Factors Affecting the Corrosive Behavior of Used Cooking Oils and a Non-Edible Fish Oil That Are in Contact with Ferrous Metals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Setup and Procedure
2.2.2. Analytical Methods
- ▪
- Iron Content Measurement
- ▪
- Acid Number Measurement
- ▪
- Water Content Analysis
- ▪
- SEM-EDS Analysis
- ▪
- Oil Composition Measurements
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Oil Composition
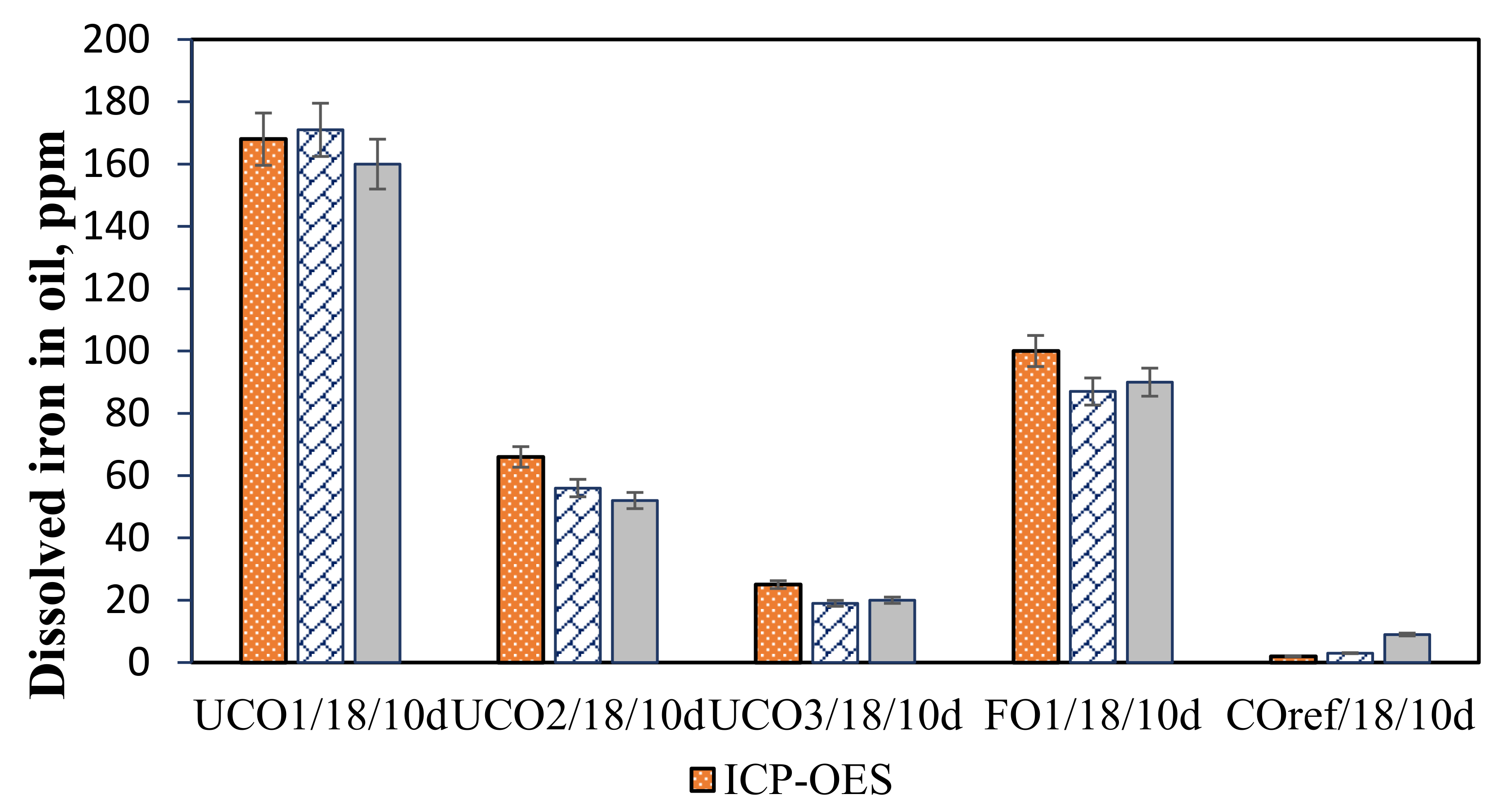
3.3. Effect of Immersion Time
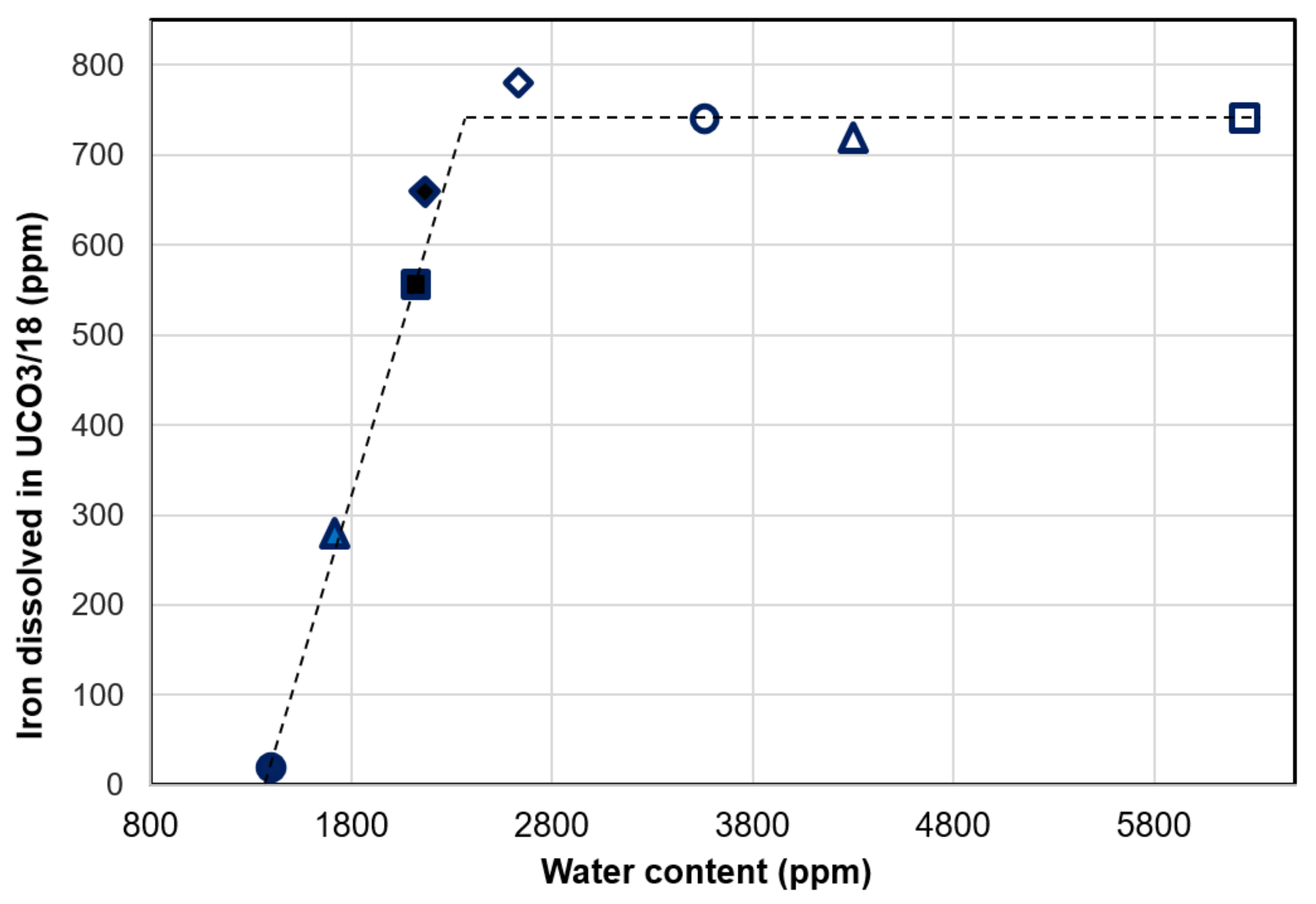
3.4. Effect of Water Content
3.5. Effect of Oleic Acid and Glycerol
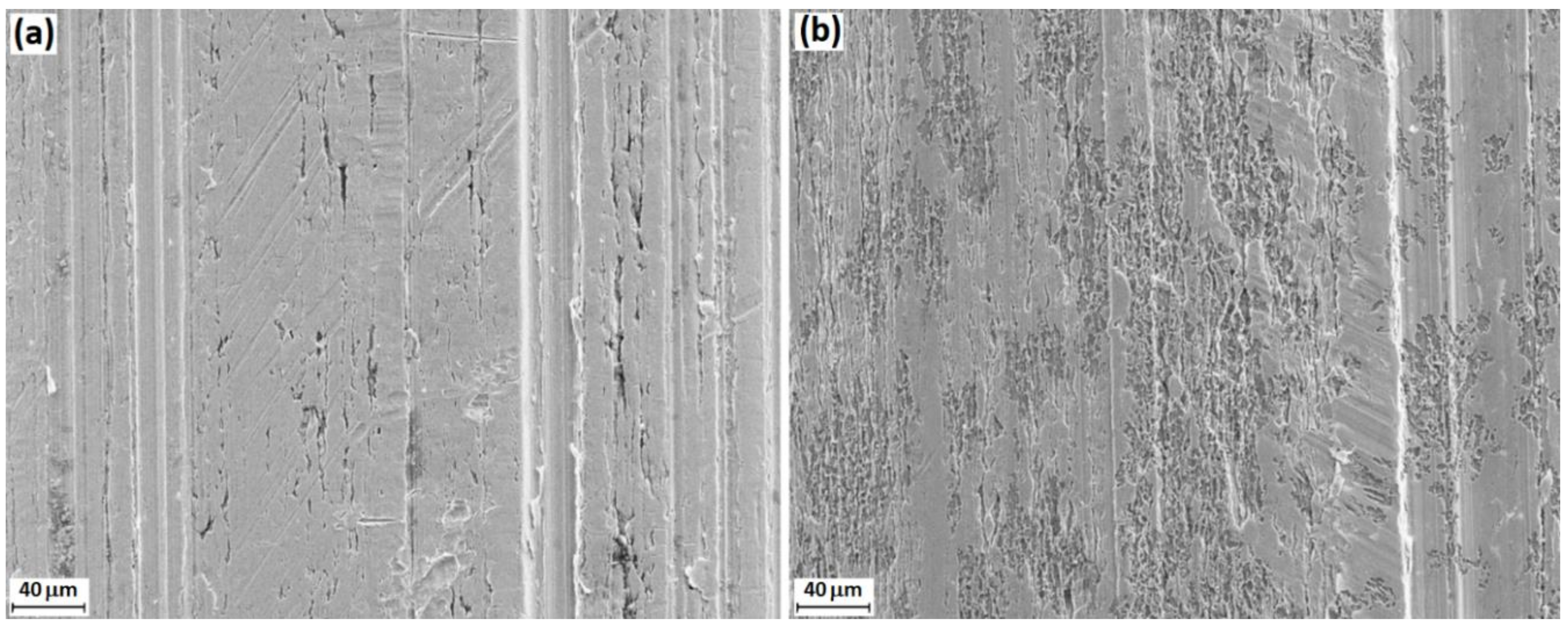
3.6. SEM-EDS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Haseeb, A.S.M.A.; Masjuki, H.H.; Ann, L.J.; Fazal, M.A. Corrosion characteristics of copper and leaded bronze in palm biodiesel. Fuel Process. Technol. 2010, 91, 329–334. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Daggett, D.; Hadaller, O.; Hendricks, R.; Walther, R. Alternative Fuels and Their Potential Impact on Aviation; (Technical Memorandum; NASA/TM-2006-214365); NASA: Cleveland, OH, USA, 2006. [Google Scholar]
- Preto, F.; Zhang, F.; Wang, J. A study on using fish oil as an alternate fuel for conventional combustors. Fuel 2008, 87, 2258–2268. [Google Scholar] [CrossRef]
- Ushakov, S.; Valland, H.; Aesoy, V. Combustion and emissions characteristics of fish oil fuel in a heavy duty diesel engine. Energy Convers. Manag. 2013, 65, 228–238. [Google Scholar] [CrossRef]
