Effect of A-/B-site Doping on Oxygen Non-Stoichiometry, Structure characteristics, and O2 Releasing Behavior of La1−xCaxCo1−yFeyO3−δ Perovskites
Abstract
:1. Introduction
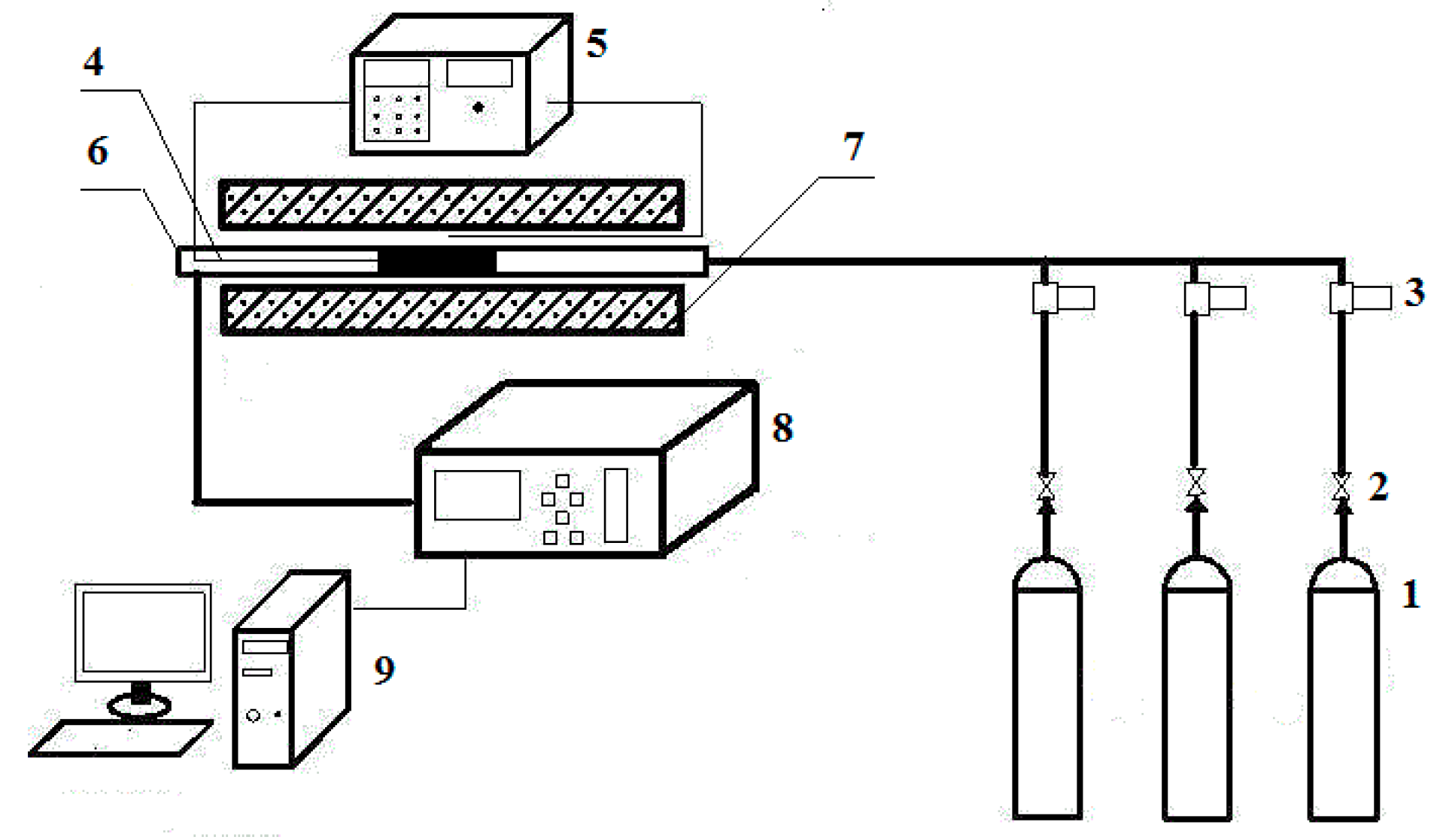
2. Experimental Methods
2.1. Sample Synthesis and Characterization
2.2. Measurements of Oxygen Stoichiometry
2.2.1. Iodometric Titration
2.2.2. Measurements of δ
