Rheological Characteristics of Molten Salt Seeded with Al2O3 Nanopowder and Graphene for Concentrated Solar Power
Abstract
:1. Introduction
2. Materials and Method
2.1. Preparation of Samples
2.2. Characterization of Nanocomposites and Nanoparticles
2.3. Measurement of Viscosity
3. Results and Discussion
3.1. Characterization of Nanocomposites
3.2. Rheological Behaviour of Pure Salt
Comparison with Literature Correlations
3.3. Rheological Behaviour of Nanocomposite
3.3.1. Size of Nanoparticles
3.3.2. Viscosity of Nanocomposites
4. Conclusions
- (1)
- The viscosities of pure HITEC salt and solar salt regularly decrease with the increase of temperatures and demonstrate the Newtonian behaviour at different temperature ranges. The present results show reasonable agreement with the calculation results from the literature correlations to some extent, while the deviation was mainly caused by parallel plate and purity of salt.
- (2)
- Both Al2O3 nanopowder and graphene are compatible with molten salt and can generate structural interaction with salt molecules. Agglomeration of graphene happens on the surface of the salts seeded with graphene. A better method to synthetize the nanocomposites seeded with graphene should be investigated in the future.
- (3)
- The viscosities of all the nanocomposites show temperature dependence. The addition of Al2O3 nanopowder into the salt has relatively little effect on the viscosity of the salt, e.g., the viscosities vary with the range of −35.4~8.1% for the HITEC salt nanocomposites and with the range of −9.2~68.1% for the solar salt nanocomposites. Whereas graphene leads to a remarkable increase in viscosities, especially at low temperature below 400 °C, which should be due to the larger size and different structural characteristics of graphene.
- (4)
- Experimental results reveal that the addition of graphene into HITEC salt induces the non-Newtonian behaviours of the nanocomposites, and the ternary melting characteristics of HITEC salt might be changed with graphene. While solar salt seeded with graphene shows Newtonian behaviour at different shear rates and temperatures, indicating that the binary melting characteristics of solar salt can be kept well.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| T | temperature, °C |
| R | ideal gas constant, J/(K·mol) |
| Greek letters | |
| μ | dynamic viscosity, mPa·s |
| Acronyms | |
| BSE | backscattered electrons |
| CSP | concentrated solar power |
| CTD | convection temperature device |
| DLS | dynamic light scatting |
| EDX | energy dispersive X-ray spectrometer |
| HTF | heat transfer fluid |
| MWCNT | multi-walled carbon nanotubes |
| PP | parallel plate |
| SEM | scanning electron microscope |
References
- Wang, S.; Brian Tarroja, B.; Schell, L.S.; Shaffer, B.; Samuelsen, S. Prioritizing among the end uses of excess renewable energy for cost-effective greenhouse gas emission reductions. Appl. Energy 2019, 235, 284–298. [Google Scholar] [CrossRef]
- Romeroa, M.; Steinfeld, A. Concentrating solar thermal power and thermochemical fuels. Energy Environ. Sci. 2012, 5, 9234–9245. [Google Scholar] [CrossRef]
- Maldonado, J.M.; Fullana-Puig, M.; Martín, M.; Solé, A.; Fernández, A.G.; Gracia, A.; Cabeza, L.F. Phase change material selection for thermal energy storage at high temperature range between 210 °C and 270 °C. Energies 2018, 11, 861. [Google Scholar] [CrossRef]
- Fernandez-García, A.; Rojas, E.; Perez, M.; Silva, R.; Hernandez-Escobedo, Q.; Manzano-Agugliaro, F. A parabolic-trough collector for cleaner industrial process heat. J. Clean. Prod. 2015, 89, 272–985. [Google Scholar] [CrossRef]
- Fernandez-García, A.; Juaidi, A.; Sutter, F.; Martinez-Arcos, L.; Manzano-Agugliaro, F. Solar reflector materials degradation due to the sand deposited on the backside protective paints. Energies 2018, 11, 808. [Google Scholar] [CrossRef]