- Munoz, R.A.A.; Fernandes, D.M.; Santos, D.Q.; Barbosa, T.G.G.; Sousa, R.M.F. Biodiesel: Production, Characterization, Metallic Corrosion and Analytical Methods for Contaminants. Biodiesel Feed. Prod. Appl. 2012, 48. [Google Scholar] [CrossRef] [Green Version]
- Johnston, P.A.; Brown, R.C. Evaluation of Bio-oil Corrosion Characteristics. In Proceedings of the 2012 AIChE Annual Meeting, Pittsburg, PA, USA, 28 October–2 November 2012. [Google Scholar]
- Hau, J.L.; Yepez, O.J.; Torres, L.H.; Vera, J.R. Measuring naphthenic acid corrosion potential with the Fe powder test. Rev. Metal. 2003, 39, 116–123. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Shiotani, H.; Goto, S.; Sugiyama, G.; Maeda, A. Japanese standards for diesel fuel containing 5% fame blended diesel fuels and its impact on corrosion. SAE Tech. Pap. 2006, 1, 3303. [Google Scholar] [CrossRef]
- Barabás, I.; Todorut, I.-A. Biodiesel Quality, Standards and Properties. In Biodiesel Quality, Emissions and By-Products; InTech: Rijeka, Croatia, 2011; pp. 5–12. [Google Scholar]
- Kovács, A.; Tóth, J.; Isaák, G.; Keresztényi, I. Aspects of storage and Determination of bio-oil induced corrosion characteristics of biodiesel. Fuel Process. Technol. 2015, 134, 59–64. [Google Scholar] [CrossRef]
- Kaul, S.; Saxena, R.C.; Kumar, A.; Negi, M.S.; Bhatnagar, A.K.; Goyal, H.B.; Gupta, A.K. Corrosive behaviour of biodiesel from seed oils of Indian origin on diesel engine parts. Fuel Process. Technol. 2007, 88, 303–307. [Google Scholar] [CrossRef]
