Frosting Performance of a Nanoporous Hydrophilic Aluminum Surface
Abstract
:1. Introduction
2. Physics Behind Ice Formation and Materials Preparation
2.1. Physics behind Ice Formation
2.2. Materials Production
2.3. Surfaces Characteristics
3. Testing Rig
3.1. Construction of the Testing Rig
3.2. System Description
4. Test Results and Analyses
4.1. The Structure of the Frost Layer
4.1.1. The Structure of the Frost Layer on the Aluminum Surfaces
- When frosting at 1 min, there were some spherical droplets, with different sizes, formed on the surface of Sample 1. The average radius of the droplets was the biggest on Sample 1. Many irregular droplets formed on the surface of Sample 2. There were many small spherical droplets on the surface of Sample 3. However, there were no obvious droplets on the surface of Sample 4; but we could see some sparse white spots on Sample 4, which were actually tiny droplets. Droplet density increased from Sample 1 to Sample 4, respectively. It revealed that when the surface contact angle decreased, the droplet density on the aluminum surfaces increased.
- With time passing by, at 5 min of the test, droplets on Sample 1 gradually merged to form larger spherical droplets. Meanwhile, new tiny droplets grew continuously between frozen droplets; however, we could still see some areas that were not covered by frost crystals on the surface of Sample 1. By contrast, frost crystals filled the surface of Sample 2 and 3. We could see many granular ice crystals on Sample 4, and there was still some surface area that was not covered by the frost crystals.
- After 10 min of frosting, Sample 1, 2, and 3 were all covered by a sheet of frost layer, but some small ice crystals could be seen, showing that the frost layers were inhomogeneous. Granular ice crystals were larger on Sample 4, and there was still some surface area that was not covered by the frost crystals.
4.1.2. The Structure of Frost Layer on the Side of the Aluminum Sheets
- At 1 min of the test, droplets formed on all aluminum surfaces. The phenomenon is the same as the analysis in Table 4.
- With time passing by, at 5 min of the test, dendritic frost, with different heights, grew on the surface of Sample 1 and they spread to the surrounding surface, connected in pieces. On the surface of Sample 2, needlelike frost, with different heights, was formed, whose density was smaller than the frost on Sample 1. Conversely, there was a dense and smooth frost layer filled up the surface on Sample 3. Different from the other, many newly frozen droplets laid on the surface of Sample 4, and the shape of these droplets were close to a sphere.
- At 10 min of frosting, bigger and higher dendritic frost, with different heights, grew on the surface of Sample 1. The frost on Sample 2 was slanted because of the gravitation of its slim ice. Lamellar frost was piled on Sample 3. And a smooth frost layer could be seen on Sample 4, but the frost layer was still thin and transparent, such that the material of the surface could still be seen clearly.
- The frost thickness decreased with the reduction of the surface contact angle. In this experiment, the frost thickness of Sample 4 was the smallest. This was attributed to the fact that a surface with a lower contact angle surface process better hydrophilic characteristic. In the early stages of the frosting experiment, for the hydrophilic aluminum sheet with the larger contact angle, the water vapor condensed from the air to the surface of the aluminum sheet, forming lots of hemispherical droplets. But on the surface of the hydrophilic aluminum sheet with a smaller contact angle, water vapor condensed to form a thin water film without significant droplets. Therefore, Sample 4 had a frost layer that was thinner and its surface of frost layer was smoother than the other aluminum sheets.
4.2. Frost Thickness
4.2.1. Frost Thickness
- It can be seen that the frost thickness on the four types of surfaces increased continually. With time passing by, frost thickness increased rapidly at first and then increased gently.
- The maximum frost thicknesses on Sample 1, Sample 2, Sample 3, and Sample 4 in 60 min were 1.69 mm, 1.31 mm, 1.01 mm, and 0.47 mm, respectively. Obviously, compared with the polished aluminum sheet, the nanoporous structure had a great effect on reducing the frost thickness on the aluminum surface. And when the surface contact angle decreased, the frost thickness on the surface also decreased.