- Fernandez-García, A.; Valenzuela, L.; Zarza, E.; Rojas, E.; Perez, M.; Hernandez-Escobedo, Q.; Manzano-Agugliaro, F. SMALL-SIZED parabolic-trough solar collectors: Development of a test loop and evaluation of testing conditions. Energy 2018, 152, 401–415. [Google Scholar] [CrossRef]
- Mostafavi Tehrani, S.S.; Taylor, R.A.; Nithyanandam, K.; Shafiei Ghazani, A. Annual comparative performance and cost analysis of high temperature, sensible thermal energy storage systems integrated with a concentrated solar power plant. Sol. Energy 2017, 153, 153–172. [Google Scholar] [CrossRef]
- Xu, B.; Li, P.W.; Chan, C. Application of phase change materials for thermal energy storage in concentrated solar thermal power plants: A review to recent developments. Appl. Energy 2015, 160, 286–307. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Zhang, P.; Li, M. Thermal characterization of nitrates and nitrates/expanded graphite mixture phase change materials for solar energy storage. Energy Convers. Manag. 2013, 73, 86–94. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, X.; Meng, Z.N.; Li, M. Heat transfer characteristics of a molten-salt thermal energy storage unit with and without heat transfer enhancement. Appl. Energy 2015, 137, 758–772. [Google Scholar] [CrossRef]
- Bonka, A.; Sau, S.; Uranga, N.; Hernaiz, M.; Bauer, T. Advanced heat transfer fluids for direct molten salt line-focusing CSP plants. Prog. Energy Combust. 2018, 67, 69–87. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P.; Li, M. Experimental and numerical study of heat transfer performance of nitrates/expanded graphite composite PCM for solar energy storage. Energy Convers. Manag. 2015, 105, 272–284. [Google Scholar] [CrossRef]
- Siegel, N.P.; Bradshaw, R.W.; Cordaro, J.B.; Kruizenga, A.M. Thermophysical property measurement of nitrate salt heat transfer fluids. In Proceedings of the ASME 2011 5th International Conference on Energy Sustainability, Washington, DC, USA, 7–10 August 2011. [Google Scholar]
- Ni, H.O.; Wu, J.; Sun, Z.; Lu, G.M.; Yu, J.G. Insight into the viscosity enhancement ability of Ca(NO3)2 on the binary molten nitrate salt: A molecular dynamics simulation study. Chem. Eng. J. 2018. [Google Scholar] [CrossRef]
- Wu, Y.T.; Li, Y.; Ren, N.; Ma, C.F. Improving the thermal properties of NaNO3-KNO3 for concentrating solar power by adding additives. Sol. Energy Mater. Sol. Cells 2018, 176, 357–373. [Google Scholar]
- Owolabi, A.L.; Al-Kayiem, H.H.; Baheta, A.T. Nanoadditives induced enhancement of the thermal properties of paraffin-based nanocomposites for thermal energy storage. Sol. Energy 2016, 135, 644–653. [Google Scholar] [CrossRef]
- Myers, P.D., Jr.; Alam, T.E.; Kamal, R.; Goswami, D.Y.; Stefanakos, E. Nitrate salts doped with CuO nanoparticles for thermal energy storage with improved heat transfer. Appl. Energy 2016, 165, 225–233. [Google Scholar] [CrossRef]
- Arthur, O.; Karim, M.A. An investigation into the thermophysical and rheological properties of nanofluids for solar thermal applications. Renew. Sustain. Energy Rev. 2016, 55, 739–755. [Google Scholar] [CrossRef]
- Al-Jethelah, M.; Tasnim, S.H.; Mahmud, S.; Dutta, A. Nano-PCM filled energy storage system for solar-thermal applications. Renew. Energy 2018, 126, 137–155. [Google Scholar] [CrossRef]
- Elbahjaoui, R.; QarniaCad, H.E. Thermal analysis of nanoparticle-enhanced phase change material solidification in a rectangular latent heat storage unit including natural convection. Energy Build. 2017, 153, 1–17. [Google Scholar] [CrossRef]
- Shin, D.Y.; Banerjee, D. Specific heat of nanofluids synthesized by dispersing alumina nanoparticles in alkali salt eutectic. Int. J. Heat Mass Transfer 2014, 74, 210–214. [Google Scholar] [CrossRef]
- Huminic, A.; Huminic, G.; Fleaca, C.; Dumitrache, F.; Morjan, I. Thermal conductivity, viscosity and surface tension of nanofluids based on FeC nanoparticles. Powder Technol. 2015, 284, 78–84. [Google Scholar] [CrossRef]
- Bashirnezhad, K.; Bazri, S.; Safaei, M.R.; Goodarzi, M.; Dahari, M.; Mahian, O.; Dalkiliça, A.S.; Wongwises, S. Viscosity of nanofluids: A review of recent experimental studies. Int. Commun. Heat Mass Transf. 2016, 73, 114–123. [Google Scholar] [CrossRef]