- Maru, M.M.; Lucchese, M.M.; Legnani, C.; Quirino, W.G.; Balbo, A.; Aranha, I.B.; Costa, L.T.; Vilani, C.; DeSena, L.A.; Damasceno, J.C.; et al. Biodiesel compatibility with carbon steel and HDPE parts. Fuel Process. Technol. 2009, 90, 1175–1182. [Google Scholar] [CrossRef]
- Diaz-Ballote, L.; Lopez-Sansores, J.F.; Maldonado-Lopez, L.; Garfias-Mesias, L.F. Corrosion behaviour of aluminium exposed to a biodiesel. Electrochem. Commun. 2009, 11, 41–44. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.S.M.A.; Masjuki, H.H. Comparative corrosive characteristics of petroleum diesel and palm biodiesel for automotive materials. Fuel Process. Technol. 2010, 91, 1308–1315. [Google Scholar] [CrossRef]
- Bruun, N.; Khazraie Shoulaifar, T.; Hemming, J.; Willför, S.; Hupa, L. Characterization of waste bio-oil as an alternate source of renewable fuel for marine engines. J. Biofuels 2019, 1–10. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef] [Green Version]
- Swinehart, D.F. The Beer-Lambert Law. J. Chem. Educ. 1962, 39, 333–335. [Google Scholar] [CrossRef]
- Canakci, M.; Sanli, H. Biodiesel Production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biotechnol. 2008, 35, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.Y.; Reitz, R.D. A numerical analysis of the emissions characteristics of biodiesel blended fuels. J. Eng. Gas Turb. Power 1999, 121, 31–37. [Google Scholar] [CrossRef]
- Wexler, H. Polymerization of drying oils. Chem. Rev. 1964, 64, 591–611. [Google Scholar] [CrossRef]
- European Biomass Industry Association. Transformation of used cooking oil into biodiesel: From waste to resource. In UCO to Biodiesel 2030; EUBIA: Brussels, Belgium, 2015. [Google Scholar]
