Effects of Nitrogen Forms and Supply Mode on Lipid Production of Microalga Scenedesmus obliquus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alga Strain
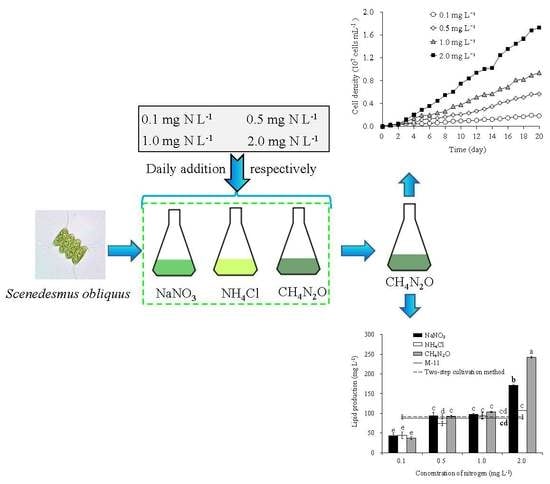
2.2. Experimental Design
2.3. Culture Conditions
2.4. Analytical Methods
2.4.1. Analysis of Cell Density
2.4.2. Chemical Analysis
2.4.3. Total Organic Carbon Analysis
2.4.4. Analysis of Quantum Yield and Lutein
2.4.5. Lipid Content Analysis
2.4.6. Fatty acid Composition Analysis
2.5. Statistics
3. Results and Discussion
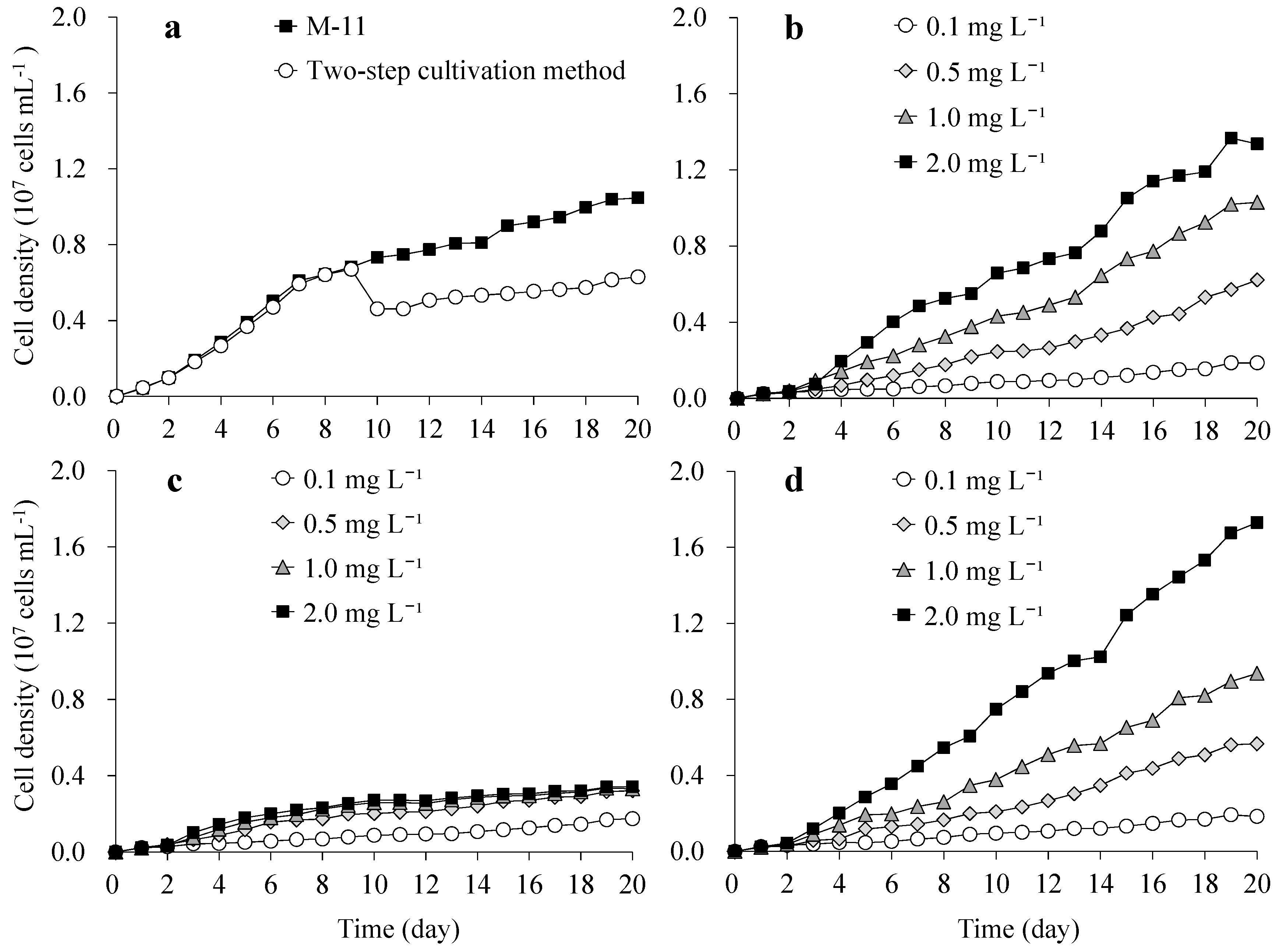
3.1. Growth of S. obliquus
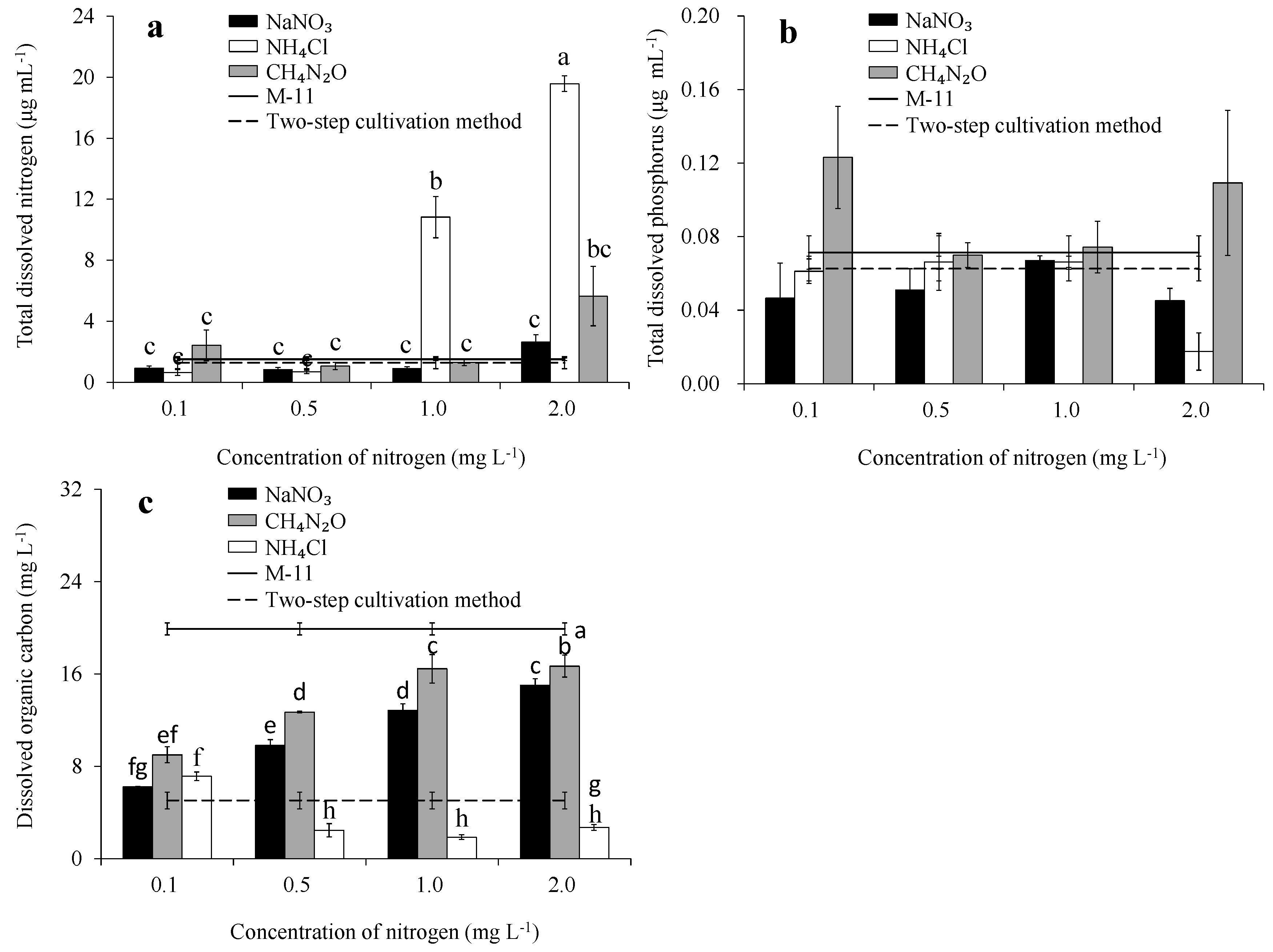
3.2. TDN, TDP, and DOC Concentrations in Medium Filtrate
3.3. Total Organic Carbon Concentration, and Content
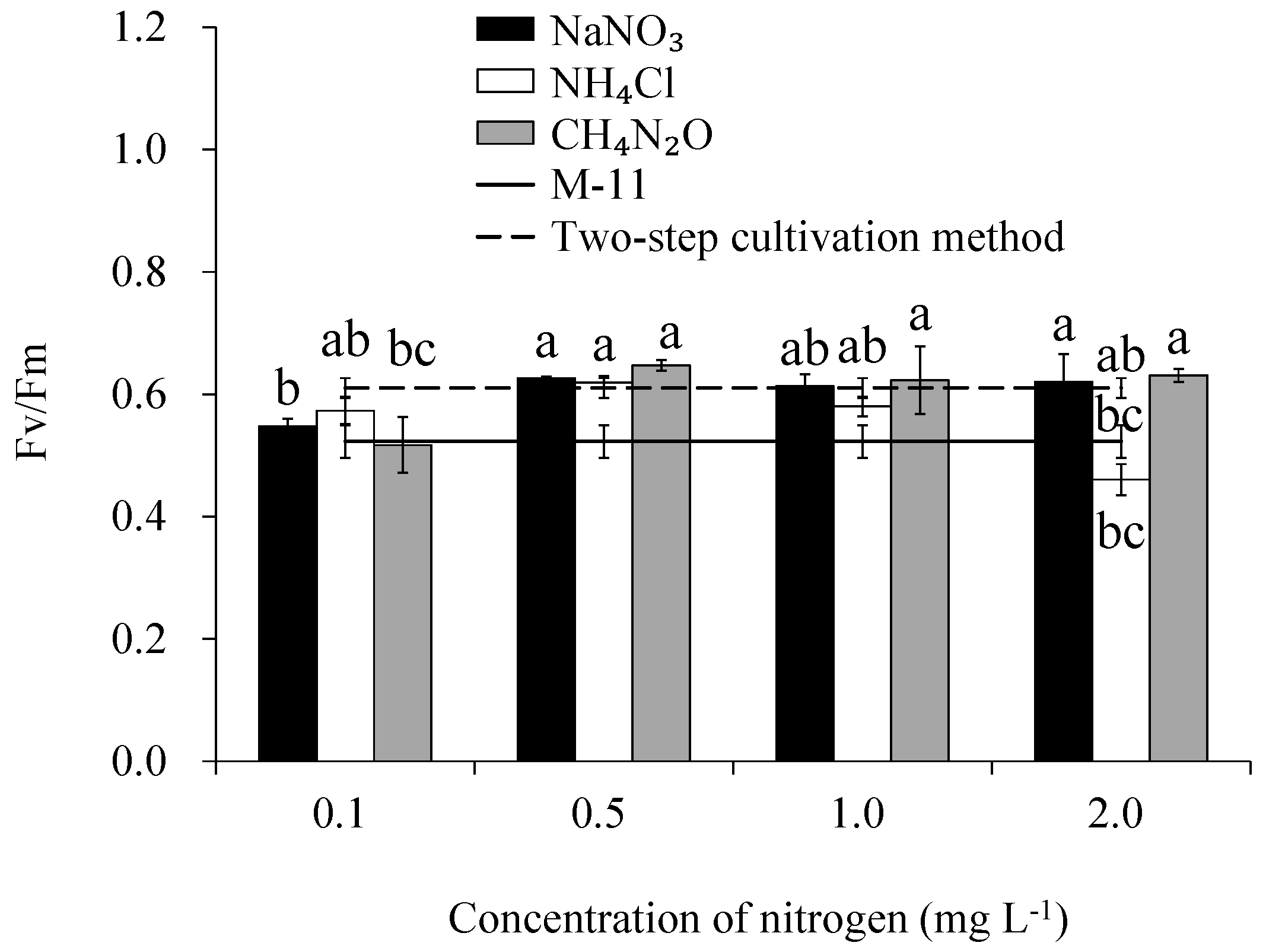
3.4. Chlorophyll Fluorescence
3.5. Lipid Content, Production, and Productivity
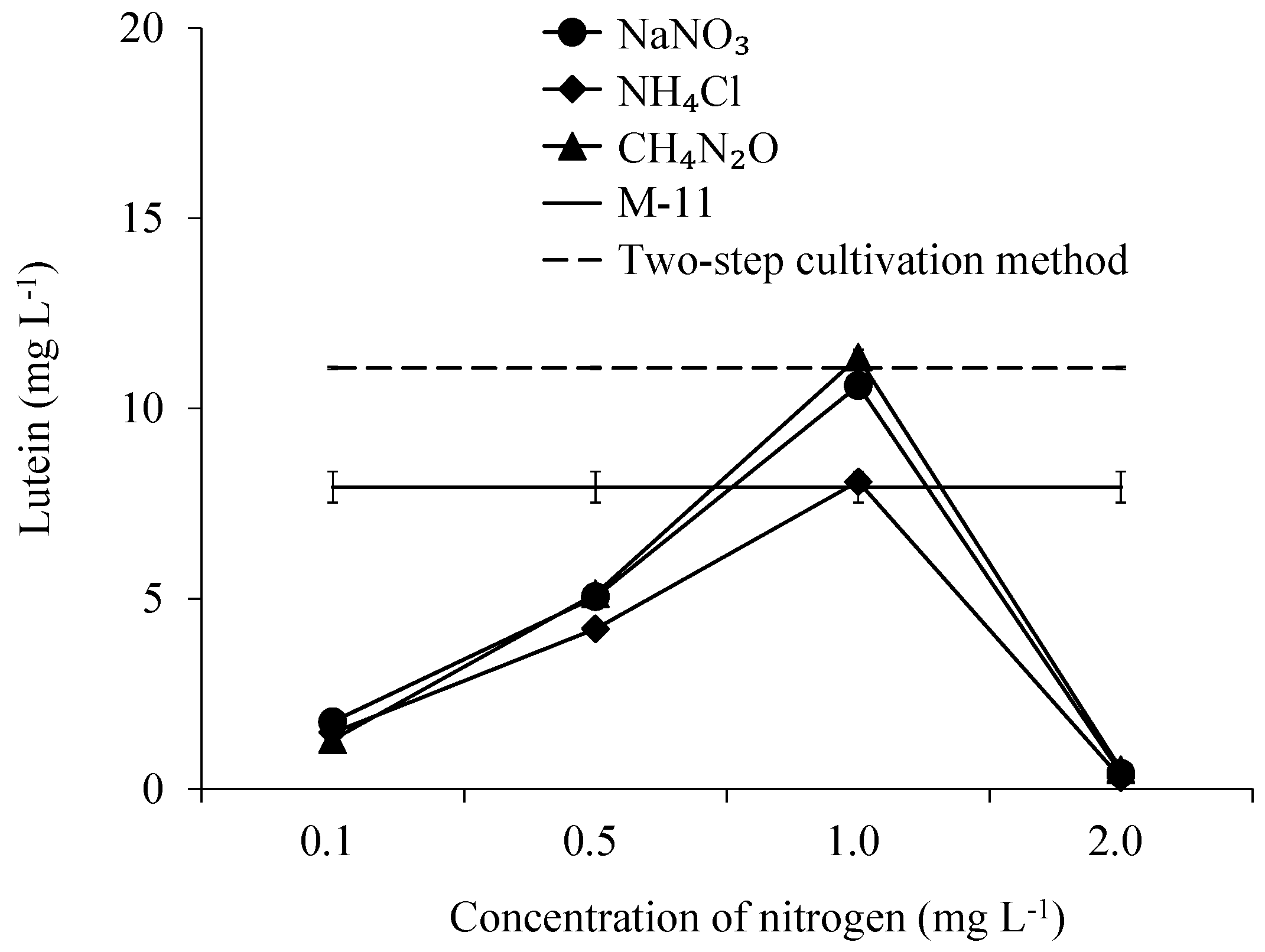
3.6. The Concentration of Lutein in Scenedesmus obliquus
3.7. Fatty Acid Composition Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sivaramakrishnan, R.; Incharoensakdi, A. Microalgae as feedstock for biodiesel production under ultrasound treatment—A review. Bioresour. Technol. 2018, 250, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.; Paddock, M.B.; VanderGheynst, J.S.; Higgins, B.T. Algal photosynthetic aeration increases the capacity of bacteria to degrade organics in wastewater. Biotechnolo. Bioeng. 