- Prasher, R.; Song, D.; Wang, J.; Phelan, P. Measurements of nanofluid viscosity and its implications for thermal applications. Appl. Phys. Lett. 2006, 89, 133108. [Google Scholar] [CrossRef]
- Jin, Y.; Cheng, J.H.; An, X.H.; Su, T.; Zhang, P.; Li, Z. Accurate viscosity measurement of nitrates/nitrites salts for concentrated solar power. Sol. Energy 2016, 137, 385–392. [Google Scholar] [CrossRef]
- Chen, H.S.; Ding, Y.L.; Tan, C.Q. Rheological behaviour of nanofluids. New J. Phys 2007, 9, 367. [Google Scholar] [CrossRef]
- Lasfargues, M.; Cao, H.; Geng, Q.; Ding, Y.L. Rheological analysis of binary eutectic mixture of sodium and potassium nitrate and the effect of low concentration CuO nanoparticle addition to its viscosity. Materials 2015, 8, 5194–5204. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Sancheza, B.; Nieto-Maestrea, J.; Veca, E.; Liberatore, R.; Sau, S.; Navarro, H.; Ding, Y.L.; Navarrete, N.; Enrique, J.J.; Ángel, G.; et al. Rheology of solar-salt based nanofluids for concentrated solar power. Influence of the salt purity, nanoparticle concentration, temperature and rheometer geometry. Sol. Energy Mater. Sol. Cells 2018, 176, 357–373. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Viscosity measurements of multi-walled carbon nanotubes-based high temperature nanofluids. Mater. Lett. 2014, 122, 212–215. [Google Scholar] [CrossRef]
- Kumar, R.; Joanni, E.; Singh, R.K.; Singh, D.P.; Moshkalev, S.A. Recent advances in the synthesis and modification of carbon-based 2D materials for application in energy conversion and storage. Prog. Energy Combust. 2018, 67, 115–157. [Google Scholar] [CrossRef]
- Choi, H.J.; Jung, S.M.; Seo, J.M.; Chang, D.W.; Dai, L.M.; Baek, J.B. Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 2012, 1, 534–551. [Google Scholar] [CrossRef]
- Chen, D.Z.; Qin, S.Y.; Tsui, G.C.P.; Tang, C.Y.; Ouyang, X.; Liu, J.H.; Tang, J.N.; Zuo, J.D. Fabrication, morphology and thermal properties of octadecylamine-grafted graphene oxide-modified phase-change microcapsules for thermal energy storage. Compos. Part B 2019, 157, 239–247. [Google Scholar] [CrossRef]
- Kholmanov, I.; Kim, J.Y.; Ou, E.; Ruoff, R.S.; Shi, L. Continuous carbon nanotube-ultrathin graphite hybrid foams for increased thermal conductivity and suppressed subcooling in composite phase change materials. ACS Nano 2015, 9, 11699–11707. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zheng, K.; Yan, Y.; Cai, Z.H.; Lin, S.X.; Hu, X.B. Graphene aerogels enhanced phase change materials prepared by one-pot method with high thermal conductivity and large latent energy storage. Sol. Energy Mater. Sol. Cells 2018, 185, 487–493. [Google Scholar] [CrossRef]
- Kant, K.; Shukla, A.; Sharma, A.; Biwole, P.H. Heat transfer study of phase change materials with graphene nanoparticle for thermal energy storage. Sol. Energy 2017, 146, 453–463. [Google Scholar] [CrossRef]
- Grosu, Y.; Nithiyanantham, U.; Zaki, A.; Faik, A. A simple method for the inhibition of the corrosion of carbon steel by molten nitrate salt for thermal storage in concentrating solar power applications. Mater. Degrad. 2018, 34, 1–8. [Google Scholar] [CrossRef]
- Xiao, X.; Wen, D.S. Preparation and characterization of salt/nickel foam composite seeded with graphene. In Proceedings of the 6th International Conference on Cryogenics and Refrigeration, Shanghai, China, 12–14 April 2018. [Google Scholar]
- Xiao, X.; Wen, D.S. Investigation on thermo-physical properties of molten salt enhanced with nanoparticle and copper foam. IEEE Xplore Digital Library 2018. [Google Scholar] [CrossRef]
- Award, A.; Navarro, H.; Ding, Y.L.; Wen, D.S. Thermal-physical properties of nanoparticle-seeded nitrate molten salts. Renew. Energy 2018, 120, 275–588. [Google Scholar]
- HITEC Heat Transfer Salt. Costal Chemical Co., L.L.C., Brenntag Company. Available online: http://www.coastalchem.com (accessed on 18 December 2018).