2.3. Oxygen Adsorption/Desorption Experiments
3. Results and Discussion
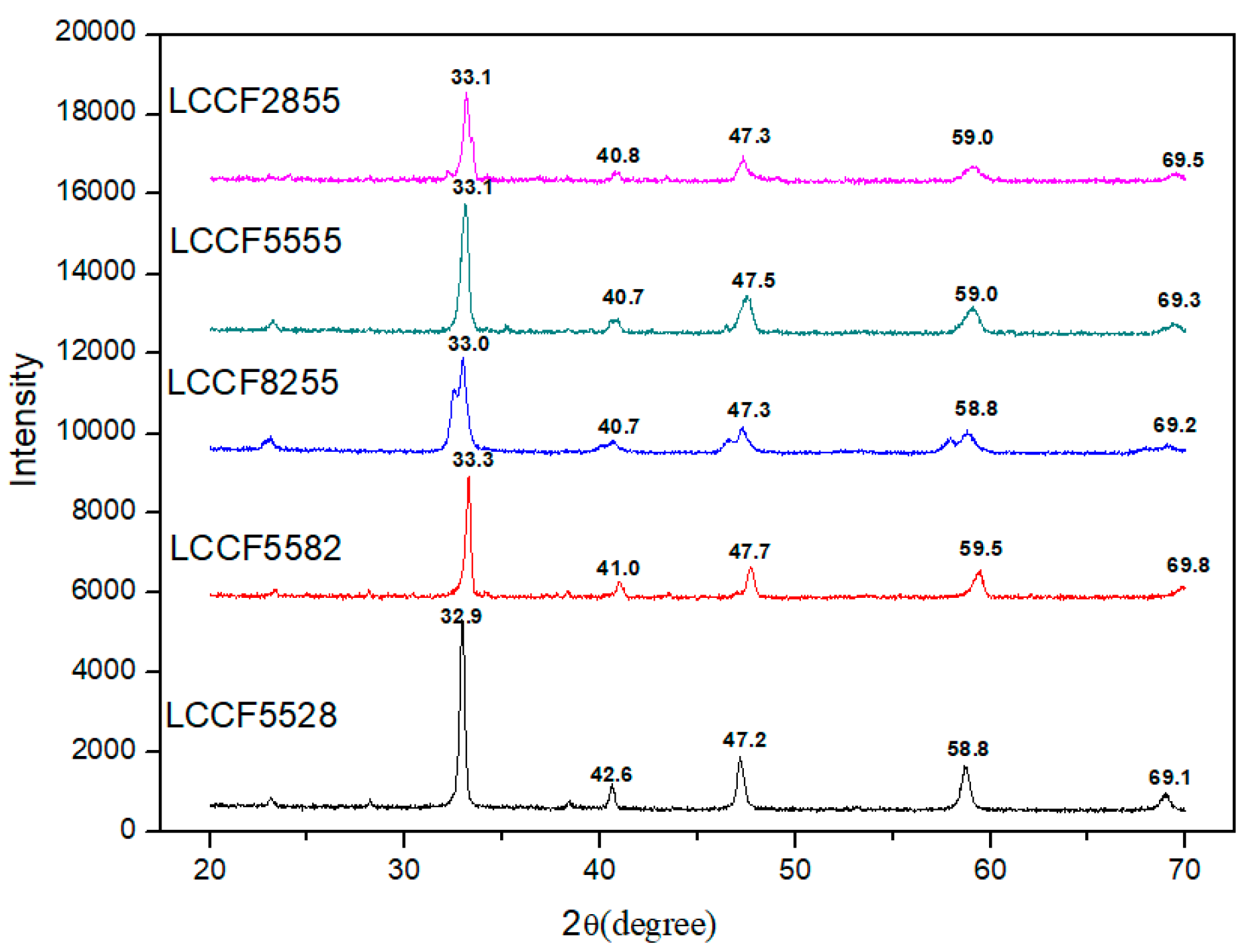
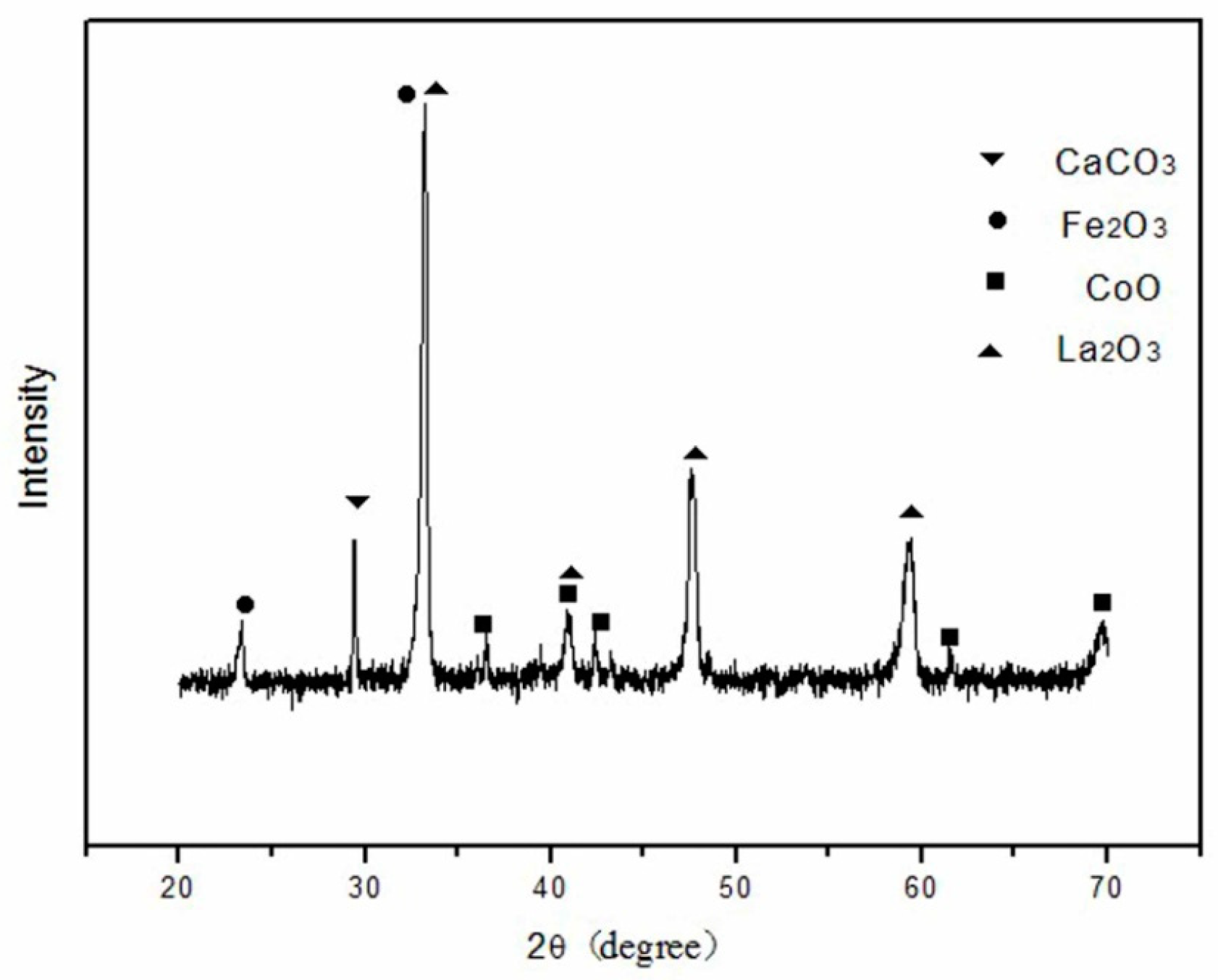
3.1. XRD Analysis and Tolerance Factor
3.2. Oxygen Non-Stoichiometry
3.3. Oxygen Adsorption/Desorption Experiments of La1−xCaxCo1−yFeyO3−δ
3.4. Effect of A-/B-Site Doping on Performance of LCCF
4. Conclusions
- The perovskite La1−xCaxCo1−yFeyO3−δ (LCCF) sorbents with different A-/B-site doping amounts were synthesized by the liquid citrate method. XRD patterns indicate that all the samples have a perovskite structure with good crystallization. In addition, their tolerance factors are close to 1. The tolerance factor suggests that LCCF5582 has the most stable perovskite-type structure.