2020, 117, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Duan, P.; Cao, X.; Liu, M.; Lin, L.; Li, M. Comparison of monoculture and mixed culture (Scenedesmus obliquus and wild algae) for C, N, and P removal and lipid production. Environ. Sci. Pollut. Res. 2019, 26, 20961–20968. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhao, M.; Shen, G.; Gan, X.; Li, M. Linear alkylbenzene sulfonate (LAS) promotes sedimentation and lipid accumulation in Scenedesmus obliquus. RSC Adv. 2017, 7, 9244–9250. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, C.Y.B.; Viegas, T.L.; Lopes, R.G.; Cella, H.; Menezes, R.S.; Soares, A.T.; Filho, N.R.A.; Derner, R.B. A comparison of harvesting and drying methodologies on fatty acids composition of the green microalga Scenedesmus obliquus. Biomass Bioenergy 2020, 132. [Google Scholar] [CrossRef]
- Yu, Z.; Pei, H.; Jiang, L.; Hou, Q.; Nie, C.; Zhang, L. Phytohormone addition coupled with nitrogen depletion almost tripled the lipid productivities in two algae. Bioresour. Technol. 2018, 247, 904–914. [Google Scholar] [CrossRef]
- Singh, K.; Kaloni, D.; Gaur, S.; Kushwaha, S.; Mathur, G. Current research and perspectives on microalgae-derived biodiesel. Biofuels 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Nayak, M.; Suh, W.I.; Chang, Y.K.; Lee, B. Exploration of two-stage cultivation strategies using nitrogen starvation to maximize the lipid productivity in Chlorella sp. HS2. Bioresour. Technol. 2019, 276, 110–118. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; George, B.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2014, 156, 146–154. [Google Scholar] [CrossRef]