- Kirst, W.E.; Nagle, W.M.; Castner, J.B. A new heat transfer medium for high temperatures. Trans. Am. Inst. Chem. Eng. 1940, 36, 371–394. [Google Scholar]
- Gaune, P.G. Viscosity of potassium nitrate-sodium nitrite-sodium nitrate mixtures. J. Chem. Eng. Data 1982, 27, 151–153. [Google Scholar] [CrossRef]
- Chen, Y.C.; Wu, Y.T.; Ren, N.; Ma, C.F. Experimental study of viscosity characteristics of high-temperature heat transfer molten salts. Sci. China Technol. Sci. 2011, 54, 3022–3026. [Google Scholar] [CrossRef]
- Yang, Z.; Garimella, S.V. Thermal analysis of solar thermal energy storage in a molten-salt thermocline. Sol. Energy 2010, 84, 974–985. [Google Scholar] [CrossRef] [Green Version]
- Coscia, K.; Neti, S.; Oztekin, A.; Nelle, S.; Mohapatra, S.; Elliott, T. The thermophysical properties of the NaNO3-KNO3, LiNO3-NaNO3, and LiNO3-KNO3 systems. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Denver, CO, USA, 11–17 November 2012. [Google Scholar]
- Nissen, D.A. Thermophysical properties of the equimolar mixture NaNO3-KNO3 from 300 to 600 °C. J. Chem. Eng. Data 1982, 27, 269–273. [Google Scholar] [CrossRef]