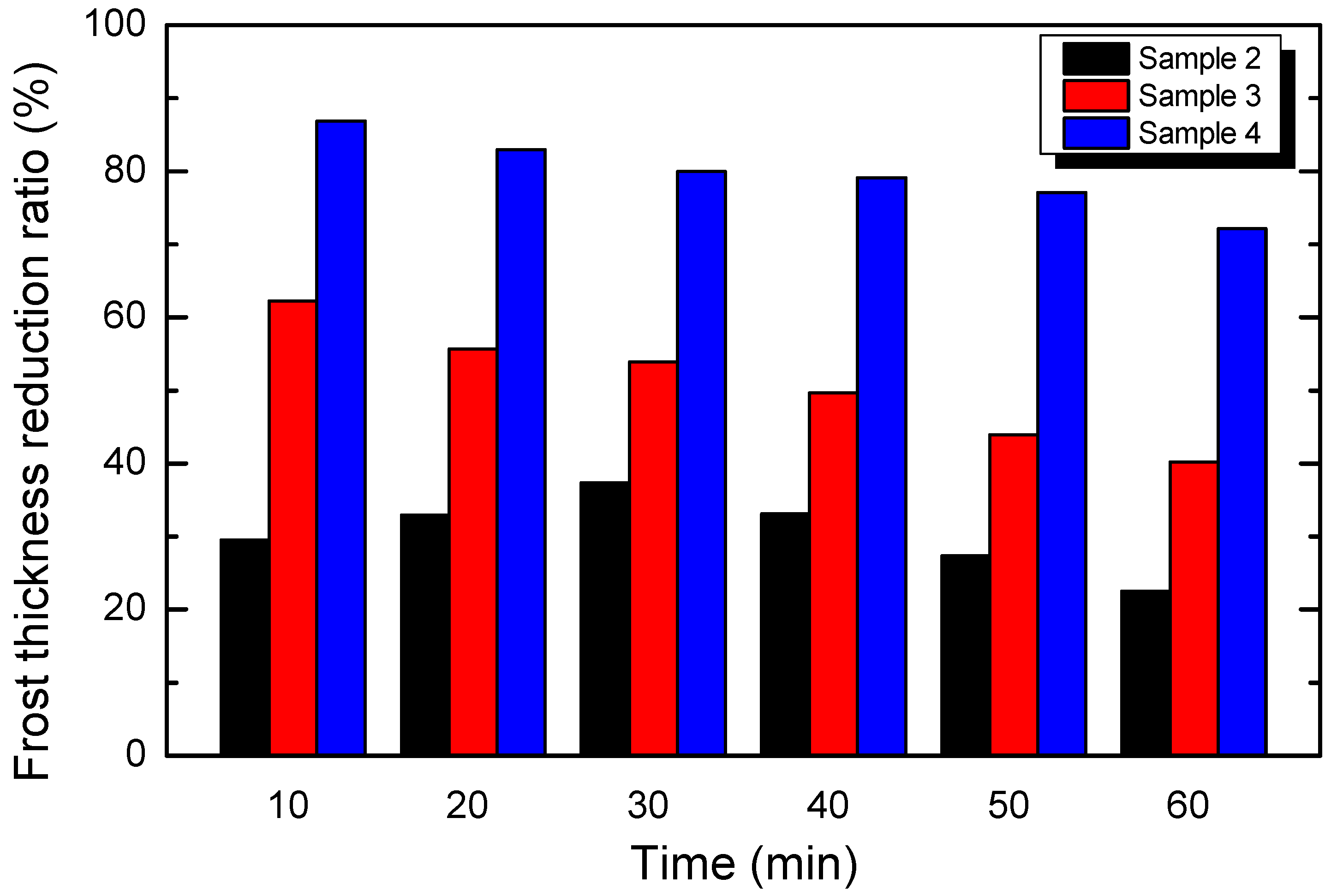
4.2.2. Frost Thickness Reduction Ratio
- The frost thickness reduction ratios of Sample 3 and Sample 4 decreased with time. The frost thickness reduction ratio of Sample 2 increased firstly and then decreased gradually. It was suggested that, compared with the polished aluminum fin (Sample 1), Sample 3 and Sample 4 showed to best restrict frost thickness growth at 10 min, whereas Sample 2 showed to be best at 30 min.
