioenergy using thermal process. Energy Explor. Exploit. 2019, 37, 544–557. [Google Scholar] [CrossRef]
- Gao, W.; Chen, K.; Zeng, J.; Xu, J.; Wang, B. Thermal pyrolysis characteristics of macroalgae Cladophora glomerata. Bioresour. Technol. 2017, 243, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Ramachandran, S.; Subbiah, S. Determination of kinetic parameters in the pyrolysis operation and thermal behavior of Prosopis juliflora using thermogravimetric analysis.pdfra using thermogr. Bioresour. Technol. 2017, 233, 413–422. [Google Scholar] [CrossRef]
- Daood, S.S.; Munir, S.; Nimmo, W.; Gibbs, B.M. Char oxidation study of sugar cane bagasse, cotton stalk and Pakistani coal under 1% and 3% oxygen concentrations. Biomass Bioenergy 2010, 34, 263–271. [Google Scholar] [CrossRef]
- Carpio, R.B.; Zhang, Y.; Kuo, C.T.; Chen, W.T.; Schideman, L.C.; Leon, R.L. Characterization and thermal decomposition of demineralized wastewater algae biomass. Algal Res. 2019, 38, 1–12. [Google Scholar] [CrossRef]
- Kantarli, İ.C.; Pala, M.; Yildirim, Y.; Yanik, J.; Abreu, M.H. Fuel characteristics and combustion behavior of seaweed-derived hydrochars. Turk. J. Chem. 2019, 43, 475–491. [Google Scholar] [CrossRef]
- Irini, A.; Dimitar, K.; Alvarado-Morales, M. Anaerobic Co-digestion of Cast Seaweed and Organic Residues; Technical University of Denmark: Lyngby, Denmark, 2017. [Google Scholar]
- Heaven, S.; Milledge, J.; Zhang, Y. Comments on Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 2011, 29, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.; Harvey, P. Grand Challenges in Marine Biotechnology; Springer Nature: Basingstoke, UK, 2018; ISBN 978-3-319-69074-2. [Google Scholar]
- Kök, M.V.; Pamir, R. Pyrolysis kinetics of oil shales determined by DSC and TG/DTG. Oil Shale 2003, 20, 57–68. [Google Scholar]
- Zhou, L.; Zou, H.; Wang, Y.; Le, Z.; Liu, Z.; Adesina, A.A. Effect of potassium on thermogravimetric behavior and co-pyrolytic kinetics of wood biomass and low density polyethylene. Renew. Energy 2017, 102, 134–141. [Google Scholar] [CrossRef]
- Lehner, T.; Challacombe, S.J.; Caldwell, J. An immunological investigation into the prevention of caries in deciduous teeth of rhesus monkeys. Arch. Oral Biol. 1975, 20, 305–310. [Google Scholar] [CrossRef]
- Paul, O.U.; John, I.H.; Ndubuisi, I.; Peter, A.; Godspower, O. Calorific Value of Palm Oil Residues for Energy Utilisation. Int. J. Eng. Innov. Res. 2015, 4, 2277–5668. [Google Scholar]
- Yamaji, G.T.N.; Mariana, P.M.; Larissa, S.H.; Yamamoto, H.F.M. Sugarcane trash for energy purposes: Storage time and particle size can improve the quality of biomass for fuel? Ind. Crops Prod. 2017, 108, 641–648. [Google Scholar]
- Shen, J.; Zhu, S.; Liu, X.; Zhang, H.; Tan, J. Measurement of Heating Value of Rice Husk by Using Oxygen Bomb Calorimeter with Benzoic Acid as Combustion Adjuvant. Energy Procedia 2012, 17, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Mhilu, C.F. Analysis of Energy Characteristics of Rice and Coffee Husks Blends. ISRN Chem. Eng. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Kumar, R.; Chandrashekar, N. Fuel properties and combustion characteristics of some promising bamboo species in India. J. For. Res. 2014, 25, 471–476. [Google Scholar] [CrossRef]