- Ollus, R.; Juoperi, K. Alternative Fuels Experiences for Medium-Speed Diesel Engines. In Proceedings of the 25th CIMAC Congress on Combustion Engine Technology, Vienna, Austria, 21–27 May 2007; International Council on Combustion Engines: Frankfurt, Germany, 2007. [Google Scholar]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Juoperi, K.; Ollus, R. Alternative fuels for medium-speed diesel engines. Wärtsilä Tech. J. 2008, 1, 24–28. [Google Scholar]
- Komatsu, D.; Souza, E.C.; de Souza, E.C.; Canale, L.C.F.; Totten, G.E. Effect of Antioxidants and Corrosion Inhibitor Additives on the Quenching Performance of Soybean Oil. Stroj. Vestn. J. Mech. Eng. 2010, 56, 121–130. [Google Scholar]
- Leng, L.; Yuan, X.; Zeng, G.; Chen, X.; Wang, H.; Li, H.; Fu, L.; Xiao, Z.; Jiang, L.; Lai, C. Rhamnolipid based glycerol-in-diesel microemulsion fuel: Formation and characterization. Fuel 2015, 147, 76–81. [Google Scholar] [CrossRef]
- Eaton, S.J.; Harakas, G.N.; Kimball, R.W.; Smith, J.A.; Pilot, K.A.; Kuflik, M.T. Formulation and combustion of glycerol–diesel fuel emulsions. Energy Fuels 2014, 28, 3940–3947. [Google Scholar] [CrossRef]
- Mize, E.; Lucio, A.J.; Fhaner, C.J.; Pratama, F.S.; Robbins, L.A.; Karpovich, D.S. Emulsions of crude glycerin from biodiesel processing with fuel oil for industrial heating. J. Agric. Food Chem. 2013, 61, 1319–1327. [Google Scholar] [CrossRef] [PubMed]




| Sample | Density 21 °C (kg/m3) | Kinematic Viscosity 40 °C (mm²/s) | Water Content (ppm) | Acid Number (mg KOH/g Oil) |