- The maximum frost thickness reduction ratios on the three types of nanopores sheets (Samples 2–4) were 37.4%, 62.3%, and 86.9%, respectively, which increased with the decrease in surface contact angle. This experiment showed that nanoporous hydrophilic aluminum sheets can effectively reduce frost thickness. As is universally known, high frost thickness between fins will block the duct of exchanger and, accordingly, increase the air pressure. To ensure that the equipment operates under stable air pressure, using the nanoporous hydrophilic aluminum, with a low frost thickness, as the material for the fin could be a good alternative.
4.3. Frost Mass
4.3.1. Frost Mass
- It was shown that under all the test conditions, the frost mass of the different samples increased linearly. The frost mass increased as the surface temperature decreased.
- The frost mass of Sample 1 was far higher than Sample 2, Sample 3, and Sample 4 from the first 10 min and under all the test conditions, which showed that, relative to the polished aluminum, nanoporous hydrophilic aluminum can decrease the formation of frost mass during the frosting process. The highest frost mass was 0.0806 g, which was formed by Sample 1 at a surface temperature of −20 °C, while the lowest frost mass was 0.0179 g, which was formed by Sample 2 at a surface temperature of −5 °C.
- The frost mass of the different nanoporous hydrophilic aluminum sheets was similar at 10 min of frosting. But the difference of the frost mass on the three nanoporous hydrophilic aluminum sheets was obviously increased at the later frosting stage. This was because, in the early frosting stage, the frost mass was mainly affected by the surface characteristics of the material. Samples 2, 3, and 4 showed similar frost resistant performance in the early frosting stage. In the later stage of the experiment, frost mainly continued to grow on the initial frost branch. New frost crystals formed more easily on the sharp frost branch than on the flat frost layer. Therefore, the difference of the frost mass on the nanoporous hydrophilic surfaces, with different frost shapes, increased in the later stage of frost formation.
4.3.2. Frost Mass Reduction Ratio
- The frost mass reduction rate of the nanoporous hydrophilic aluminum sheets increased firstly and then decreased with the increase of the surface temperature. All of the samples reached the maximum frost mass reduction ratio when the surface temperature was −15 °C. As a result, for the nanoporous hydrophilic aluminum sheets, −15 °C was the optimum surface temperature for delaying frost at Ta = 5 °C, φ = 60%.
- Among the three kinds of nanoporous hydrophilic aluminum sheets (Samples 2–4), the frost reduction rate of Sample 2 was the highest, reaching a maximum of 65.9% at the surface temperature of −15 °C. And the frost reduction rate of Sample 3 was the lowest, with a minimum value of 13.7% when the surface temperature was −5 °C.