- Mar, R.W.; Bradshaw, R.W.; Carling, R.W.; Goods, S.H.; Nagelberg, A.S.; Nissen, D.A. Progress Report: Molten Nitrate Salt Technology Development; SAND82-8220; Sandia National Labs: Livermore, CA, USA, 1982.
- Murgulescu, I.G.; Zuca, S. Viscosity of binary mixtures of molten nitrates as a function of ionic radius-II. Electrochim. Acta 1969, 14, 519–526. [Google Scholar] [CrossRef]


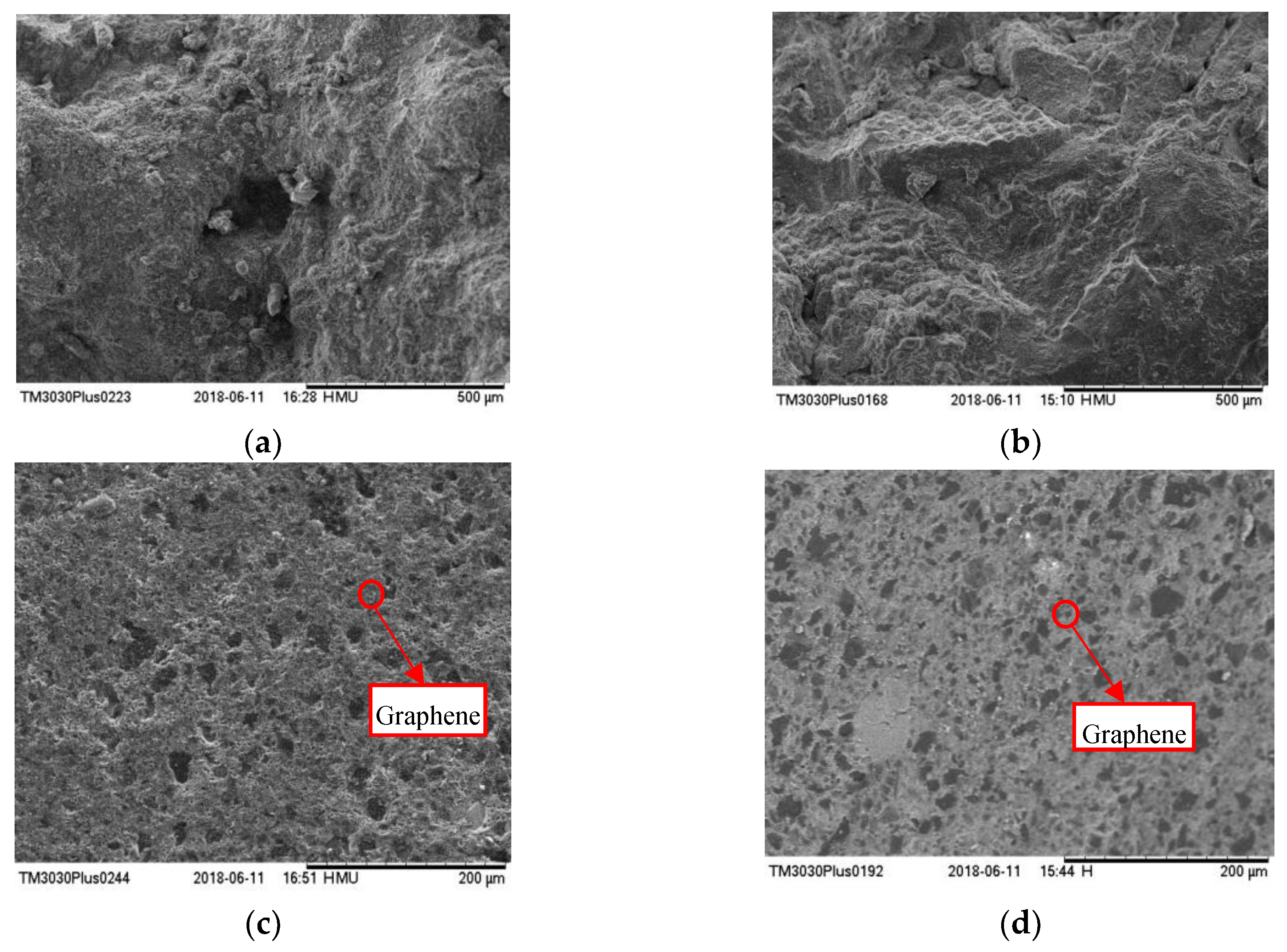



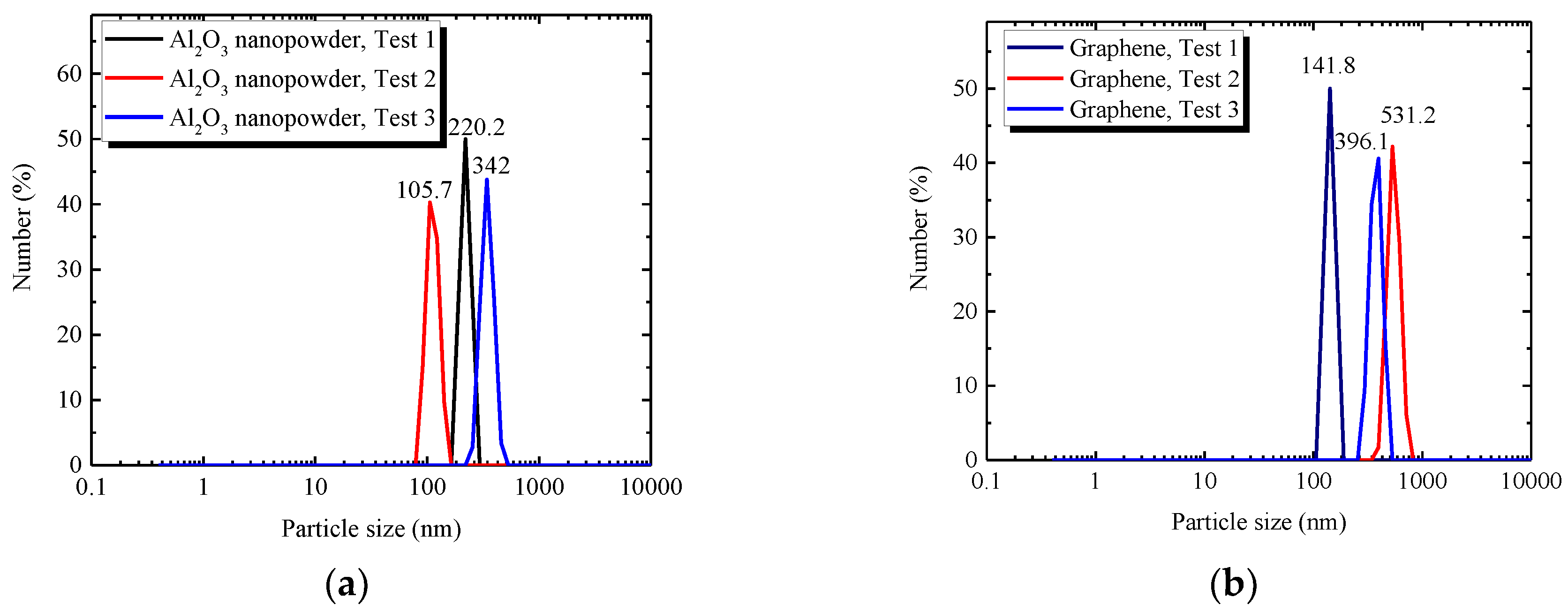
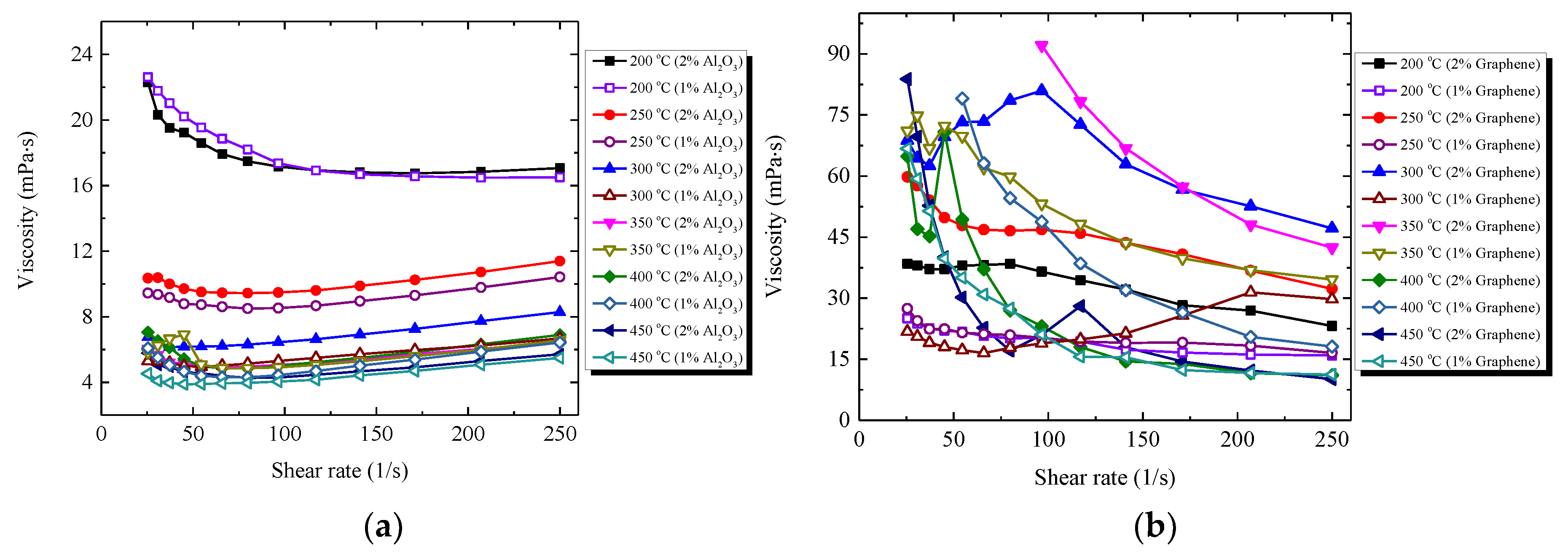


| Material Type | Nanoparticle (Al2O3 Nanopowder, Graphene) | Temperature (°C) | ||
|---|---|---|---|---|
| HITEC salt | 0% | 1 wt.% | 2 wt.% | 200, 250, 300, 350, 400, 450 |
| solar salt | 0% | 1 wt.% | 2 wt.% | 250, 300, 350, 400, 450, 500 |
| Material Type | Fitting Curves | Measure Methods | Temperature Range | Reference |
|---|---|---|---|---|
| HITEC salt | Rotating method | 150~500 °C | [40] | |
| Rotational coaxial cylinder method | 147~422 °C | [25] | ||
| Ostwald rheometer | 182~507 °C | [41] | ||