- Jia, J.; Han, D.; Gerken, H.G.; Li, Y.; Sommerfeld, M.; Hu, Q.; Xu, J. Molecular mechanisms for photosynthetic carbon partitioning into storage neutral lipids in Nannochloropsis oceanica under nitrogen-depletion conditions. Algal Res. 2015, 7, 66–77. [Google Scholar] [CrossRef]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Borghi, M.D. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Lv, J.-M.; Cheng, L.-H.; Xu, X.-H.; Zhang, L.; Chen, H.-L. Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour. Technol. 2010, 101, 6797–6804. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Pei, H.; Ma, G.; Zhang, S.; Mu, R. Improving productivity and quality of biodiesel from Chlorella vulgaris SDEC-3M through customized process designs. Energy Convers. Manag. 2016, 129, 100–107. [Google Scholar] [CrossRef]
- Sonkar, S.; Mallick, N. Development of a single phase nitrate feeding strategy for enhanced lipid productivity from green microalgae for biodiesel production. Environ. Prog. Sustain. Energy 2017, 36, 222–231. [Google Scholar] [CrossRef]
- Han, F.; Huang, J.; Li, Y.; Wang, W.; Wan, M.; Shen, G.; Wang, J. Enhanced lipid productivity of chlorella pyrenoidosa through the culture strategy of semi-continuous cultivation with nitrogen limitation and pH control by CO2. Bioresour. Technol. 2013, 136, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wan, L.; Li, A.; Zhang, C. Responses in growth, lipid accumulation, and fatty acid composition of four oleaginous microalgae to different nitrogen sources and concentrations. Chin. J. Oceanol. Limnol. 2013, 31, 1306–1314. [Google Scholar] [CrossRef]