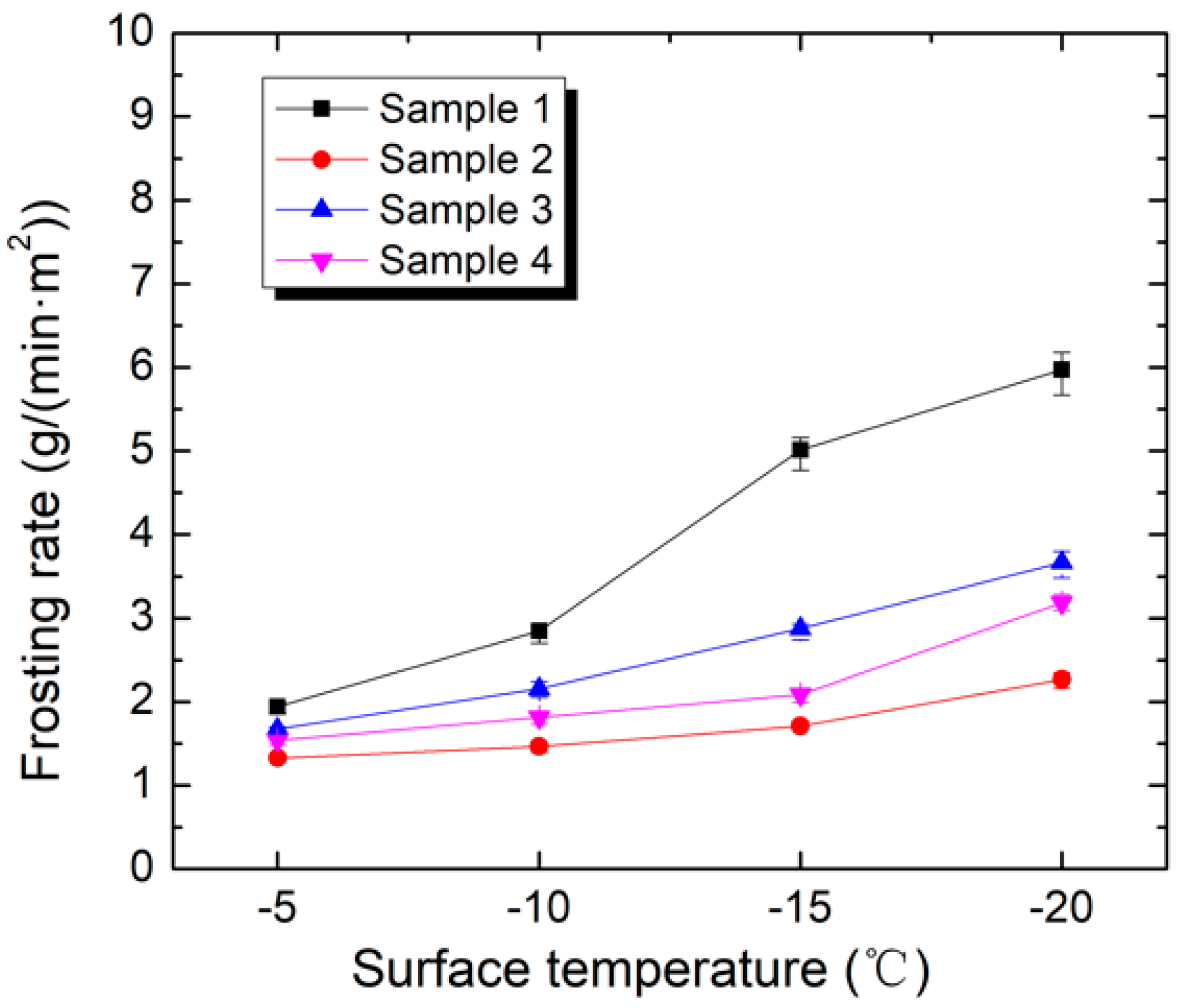
4.4. Frosting Rate
4.5. The Effect of Surface Properties on Frost Formation
5. Conclusions
- By observing the appearance of frost on the aluminum surface, it was found that, compared with the ordinary polished aluminum sheet, the droplets that were condensed on the surface of the nanoporous aluminum sheets had smaller diameters.
- The topography of the frost layers on the sides of the aluminum sheets were different. The frost layer thickness decreased with the decrease of the aluminum surface contact angle. At the end of frosting, Sample 4 had the smallest frost layer thickness. To ensure that equipment operates under a stable air pressure, using nanoporous hydrophilic aluminum, with a low frost thickness, as the material for the fin could be a good alternative.
- Under all the test conditions, the frost mass measurements of the nanoporous hydrophilic aluminum sheets were lower than the polished aluminum sheet, from the early stage of frost formation. For all the nanoporous hydrophilic aluminum sheets, −15 °C was the optimum surface temperature for delaying frost formation; at Ta = 5 °C, φ = 60%. Furthermore, the frost mass reduction rate of Sample 2 was the highest, reaching a maximum of 65.9% at a surface temperature of −15 °C. The study showed that the nanoporous hydrophilic aluminum sheets could reduce the frost mass to delay frost formation, decrease the defrosting frequency, and, consequently, could conserve energy.