| Oscillating rheometer | 184~482 °C | [42] | ||
| Oscillation cup method | 250~450 °C | [43] | ||
| Oscillation cup method | 247~500 °C | [44] | ||
| solar salt | Rotational coaxial cylinder method | 250~550 °C | [27] | |
| Rotational coaxial cylinder method (commercial) | 243~447 °C | [25] | ||
| Rotational coaxial cylinder method | 233~440 °C | [25] | ||
| Rheometric ARES rheometer | 222~547 °C | [45] | ||
| Oscillation cup method | 275~600 °C | [46,47] | ||
| Oscillating sphere method | 250~450 °C | [48] |
| Material Type | Viscosities (mPa·s) | ||||||
|---|---|---|---|---|---|---|---|
| 200 °C | 250 °C | 300 °C | 350 °C | 400 °C | 450 °C | 500 °C | |
| HITEC salt | 19.41 | 14.08 | 7.80 | 5.90 | 5.30 | 4.97 | - |
| HITEC salt/1 wt.% Al2O3 nanopowder | 18.67 | 9.09 | 5.48 | 5.68 | 5.08 | 4.32 | - |
| HITEC salt/2 wt.% Al2O3 nanopowder | 18.22 | 10.01 | 6.73 | 5.44 | 5.74 | 4.84 | - |
| HITEC salt/1 wt.% Graphene | 20.12 | 21.00 | 21.39 | 56.37 | 42.35 | 30.62 | - |
| HITEC salt/2 wt.% Graphene | 34.36 | 46.82 | 66.41 | 64.18 | 33.32 | 32.30 | - |
| solar salt | - | 11.46 | 7.69 | 6.13 | 5.33 | 4.60 | 3.42 |