- Amin, N.F.; Khalafallah, M.A.; Ali, M.A.; Abou-Sdera, S.A.; Matter, I.A. Effect of some nitrogen sources on growth and lipid of microalgae Chlorella sp. for biodiesel production. J. Appl. Sci. Res. 2013, 9, 4845–4855. [Google Scholar]
- Zhao, M.; Qu, D.; Shen, W.; Li, M. Effects of dissolved organic matter from different sources on Microcystis aeruginosa growth and physiological characteristics. Ecotoxicol. Environ. Saf. 2019, 176, 125–131. [Google Scholar] [CrossRef]
- Gan, X.; Shen, G.; Xin, B.; Li, M. Simultaneous biological desalination and lipid production by Scenedesmus obliquus cultured with brackish water. Desalination 2016, 400, 1–6. [Google Scholar] [CrossRef]
- Tian, Y.; Gao, L.; Deng, J.; Li, M. Characterization of phytoplankton community in a river ecosystem using pigment composition: A feasibility study. Environ. Sci. Pollut. R. 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, H.; Gan, K.; Sun, Y. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga scenedesmus sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar]
- Ho, S.-H.; Chen, C.-Y.; Chang, J.-S. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.K.; Lim, P.E.; Vello, V.; Sim, K.S.; Majid, N.A.; Mustafa, E.M.; Nik, S.N.M.; Liew, K.E.; Chen, B.J.T.; Phang, S.M. Metabolic and physiological regulation of Chlorella sp.(Trebouxiophyceae, Chlorophyta) under nitrogen deprivation. J. Oceanol. Limnol. 2019, 37, 186–198. [Google Scholar] [CrossRef]