- Günther, B.; Gebauer, K.; Barkowski, R.; Rosenthal, M.; Bues, C.T. Calorific value of selected wood species and wood products. Eur. J. Wood Wood Prod. 2012, 70, 755–757. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, H.X.; Liu, M.; Sun, B.B.; Zhang, Y.; Dong, S.S.; Qi, L.B.; Qin, S. Production of bio-oil from fast pyrolysis of macroalgae enteromorpha prolifera powder in a free-fall reactor. Energy Sources Part A Recover. Util. Environ. Eff. 2013, 35, 859–867. [Google Scholar] [CrossRef]
- Choia, J.H.; Woob, H.C.; Suha, D.J. Pyrolysis of Seaweeds for Bio-oil and Bio-char Production. Chem. Eng. Trans. 2014, 37, 121–126. [Google Scholar]
- Melendres, A.R., Jr.; Ilano, A.S. Bio-oil Product from Wild Brown Macro-algae Dunggandunggan (Padinasp) in Asturias and Carmen, Cebu, Philippines. Int. J. Med. Plants Nat. Prod. 2017, 3, 27–36. [Google Scholar]
- Wang, S.; Jiang, X.M.; Wang, Q.; Ji, H.S.; Wu, L.F.; Wang, J.F.; Xu, S.N. Research of specific heat capacities of three large seaweed biomass. J. Therm. Anal. Calorim. 2014, 115, 2071–2077. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Hussein, R.; Ong, M.Y. Sustainability of biodiesel production in Malaysia by production of bio-oil from crude glycerol using microwave pyrolysis: A review. Green Chem. Lett. Rev. 2018, 11, 135–157. [Google Scholar] [CrossRef]
- Kalla, A.M.; Devaraju, R. Microwave energy and its application in food industry: A reveiw. Asian J. Dairy Food Res. 2017, 36, 37–44. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Du, F.; Wang, Y.; Pan, L.; Chen, D. Microwave-assisted hydrothermal liquefaction of lignin for the preparation of phenolic formaldehyde adhesive. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, M.; Zhu, X.; Guo, D.; Liu, S.; Hu, Z.; Xiao, B.; Wang, J.; Laghari, M. Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour. Technol. 2015, 192, 441–450. [Google Scholar] [CrossRef]
- Gai, C.; Zhang, Y.; Chen, W.T.; Zhang, P.; Dong, Y. Thermogravimetric and kinetic analysis of thermal decomposition characteristics of low-lipid microalgae. Bioresour. Technol. 2013, 150, 139–148. [Google Scholar] [CrossRef]
- Idris, S.S.; Rahman, N.A.; Ismail, K.; Alias, A.B.; Rashid, Z.A.; Aris, M.J. Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Bioresour. Technol. 2010, 101, 4584–4592. [Google Scholar] [CrossRef]
- Slopiecka, K.; Bartocci, P.; Fantozzi, F. Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl. Energy 2012, 97, 491–497. [Google Scholar] [CrossRef]
- Waheed, Q.M.K.; Maqsood, S.; Nawaz, R.; Aqeel, A.B. Investigation of thermal behaviour and kinetic analysis of Pakistani biomass: Rice husk, sugarcane bagasse and wheat straw using thermogravimetric analysis. In Proceedings of the 2015 Power Generation System and Renewable Energy Technologies (PGSRET), Islamabad, Pakistan, 10–11 June 2015. [Google Scholar]
- Xiong, S.; Zhuo, J.; Zhou, H.; Pang, R.; Yao, Q. Study on the co-pyrolysis of high density polyethylene and potato blends using thermogravimetric analyzer and tubular furnace. J. Anal. Appl. Pyrolysis 2015, 112, 66–73. [Google Scholar] [CrossRef]
- Olajire, A.; Zhi, C.; Hanson, S.; Wai, C. Thermogravimetric analysis of the pyrolysis characteristics and kinetics of plastics and biomass blends. Fuel Proc. Technol. 2014, 128, 471–481. [Google Scholar]
- Vernieres-hassimi, L. Comparative investigation for the determination of kinetic parameters for biomass pyrolysis by thermogravimetric analysis. J. Ther. Anal. Calorim. 2017, 129, 1201–1213. [Google Scholar]