- When the surface temperature was −15 °C, the frosting rate of Sample 1 reached 5.01 g/(m2·min) and the frosting rate of Sample 2 was 1.71 g/(m2·min). The frosting rate of Sample 2 was about one-third of that of Sample 1.
- Based on the classical nucleation theory, frosting is related to the surface contact angle and roughness. In general, the frost thickness and frost mass of the nanoporous hydrophilic aluminum was smaller than that of the polished aluminum. For the nanoporous hydrophilic aluminum sheets, Sample 2 and Sample 4 were better for lessening the formation of frost. The experiment revealed that manufacturing nanopores and promoting hydrophilicity can delay the formation of frost.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| T | temperature (°C) |
| φ | air relative humidity (%) |
| θ | contact angle (°) |
| d | pore diameter (nm) |
| I | current (A) |
| L | length (mm) |
| W | width (mm) |
| H | height (mm) |
| M | mass (g) |
| Z | frost thickness (mm) |
| α | frost thickness reduction ratio (%) |
| t | time (min) |
| ΔG | change of Gibbs free energy (J) |
| ΔGx | change of Gibbs free energy per unit volume (J/m3) |
| ΔG* | activation energy to reach the critical nucleus size (J) |
| ΔGf,v | volumetric Gibbs free energy per unit volume between water and ice (J/m3) |
| r | nucleation radius (m) |
| γ | interfacial energy between two phases (J/m2) |
| f | geometrical factor relating to the energy barrier for homogeneous nucleation to heterogeneous nucleation |
| R | roughness curvature radius of the substrate |
| Subscripts | |
| s | surface |
| a | air |
| u | unfrosted |
| f | frost |
| ave | average value |
| max | maximum value |
| min | minimum value |
| 1 | sample 1 |
| n | number of samples, n = 2, 3, 4 |
| c | critical ice nucleus size |
| A | phase A |
| B | phase B |
| I | ice |
| W | water |
| het | heterogeneous |
| hom | homogeneous |
References
- Yang, L.; Yan, H.; Lam, J.C. Thermal comfort and building energy consumption implications—A review. Appl. Energy 2014, 115, 164–173. [Google Scholar] [CrossRef]
- Pérez-Lombard, L.; Ortiz, J.; Pout, C. A review on buildings energy consumption information. Energy Build. 2008, 40, 394–398. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Dong, J.; Yao, Y. Advances in vapor compression air source heat pump system in cold regions: A review. Renew. Sustain. Energy Rev. 2018, 81, 353–365. [Google Scholar] [CrossRef]