| solar salt/1 wt.% Al2O3 nanopowder | - | 14.43 | 9.05 | 6.36 | 5.57 | 5.13 | 4.50 |
| solar salt/2 wt.% Al2O3 nanopowder | - | 19.26 | 9.87 | 6.22 | 5.46 | 4.47 | 3.11 |
| solar salt/1 wt.% Graphene | - | 21.38 | 15.88 | 6.26 | 4.06 | 4.24 | 3.96 |
| solar salt/2 wt.% Graphene | - | 17.25 | 26.76 | 16.98 | 7.41 | 4.75 | 3.60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Zhang, G.; Ding, Y.; Wen, D. Rheological Characteristics of Molten Salt Seeded with Al2O3 Nanopowder and Graphene for Concentrated Solar Power. Energies 2019, 12, 467. https://doi.org/10.3390/en12030467
Xiao X, Zhang G, Ding Y, Wen D. Rheological Characteristics of Molten Salt Seeded with Al2O3 Nanopowder and Graphene for Concentrated Solar Power. Energies. 2019; 12(3):467. https://doi.org/10.3390/en12030467
Chicago/Turabian StyleXiao, Xin, Gan Zhang, Yulong Ding, and Dongsheng Wen. 2019. "Rheological Characteristics of Molten Salt Seeded with Al2O3 Nanopowder and Graphene for Concentrated Solar Power" Energies 12, no. 3: 467. https://doi.org/10.3390/en12030467