- Yaman, E. Pyrolysis kinetics of walnut shell and waste polyole fi ns using thermogravimetric analysis. J. Energy Inst. 2016, 1–13. [Google Scholar] [CrossRef]
- Azizi, K.; Moraveji, K.M.; Najafabadi, A.H. Characteristics and kinetics study of simultaneous pyrolysis of microalgae Chlorella vulgaris, wood and polypropylene through TGA. Bioresour. Technol. 2017, 243, 481–491. [Google Scholar] [CrossRef]
- Bartošová, A.; Blinová, L.; Gerulová, K. Characterisation Of Polysacharides And Lipids From Selected Green Algae Species By FTIR-ATR Spectroscopy. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2015, 23, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Stehfest, K.; Toepel, J.; Wilhelm, C. The application of micro-FTIR spectroscopy to analyze nutrient stress-related changes in biomass composition of phytoplankton algae. Plant Physiol. Biochem. 2005, 43, 717–726. [Google Scholar] [CrossRef]
- Jebsen, C.; Norici, A.; Wagner, H.; Palmucci, M.; Giordano, M.; Wilhelm, C. FTIR spectra of algal species can be used as physiological fingerprints to assess their actual growth potential. Physiol. Plant. 2012, 146, 427–438. [Google Scholar] [CrossRef]

| Sample | Composition (%) | Calorific Value (MJ/kg) | ||||
|---|---|---|---|---|---|---|
| Carbon | Hydrogen | Nitrogen | Sulphur | Oxygen | ||
| Caulerpa lentifilla | 35.76 | 3.63 | 2.71 | 1.32 | 30.11 | 13.3538 |
| Gracillaria coronopifolia | 23.08 | 4.01 | 1.76 | 7.82 | 29.43 | 10.6661 |
| Chaetomorpha linum | 27.81 | 5.69 | 3.19 | 0.85 | 29.67 | 12.7278 |
| Samples | Heating Rates (°C/min) | Temperature Range (°C) | R2 | Activation Energy, E (kJ/mol) | Average E (kJ/mol) | Pre-Exponential Factor, A (min−1) | Average A (min−1) |
|---|---|---|---|---|---|---|---|
| n = 1 | |||||||
| Caulerpa lentillifera | 10 | 100–220 | 0.9584 | 18.565 | 18.8707 ± 0.3735 | 6.00 × 107 | (1.11 ± 1.61) × 109 |
| 20 | 105–320 | 0.9830 | 19.287 | 2.96 × 108 | |||
| 30 | 110–390 | 0.9504 | 18.760 | 2.96 × 109 | |||
| Gracilaria coronopifolia | 10 | 100–180 | 0.9938 | 29.929 | 22.3727 ± 6.6656 | 2.00 × 106 | (1.85 ± 2.17) × 108 |
| 20 | 105–240 | 0.9982 | 19.862 | 1.28 × 108 | |||
| 30 | 110–280 | 0.9600 | 17.327 | 4.25 × 108 | |||
| Chaetomorpha linum | 10 | 100–180 | 0.9975 | 27.809 | 20.1583 ± 6.6632 | 6.00 × 106 | (3.48 ± 3.73) × 108 |
| 20 | 105–260 | 0.9719 | 17.039 | 2.93 × 108 | |||
| 30 | 110–300 | 0.9882 | 15.627 | 7.45 × 108 | |||
| n = 2 | |||||||
| Caulerpa lentillifera | 10 | 100–220 | 0.9953 | 40.313 | 33.1827 ± 6.7652 | 1.38 × 105 | (1.92 ± 2.50) × 107 |
| 20 | 105–320 | 0.9450 | 32.381 | 1.00 × 107 | |||
| 30 | 110–390 | 0.6883 | 26.854 | 4.76 × 107 | |||
| Gracilaria coronopifolia | 10 | 100–180 | 0.9426 | 69.248 | 48.6120 ± 17.9179 | 3.00 × 108 | (1.01 ± 1.72) × 108 |
| 20 | 105–240 | 0.9366 | 39.586 | 6.28 × 105 | |||
| 30 | 110–280 | 0.9776 | 37.002 | 2.75 × 106 | |||
| Chaetomorpha linum | 10 | 100–180 | 0.9834 | 45.503 | 37.7747 ± 6.7294 | 8.38 × 104 | (3.15 ± 5.13) × 106 |
| 20 | 105–260 | 0.8478 | 34.610 | 2.98 × 105 | |||
| 30 | 110–300 | 0.8761 | 33.211 | 9.07 × 106 | |||
| n = 3 | |||||||
| Caulerpa lentillifera | 10 | 100–220 | 0.9740 | 18.565 | 18.8707 ± 0.3735 | 6.00× 107 | (1.11 ± 1.61) × 109 |
| 20 | 105–320 | 0.8998 | 19.287 | 2.96 × 108 | |||
| 30 | 110–390 | 0.6627 | 18.760 | 2.96 × 109 | |||
| Gracilaria coronopifolia | 10 | 100–180 | 0.8932 | 29.929 | 22.3727 ± 6.6656 | 2.00 × 106 | (1.85 ± 2.17) × 108 |
| 20 | 105–240 | 0.8914 | 19.862 | 1.28 × 108 | |||
| 30 | 110–280 | 0.9413 | 17.327 | 4.25 × 108 | |||