- Kim, K.; Lee, K.S. Frosting and defrosting characteristics of a fin according to surface contact angle. Int. J. Heat Mass Transf. 2011, 54, 2758–2764. [Google Scholar] [CrossRef]
- Lv, J.; Song, Y.; Jiang, L.; Wang, J.V. Bio-inspired strategies for anti-icing. ACS Nano 2014, 8, 3152–3169. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liang, C.; Yang, M.; Fan, C.; Zhang, X. Effects of surface characteristic on frosting and defrosting behaviors of fin-tube heat exchangers. Appl. Therm. Eng. 2015, 75, 1126–1132. [Google Scholar] [CrossRef]
- Sheng, W.; Liu, P.; Dang, C.; Liu, G. Review of restraint frost method on cold surface. Renew. Sustain. Energy Rev. 2017, 79, 806–813. [Google Scholar] [CrossRef]
- Orejon, D.; Shardt, O.; Gunda, N.S.; Ikuta, T.; Takahashi, K.; Takata, Y.; Mitra, S.K. Simultaneous dropwise and filmwise condensation on hydrophilic microstructured surfaces. Int. J. Heat Mass Transf. 2017, 114, 187–197. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Zhang, X.; Meng, S.; Ma, C. An experimental study on minimizing frost deposition on a cold surface under natural convection conditions by use of a novel anti-frosting paint. Part I. Anti-frosting performance and comparison with the uncoated metallic surface. Int. J. Refrig. 2006, 29, 229–236. [Google Scholar] [CrossRef]
- Lee, H.; Shin, J.; Ha, S.; Choi, B.; Lee, J. Frost formation on a plate with different surface hydrophilicity. Int. J. Heat Mass Transf. 2004, 47, 4881–4893. [Google Scholar] [CrossRef]
- Okoroafor, E.U.; Newborough, M. Minimising frost growth on cold surfaces exposed to humid air by means of crosslinked hydrophilic polymeric coatings. Appl. Therm. Eng. 2000, 20, 737–758. [Google Scholar] [CrossRef]
- Shin, J.; Tikhonov, A.V.; Kim, C. Experimental study on frost structure on surfaces with different hydrophilicity: Density and thermal conductivity. J. Heat Transf. 2003, 125, 84–94. [Google Scholar] [CrossRef]
- Liu, Z.L.; Zhang, X.H.; Wang, H.Y.; Meng, S.; Cheng, S.Y. Influences of surface hydrophilicity on frost formation on a vertical cold plate under natural convection conditions. Exp. Therm. Fluid Sci. 2007, 31, 789–794. [Google Scholar] [CrossRef]
- Jhee, S.; Lee, K.S.; Kim, W.S. Effect of surface treatments on the frosting/defrosting behavior of a fin-tube heat exchanger. Int. J. Refrig. 2002, 25, 1047–1053. [Google Scholar] [CrossRef]