- Yu, Z.; Song, M.; Pei, H.; Jiang, L.; Hou, Q.; Nie, C.; Zhang, L. The effects of combined agricultural phytohormones on the growth, carbon partitioning and cell morphology of two screened algae. Bioresour. Technol. 2017, 239, 87–96. [Google Scholar] [CrossRef]
- Salama, E.-S.; Kabra, A.N.; Ji, M.-K.; Kim, J.R.; Min, B.; Jeon, B.-H. Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresour. Technol. 2014, 172, 97–103. [Google Scholar] [CrossRef]
- Kichul, C.; Kilnam, K.; Nalae, L.; Moosang, K.; Jeongchul, H.; Hyeon, H.S.; Mikyung, K.; Seong, W.R.; Daekyung, K.; Tatsuya, O. Enhanced biomass and lipid production by supplement of myo-inositol with oceanic microalga Dunaliella salina. Biomass Bioenergy 2015, 72, 1–7. [Google Scholar]
- Sangram, K.L.; Nicole, C.; Rudolph, P.; Stephen, M.M.; Ian, T.; Li, Y. Current advances in molecular, biochemical, and computational modeling analysis of microalgae triacylglycerol biosynthesis. Biotechnol. Adv. 2016, 34, 1046–1063. [Google Scholar]
- Long, B.M.; Jones, G.J.; Orr, P.T. Cellular Microcystin Content in N-Limited Microcystis aeruginosa Can Be Predicted from Growth Rate. Appl. Environ. Microbiol. 2001, 67, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.; Zhao, M.; Duan, P.; Li, M. Effects of nitrogen forms and supply modes on colony formation in Microcystis aeruginosa. Environ. Boil. Fishes 2017, 30, 831–837. [Google Scholar] [CrossRef]
- Von Ruckert, G.; Giani, A. Effect of nitrate and ammonium on the growth and protein concentration of Microcystis viridis Lemmermann (Cyanobacteria). Braz. J. Bot. 2004, 27, 325–331. [Google Scholar]