| Chaetomorpha linum | 10 | 100–180 | 0.9681 | 27.809 | 20.1583 ± 6.6632 | 6.00 × 106 | (3.48 ± 3.73) × 108 |
| 20 | 105–260 | 0.7883 | 17.039 | 2.93 × 108 | |||
| 30 | 110–300 | 0.8149 | 15.627 | 7.45 × 108 | |||
| Biomass | Heating Rates (°C/min) | Activation Energy (kJ/mol) | Pre-Exponential Factor (min−1) | Reference |
|---|---|---|---|---|
| Potato | 10 | 81.3 | 5.77 × 103 | [38] |
| 20 | 81.5 | 9.97 × 103 | [38] | |
| 30 | 80 | 9.57 × 103 | [38] | |
| Sawdust | - | 59.76 | 1.50 × 104 | [39] |
| Bamboo | - | 65.96 | 5.81 × 104 | [39] |
| EFB | - | 50.37 | 2.32 × 103 | [39] |
| Beechwood | - | 167.14 | 4.90 × 1011 | [40] |
| Walnut shell | 5 | 69.11 | 1.87 × 105 | [41] |
| 10 | 79.73 | 2.62 × 106 | [41] | |
| 15 | 61.00 | 4.91 × 104 | [41] | |
| 20 | 67.03 | 2.19 × 105 | [41] | |
| 50 | 69.71 | 6.48 × 105 | [41] | |
| Chlorella vulgaris (microalgae) | - | 131.228 | 2.80 × 1010 | [42] |
| Caulerpa lentillifera | 10 | 18.565 | 6.0 × 107 | This work |
| 20 | 19.287 | 2.96 × 108 | ||
| 30 | 18.760 | 2.96 × 109 | ||
| Gracilaria coronopifolia | 10 | 29.929 | 2.0 × 106 | |
| 20 | 19.862 | 1.28 × 108 | ||
| 30 | 17.327 | 4.25 × 108 | ||
| Chaetomorpha linum | 10 | 27.809 | 6.0 × 106 | |
| 20 | 17.039 | 2.93 × 108 | ||
| 30 | 15.627 | 7.45 × 108 |
| Wavenumber Range (cm−1) * | Typical Band Assignment * | Main Peak (cm−1) | ||
|---|---|---|---|---|
| Caulerpa lentillifera | Gracilaria coronopifolia | Chaetomorpha linum | ||
| 3700–3200 | O–H stretching of hydroxyl functional group | 3630.03 | 3481.51 | 3444.87 |
| 3000–2800 | N–H stretching (protein) or =C-H and C–H stretching (lipid carbohydrate) | 2916.37 | 2926.01 | 2906.73 |
| 1709–1583 | C=O stretching (protein amide I band) | 1662.64 | 1643.35 | 1647.21 |
| 1585–1481 | N–H bending and C–N stretching | 1544.98 | 1533.41 | 1550.77 |
| 1440–1395 | O–H bending of carboxylic acid | 1419.61 | 1411.89 | 1440.83 |
| 1356–1191 | P=O stretching of phosphodiesters (Nucleic Acid and other phosphate-containing compounds) | 1242.16 | - | 1251.80 |
| 1072–1099 | Carbohydrate C–O–C of polysaccharides (Nucleic Acid) or P=O stretching of phosphodiesters | 1166.93 | - | - |
| 1072–980 | Carbohydrate C–O–C of polysaccharides | 1029.99 | 1037.70 | 1041.56 |
| Sample | Moisture Content (%) | Ash (%) | Ash (%dw) | Ash Removal Efficiency (%) |
|---|---|---|---|---|
| Chaetomorpha linum | 78.95 | 9.87 | 46.864 | - |
| Chaetomorpha linum with 1% acetic acid | 79.72 | 8.87 | 43.733 | 6.681 |
| Chaetomorpha linum with 2% acetic acid | 80.52 | 6.83 | 35.045 | 25.220 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, M.Y.; Syahira Abdul Latif, N.-I.; Leong, H.Y.; Salman, B.; Show, P.L.; Nomanbhay, S. Characterization and Analysis of Malaysian Macroalgae Biomass as Potential Feedstock for Bio-Oil Production. Energies 2019, 12, 3509. https://doi.org/10.3390/en12183509
Ong MY, Syahira Abdul Latif N-I, Leong HY, Salman B, Show PL, Nomanbhay S. Characterization and Analysis of Malaysian Macroalgae Biomass as Potential Feedstock for Bio-Oil Production. Energies. 2019; 12(18):3509. https://doi.org/10.3390/en12183509
Chicago/Turabian StyleOng, Mei Yin, Nor-Insyirah Syahira Abdul Latif, Hui Yi Leong, Bello Salman, Pau Loke Show, and Saifuddin Nomanbhay. 2019. "Characterization and Analysis of Malaysian Macroalgae Biomass as Potential Feedstock for Bio-Oil Production" Energies 12, no. 18: 3509. https://doi.org/10.3390/en12183509