- Gohari, B.; Russell, K.; Hejazi, V.; Rohatgi, P. Role of water solidification concepts in designing nano-textured anti-icing surfaces. J. Phys. Chem. B 2017, 121, 7527–7535. [Google Scholar] [CrossRef]
- Schutzius, T.M.; Jung, S.; Maitra, T.; Eberle, P.; Antonini, C.; Stamatopoulos, C.; Poulikakos, D. Physics of icing and rational design of surfaces with extraordinary icephobicity. Langmuir 2015, 31, 4807–4821. [Google Scholar] [CrossRef]
- Sommers, A.D.; Jacobi, A.M. Creating micro-scale surface topology to achieve anisotropic wettability on an aluminum surface. J. Micromech. Microeng. 2006, 16, 1571–1578. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Wenzel, R. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Wang, W.C.; Zhao, W.; Wang, K.G.; Wang, L.; Wang, X.W.; Wang, S.; Zhang, C.; Bai, J.T. On hydrophilicity improvement of the porous anodic alumina film by hybrid nano/micro structuring. Appl. Surf. Sci. 2017, 416, 710–715. [Google Scholar] [CrossRef]
- Piucco, R.O.; Hermes, C.J.; Melo, C.; Barbosa, J.R., Jr. A study of frost nucleation on flat surfaces. Exp. Therm. Fluid Sci. 2008, 32, 1710–1715. [Google Scholar] [CrossRef]
- Chen, A.; Huang, H. Rapid fabrication of t-shaped micropillars on polypropylene surfaces with robust cassie-baxter state for quantitative droplet Collection. J. Phys. Chem. C 2016, 120, 1556–1561. [Google Scholar] [CrossRef]
- Hobbs, P.V. Ice Physics; Clarendon Press: Oxford, UK, 1974. [Google Scholar]
- Hosford, W.F. Materials Science: An Intermediate Text; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Pruppacher, H.R.; Klett, J.D.; Wang, P.K. Microphysics of clouds and precipitation. Aerosol Sci. Technol. 1998, 28, 381–382. [Google Scholar] [CrossRef]

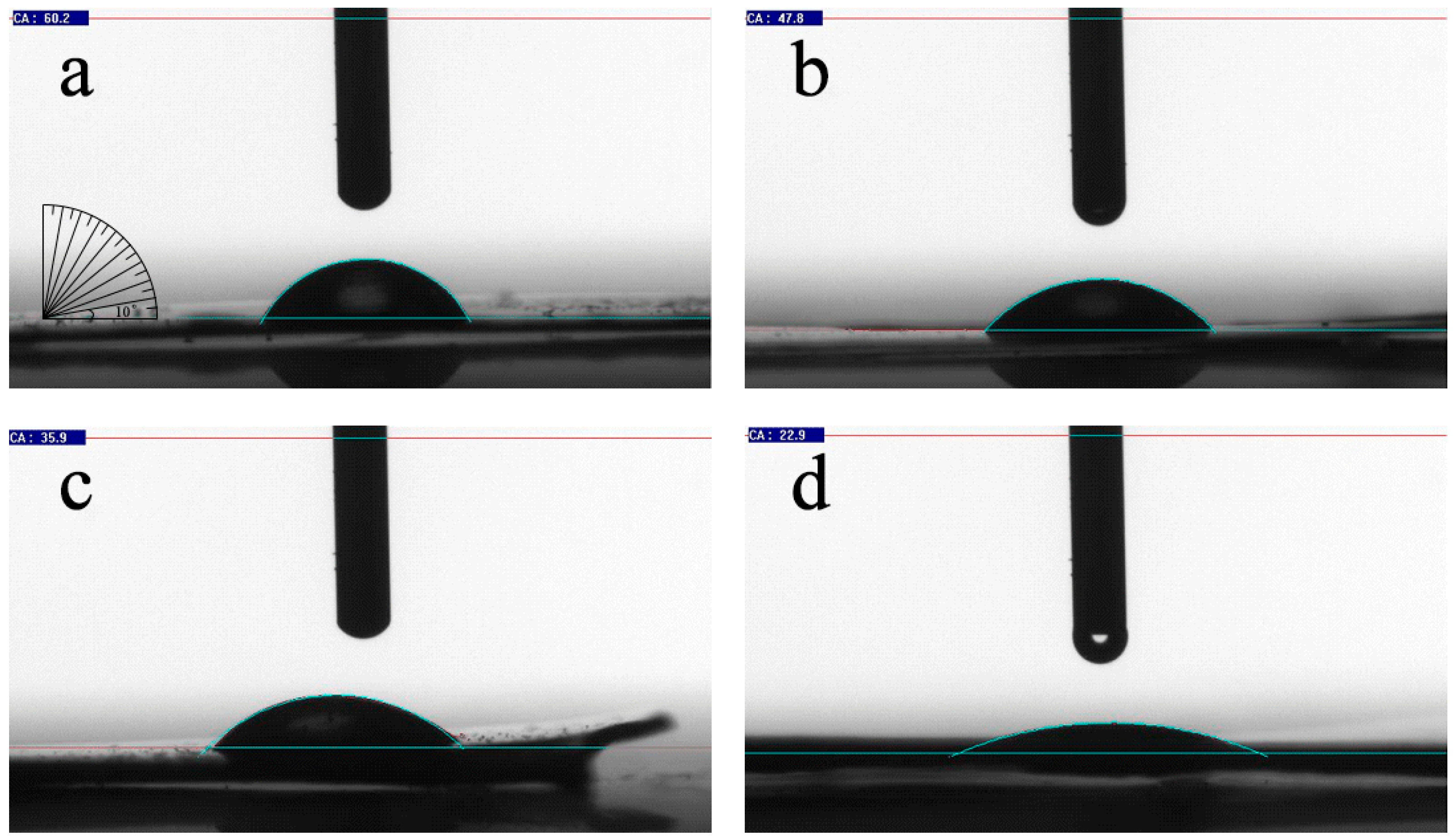




| Test Sample | Current (I/A) | Contact Angle (θ/°) | Pore Diameter (d/nm) | Size (mm × mm) |
|---|---|---|---|---|
| 1 | - | 60.2 | 0 | 15 × 15 |
| 2 | 1 | 47.8 | 150 | 15 × 15 |
| 3 | 2 | 35.9 | 350 | 15 × 15 |
| 4 | 4 | 22.9 | 700 | 15 × 15 |
| Name | Type | Parameter |
|---|---|---|
| Multichannel temperature monitor | AT4340 | Sensor: K-type thermocouple; measuring range: −200–1300 °C; accuracy: ±1 °C; |
| Double-channel temperature and humidity monitor | LS-HT211X | Temperature measurement range: −40–100 °C; temperature accuracy: ±0.5 °C; relative humidity measurement range: 0%–100%; relative humidity accuracy: ±3% |
| Pump | WH-D12220 | Power: 3.6 W; maximum head: 2 m; water flow: 2 L/min; noise: 30 dB |