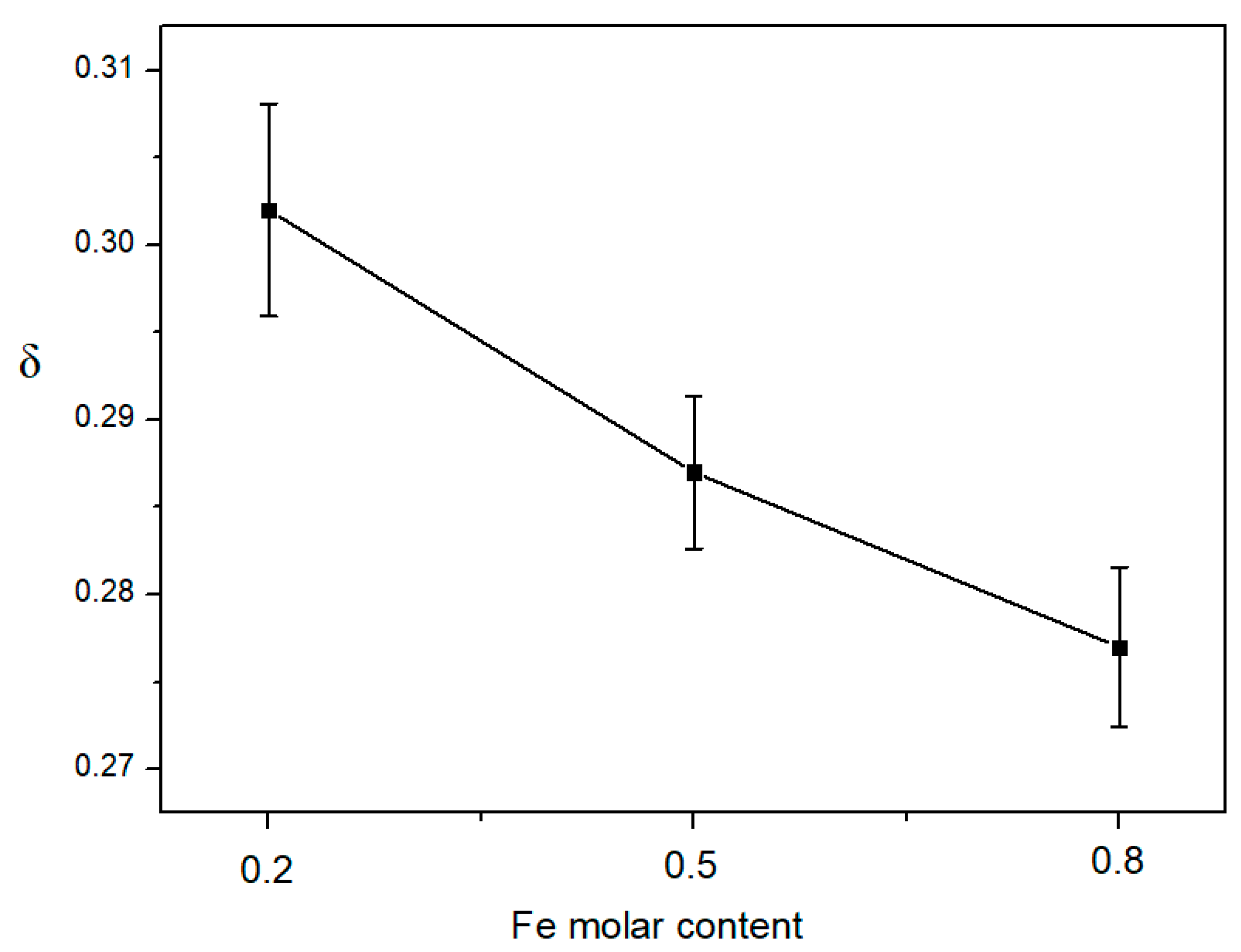
- The oxygen non-stoichiometry δ of LCCF sorbents was measured by iodometric titration. Oxygen non-stoichiometry values of LCCF by A-site doping increased with increasing Ca-doping amount, but the O2 desorption amount was nearly constant. This suggests that the O2 desorption amount is not dependent on the oxygen non-stoichiometry δ of LCCF from A-site doping.