- Kamyab, H.; Tin Lee, C.; Md Din, M.F.; Ponraj, M.; Eva Mohamad, S.; Sohrabi, M. Effects of nitrogen source on enhancing growth conditions of green algae to produce higher lipid. Desalin. Water Treat. 2014, 52, 3579–3584. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, H.; Zhao, J.; Xu, Y.; Ge, F. Mechanisms of cetyltrimethyl ammonium chloride-induced toxicity to photosystem II oxygen evolution complex of Chlorella vulgaris F1068. J. Hazard. Mater. 2020, 383. [Google Scholar] [CrossRef] [PubMed]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Babu, A.G.; Wu, X.; Kabra, A.N.; Kim, D.-P. Cultivation of an indigenous Chlorella sorokiniana with phytohormones for biomass and lipid production under N-limitation. Algal Res. 2017, 23, 178–185. [Google Scholar] [CrossRef]
- Del Campo, J. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Moreno, J.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef]
- Marella, T.K.; Datta, A.; Patil, M.D.; Dixit, S.; Tiwari, A. Biodiesel production through algal cultivation in urban wastewater using algal floway. Bioresour. Technol. 2019, 280, 222–228. [Google Scholar] [CrossRef]
- Sung, Y.J.; Patel, A.K.; Yu, B.S.; Choi, H.I.; Kim, J.; Jin, E.; Sim, S.J. Sedimentation rate-based screening of oleaginous microalgae for utilization as a direct combustion fuel. Bioresour. Technol. 2019, 293. [Google Scholar] [CrossRef]


| Algae Strain | Treatment | Lipid Content (%) | Lipid Productivity (mg L−1 day−1) | References |
|---|---|---|---|---|
| Scenedesmus obliquus FACHB-416 | BG-11 + 1.2 g L−1 NaCl | 16.2 | 9.9 | Gan et al. [20] |