|---|---|---|---|---|
| UCO1/18 | 916 | 40.7 | 1662 | 6.5 |
| UCO2/18 | 917 | 40.7 | 1449 | 8.2 |
| UCO3/18 | 916 | 40.3 | 1403 | 8.1 |
| FO1/18 | 916 | 26.6 | 2473 | 24.8 |
| COref | 873 | 8.9 | 37 | 0.02 |
| COref * | 879 | 9.1 | <100 | <0.10 |
| Sample | Total FFA (mg/g Oil) | Diglycerides (mg/g Oil) | Polymerized Triglycerides (%) |
|---|---|---|---|
| UCO1/18/0 d | 29.1 | 7.08 | 1.17 |
| UCO1/18/10 d | 33.7 | 7.55 | 1.50 |
| UCO2/18/0 d | 43.3 | 7.65 | 1.28 |
| UCO2/18/10 d | 45.2 | 7.83 | 1.40 |
| UCO3/18/0 d | 46.8 | 7.65 | 1.34 |
| UCO3/18/10 d | 47.0 | 7.75 | 1.34 |
| FO1/18/0 d | 99.7 | 9.70 | n.d. |
| FO1/18/10 d | 93.9 | 10.30 | n.d. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruun, N.; Demesa, A.G.; Tesfaye, F.; Hemming, J.; Hupa, L. Factors Affecting the Corrosive Behavior of Used Cooking Oils and a Non-Edible Fish Oil That Are in Contact with Ferrous Metals. Energies 2019, 12, 4812. https://doi.org/10.3390/en12244812
Bruun N, Demesa AG, Tesfaye F, Hemming J, Hupa L. Factors Affecting the Corrosive Behavior of Used Cooking Oils and a Non-Edible Fish Oil That Are in Contact with Ferrous Metals. Energies. 2019; 12(24):4812. https://doi.org/10.3390/en12244812
Chicago/Turabian StyleBruun, Nina, Abayneh Getachew Demesa, Fiseha Tesfaye, Jarl Hemming, and Leena Hupa. 2019. "Factors Affecting the Corrosive Behavior of Used Cooking Oils and a Non-Edible Fish Oil That Are in Contact with Ferrous Metals" Energies 12, no. 24: 4812. https://doi.org/10.3390/en12244812