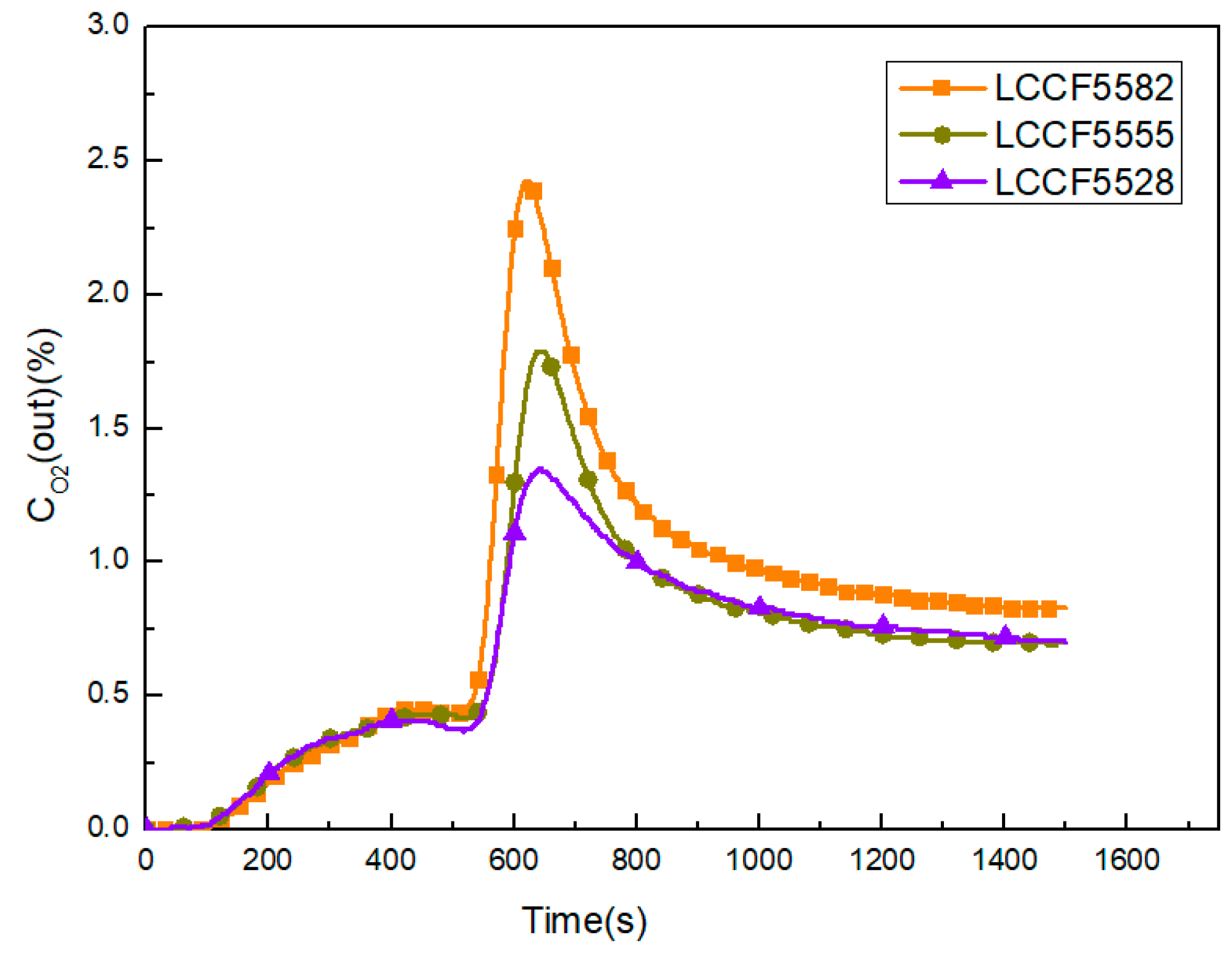
- Oxygen non-stoichiometry values of LCCF with B-site Fe-doping are nearly the same. However, B-site Fe-doping has an obvious impact on the oxygen desorption amount. In addition, the oxygen desorption amounts increase with decreasing B-site Fe molar content. As a result, suitable B-site doping for perovskite is necessary to obtain a higher O2 desorption amount.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| oxygen vacancy | |
| denote lattice oxygen | |
| electronic-hole | |
| t | tolerance factors |
| LCCF2855 | La0.2Ca0.8Co0.5Fe0.5O3−δ |
| LCCF5555 | La0.5Ca0.5Co0.5Fe0.5O3−δ |
| LCCF8255 | La0.8Ca0.2Co0.5Fe0.5O3−δ |
| LCCF5528 | La0.5Ca0.5Co0.2Fe0.8O3−δ |
| LCCF5582 | La0.5Ca0.5Co0.8Fe0.2O3−δ |
References
- Shen, Q.W.; Zhang, Y.D.; Ding, H.R.; Xu, Y.Q.; Shi, B.C.; Zheng, Y.; Yuan, J.L. Performance and stability enhancement of perovskite-type nanomaterials applied for carbon capture utilizing oxyfuel combustion. Energies 2017, 10, 164. [Google Scholar] [CrossRef]
- Shen, Q.W.; Zheng, Y.; Luo, C.; Zheng, C.G. Development and characterization of Ba1−xSrxCo0.8Fe0.2O3−δ perovskite for oxygen production in oxyfuel combustion system. Chem. Eng. J. 2014, 225, 462–470. [Google Scholar] [CrossRef]
- Shen, Q.W.; Zheng, Y.; Li, S.A.; Ding, H.R.; Xu, Y.Q. Optimize process parameters of microwave-assisted EDTA method using orthogonal experiment for novel BaCoO3−δ perovskite. J. Alloys Compd. 2016, 658, 125–131. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, Y.S. Fixed-bed performance for production of oxygen-enriched carbon dioxide stream by perovskite-type ceramic sorbent. Sep. Purif. Technol. 2006, 49, 27–35. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, Y.S.; Bulow, M. High temperature sorption separation of air for producing oxygen-enriched carbon dioxide stream. AIChE J. 2006, 52, 574–581. [Google Scholar] [CrossRef]
- Shen, Q.W.; Sun, L.N.; Wang, B.W. Numerical simulation of the effects of obstacles in gas flow fields of a solid oxide fuel cell. Int. J. Electrochem. Sci. 2019, 14, 1698–1712. [Google Scholar] [CrossRef]