| Semiconductor refrigerator | X206 | Cooling capacity: 480 W/h; applicable volume: 1–2 m2; temperature difference: 7–12 °C; |
| Low temperature humidity chamber | BYCT-TH150B | Temperature range: −40–150 °C; temperature accuracy: ±0.5 °C; relative humidity range: 20%–90%; relative humidity accuracy: ±2% |
| Digital microscope | RH-SM-10A | Maximum eyepiece resolution: 2592 × 1944; objective lens magnification: 0.7–4.5X; |
| Electronic balance | BSM−120.4 | Range: 0–120 g; accuracy: 0.1 mg. |
| Test Condition | Surface Temperature Ts (°C) | Air Temperature Ta (°C) | Air Relative Humidity φ (%) |
|---|---|---|---|
| A | −5 | 5 | 60 |
| B | −10 | 5 | 60 |
| C | −15 | 5 | 60 |
| D | −20 | 5 | 60 |
| Time (min) | Sample 1 (θ = 60.2°) | Sample 2 (θ = 47.8°) | Sample 3 (θ = 35.9°) | Sample 4 (θ = 22.9°) |
|---|---|---|---|---|
| 0 |  |  |  |  |
| 1 |  |  |  |  |
| 5 |  |  |  |  |
| 10 |  |  |  |  |
| Time (min) | Sample 1 (θ = 60.2°) | Sample 2 (θ = 47.8°) | Sample 3 (θ = 35.9°) | Sample 4 (θ = 22.9°) |
|---|---|---|---|---|
| 0 |  |  |  |  |
| 1 |  |  |  |  |
| 5 |  |  |  |  |
| 10 |  |  |  |  |
| Test Sample | Time (min) | ||||||
|---|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 30 | 40 | 50 | 60 | |
| 1 (θ = 60.2°) | 0.42 | 0.61 | 0.88 | 1.15 | 1.39 | 1.57 | 1.69 |
| 2 (θ = 47.8°) | 0.24 | 0.43 | 0.59 | 0.72 | 0.93 | 1.14 | 1.31 |
| 3 (θ = 35.9°) | 0.12 | 0.23 | 0.39 | 0.53 | 0.70 | 0.88 | 1.01 |
| 4 (θ = 22.9°) | 0.03 | 0.08 | 0.15 | 0.23 | 0.29 | 0.36 | 0.47 |
| Test Sample | θ (°) | d (nm) | Ts (°C) | |||
|---|---|---|---|---|---|---|
| −5 | −10 | −15 | −20 | |||
| 1 | 60.2 | 0 | 0.0262 | 0.0384 | 0.0677 | 0.0806 |
| 2 | 47.8 | 150 | 0.0179 | 0.0198 | 0.0231 | 0.0306 |
| 3 | 35.9 | 350 | 0.0226 | 0.0291 | 0.0388 | 0.0495 |
| 4 | 22.9 | 700 | 0.0208 | 0.0245 | 0.0282 | 0.0430 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Zeng, B.; Zhang, Y.; He, S.; Zhao, X. Frosting Performance of a Nanoporous Hydrophilic Aluminum Surface. Energies 2018, 11, 3483. https://doi.org/10.3390/en11123483
Yang W, Zeng B, Zhang Y, He S, Zhao X. Frosting Performance of a Nanoporous Hydrophilic Aluminum Surface. Energies. 2018; 11(12):3483. https://doi.org/10.3390/en11123483
Chicago/Turabian StyleYang, Wansheng, Bin Zeng, Yanmei Zhang, Song He, and Xudong Zhao. 2018. "Frosting Performance of a Nanoporous Hydrophilic Aluminum Surface" Energies 11, no. 12: 3483. https://doi.org/10.3390/en11123483