| BG-11 + 8.8 g L−1 NaCl | 20.8 | 1.8 | ||
| Scenedesmus sp. LX1 | mBG-11 + 2.5 mg N L−1 of NaNO3 | 29.7 | 8.7 | Li et al. [22] |
| mBG-11 + 5 mg N L−1 of NaNO3 | 22.5 | 8.5 | ||
| mBG-11 + 10 mg N L−1 of NaNO3 | 20.8 | 11.6 | ||
| Scenedesmus sp. SDEC-8 | BG-11 + two-step cultivation method | 18.9 | 3.8 | Yu et al. [25] |
| BG-11 + two-step cultivation method + 5 mg L−1 auxin | 46.9 | 11.9 | ||
| BG-11 + two-step cultivation method + 30 mg L−1 auxin | 16.7 | 5.2 | ||
| BG-11 + two-step cultivation method + 50 mg L−1 auxin | 14.7 | 2.3 | ||
| Scenedesmus obliquus GU732418 | BBM | — | 2.3 | Salama et al. [26] |
| BBM + 1.75 mg L−1 IAA | — | 4.6 | ||
| BBM + 2.15 mg L−1 DAH | — | 5.8 | ||
| Dunaliella salina CCAP 19/20 | ESM | — | 0.8 | Kichul et al. [27] |
| ESM + 500 mg L−1 myo-inositol | — | 0.9 | ||
| Scenedesmus obliqquus FACHB-416 | multiple-dose of 2 mg N L−1 of CH4N2O | 22.9 | 12.1 | Current study |
| Fatty Acid | M-11 | NaNO3 | NH4Cl | CH4N2O | Two-Step Cultivation Method | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.5 | 1 | 2 | 0.1 | 0.5 | 1 | 2 | 0.1 | 0.5 | 1 | 2 | |||
| C14:0 | 0.3 | 0.2 | 0.0 | 0.0 | 0.6 | 0.2 | 0.0 | 0.0 | 1.0 | 0.0 | 0.2 | 0.0 | 0.4 | 0.5 |
| C15:0 | 0.6 | 0.3 | 0.4 | 0.5 | 0.5 | 0.3 | 1.7 | 1.0 | 0.9 | 0.3 | 0.4 | 0.6 | 0.9 | 0.6 |
| C16:0 | 31.7 | 29.5 | 30.5 | 34.4 | 51.9 | 32.3 | 53.1 | 47.4 | 51.2 | 30.8 | 31.6 | 40.7 | 34.0 | 58.0 |
| C16:1 | 0.8 | 0.6 | 1.0 | 0.6 | 0.7 | 0.5 | 1.0 | 1.4 | 1.0 | 0.5 | 0.6 | 0.8 | 1.0 | 0.8 |