- Liao, Q.; Chen, Y.; Wei, Y.Y.; Zhou, L.Y.; Wang, H.H. Performance of U.-shaped BaCo0.7Fe0.2Ta0.1O3−δ hollow-fiber membranes reactor with high oxygen permeation for methane conversion. Chem. Eng. J. 2014, 237, 146–152. [Google Scholar] [CrossRef]
- Boldrini, S.; Mortalo, C.; Fasolin, S. Influence of microwave-assisted pechini method on La0.8Sr0.2 Ga0.83 Mg0.17O3–δ Ionic Conductivity. Fuel Cells 2012, 12, 54–60. [Google Scholar] [CrossRef]
- Zheng, J.Q.; Zhu, Y.J.; Xu, J.S. Microwave-assisted rapid synthesis and photocatalytic activity of mesoporous Nd-doped SrTiO3 nanospheres and nanoplates. Mater. Lett. 2013, 100, 62–65. [Google Scholar] [CrossRef]
- Guntuka, S.; Banerjee, S.; Farooq, S.; Srinivasan, M.P. A- and B-site substituted lanthanum cobaltite perovskite as high temperature oxygen sorbent. Ind. Eng. Chem. Res. 2008, 47, 154–162. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, Y.S. High-temperature sorption process for air separation and oxygen removal. Ind. Eng. Chem. Res. 2002, 41, 2775–2784. [Google Scholar] [CrossRef]
- Lin, Y.S.; Maclean, D.L.; Zeng, Y. High Temperature Adsorption Process. U.S. Patent 6059858, 9 May 2000. [Google Scholar]
- Mizusaki, J.; Yamauchi, S.; Fueki, K.; Ishikamw, A. Nonstoichiometry of perovskite type oxides La1−xSrxCoO3−δ. Solid State Ionics 1984, 12, 19–124. [Google Scholar] [CrossRef]
- Teraoka, Y.; Hua, H.M.; Furukawa, S. Oxygen permeation through perovskite-tvpe oxides. Chem. Lett. 1985, 11, 1743–1746. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, Y.S.; Zeng, Y. A semi-empirical equation for oxygen non- stoichiometry of perovskite-type ceramics. Solid State Ionics 2002, 150, 245–254. [Google Scholar] [CrossRef]
- Cherepanov, V.; Gavrilova, A.L.Y.; Aksenova, T.V. Synthesis, structure and oxygen nonstoichiometry of La0.4Sr0.6Co1−yFeyO3−δ. Prog. Solid State Chem. 2007, 35, 175–182. [Google Scholar] [CrossRef]
- Thursfield, A.; Metcalfe, I.S. The use of dense mixed ionic and electronic conducting membranes for chemical production. Chem. Mater. 2004, 14, 2475–2485. [Google Scholar] [CrossRef]
- Chen, W.M.; Hong, W.; Geng, J.F. Iodometric titration for determining the oxygen content of samples doped with Fe and Co. Phys. C 1996, 270, 349–353. [Google Scholar] [CrossRef]
- Yin, Q.; Kniep, J.; Lin, Y.S. Oxygen sorption and desorption properties of Sr–Co–Fe oxide. Chem. Eng. Sci. 2008, 63, 2211–2218. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef] [Green Version]


| Ions | Ionic Radii (Å) | Oxides | t |
|---|---|---|---|
| La3+ | 1.36 | LCCF8255 | 1.0008 |
| Ca2+ | 1.34 | LCCF5555 | 0.9986 |
| Co3+ | 0.545 | LCCF2855 | 0.9964 |
| Fe3+ | 0.55 | LCCF5582 | 0.9994 |
| LCCF5528 | 0.9979 |
| Sample | Oxygen Desorption Amount (mg O2/g·sorbent) |
|---|---|
| LCCF8255 | 9.34 |
| LCCF5555 | 8.81 |
| LCCF2855 | 9.94 |
| Sample | Oxygen Desorption Amount (mg O2/g·sorbent) |
|---|---|
| LCCF5582 | 10.71 |
| LCCF5555 | 8.81 |
| LCCF5528 | 8.50 |
| Effect of A-site Doping | δ | Oxygen Desorption Amount (mg O2/g·sorbent) |
|---|---|---|
| LCCF8255 | 0.119 | 9.34 |
| LCCF5555 | 0.287 | 8.81 |
| LCCF2855 | 0.47 | 9.44 |
| Effect of B-site Doping | δ | Oxygen Desorption Amount (mg O2/g·sorbent) |
|---|---|---|
| LCCF5582 | 0.302 | 10.7 |
| LCCF5555 | 0.287 | 8.81 |
| LCCF5528 | 0.277 | 8.50 |
| Samples | δ | Oxygen Desorption Amount (mg O2/g·sorbent) |
|---|---|---|
| LCCF5582 | 0.302 | 10.7 |
| LCCF5555 | 0.287 | 8.81 |
| LCCF5528 | 0.277 | 8.50 |
| LCCF8255 | 0.119 | 9.34 |
| LCCF2855 | 0.47 | 9.44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Q.; Li, S.; Yang, G.; Sunden, B.; Yuan, J. Effect of A-/B-site Doping on Oxygen Non-Stoichiometry, Structure characteristics, and O2 Releasing Behavior of La1−xCaxCo1−yFeyO3−δ Perovskites. Energies 2019, 12, 410. https://doi.org/10.3390/en12030410
Shen Q, Li S, Yang G, Sunden B, Yuan J. Effect of A-/B-site Doping on Oxygen Non-Stoichiometry, Structure characteristics, and O2 Releasing Behavior of La1−xCaxCo1−yFeyO3−δ Perovskites. Energies. 2019; 12(3):410. https://doi.org/10.3390/en12030410
Chicago/Turabian StyleShen, Qiuwan, Shian Li, Guogang Yang, Bengt Sunden, and Jinliang Yuan. 2019. "Effect of A-/B-site Doping on Oxygen Non-Stoichiometry, Structure characteristics, and O2 Releasing Behavior of La1−xCaxCo1−yFeyO3−δ Perovskites" Energies 12, no. 3: 410. https://doi.org/10.3390/en12030410