| C18:0 | 3.3 | 2.8 | 2.7 | 2.7 | 4.7 | 3.0 | 5.0 | 4.3 | 5.3 | 3.1 | 2.5 | 3.6 | 2.8 | 5.2 |
| C18:1 | 52.7 | 55.8 | 54.4 | 49.4 | 28.0 | 53.6 | 25.4 | 32.0 | 17.6 | 55.5 | 53.6 | 40.9 | 46.3 | 24.6 |
| C18:2 | 8.4 | 9.2 | 8.9 | 9.5 | 10.5 | 8.3 | 9.2 | 9.6 | 19.1 | 8.0 | 8.8 | 9.4 | 10.2 | 7.2 |
| C18:3 | 0.4 | 0.3 | 0.3 | 0.6 | 0.8 | 0.3 | 0.9 | 1.1 | 0.8 | 0.1 | 0.3 | 0.8 | 1.0 | 0.7 |
| C20:1 | 0.7 | 0.8 | 0.8 | 0.6 | 0.0 | 0.8 | 0.0 | 0.0 | 0.0 | 0.9 | 0.7 | 0.7 | 0.4 | 0.7 |
| C22:0 | 0.5 | 0.3 | 0.4 | 0.6 | 0.9 | 0.4 | 1.3 | 1.5 | 1.7 | 0.4 | 0.4 | 0.9 | 0.8 | 0.8 |
| C24:0 | 0.7 | 0.3 | 0.6 | 1.1 | 1.4 | 0.4 | 2.4 | 1.7 | 1.5 | 0.5 | 0.8 | 1.5 | 2.2 | 1.0 |
| Unsaturated | 63.0 | 66.7 | 65.4 | 60.7 | 40.0 | 63.5 | 36.5 | 44.1 | 38.4 | 65.0 | 64.0 | 52.6 | 58.9 | 34.0 |
| C16-C18 | 97.3 | 98.2 | 97.8 | 97.2 | 96.6 | 98.0 | 94.6 | 95.8 | 94.9 | 98.0 | 97.4 | 96.2 | 95.3 | 96.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, M.; Gao, L.; Zhao, W.; Chen, W.; Li, M. Effects of Nitrogen Forms and Supply Mode on Lipid Production of Microalga Scenedesmus obliquus. Energies 2020, 13, 697. https://doi.org/10.3390/en13030697
An M, Gao L, Zhao W, Chen W, Li M. Effects of Nitrogen Forms and Supply Mode on Lipid Production of Microalga Scenedesmus obliquus. Energies. 2020; 13(3):697. https://doi.org/10.3390/en13030697
Chicago/Turabian StyleAn, Mei, Li Gao, Wen Zhao, Weiguang Chen, and Ming Li. 2020. "Effects of Nitrogen Forms and Supply Mode on Lipid Production of Microalga Scenedesmus obliquus" Energies 13, no. 3: 697. https://doi.org/10.3390/en13030697