Evaluating the Impacts of ACP Management on the Energy Performance of Hydrothermal Liquefaction via Nutrient Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. HTL Conversion and Characterization of ACP
2.2. Theoretical Recovery of Nutrients from ACP
2.3. Experimental Recovery of Nutrients from ACP
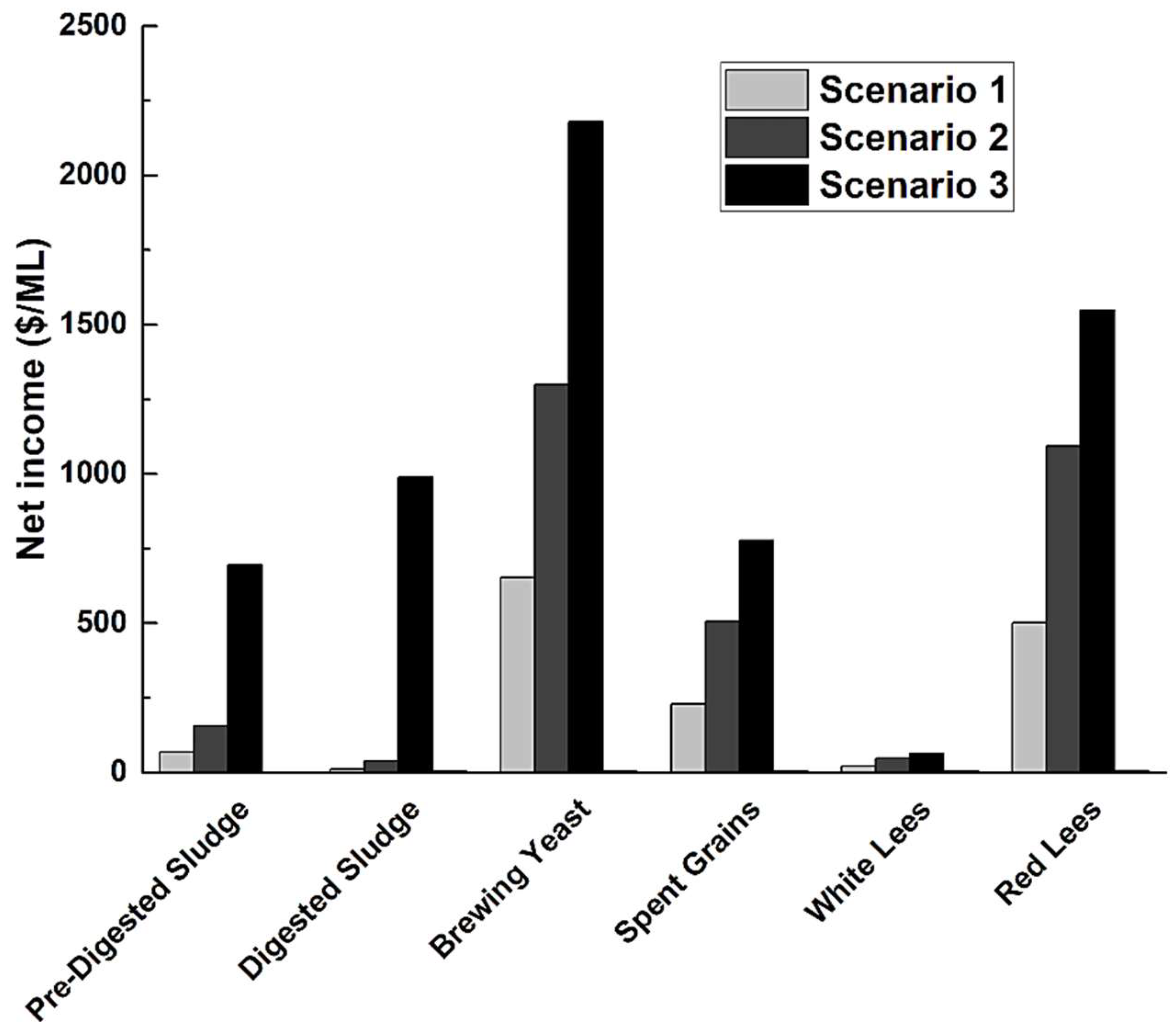
2.4. Energy Analysis of ACP Management via Struvite Precipitation
- Scenario 1: Energy output corresponds to only the amount of commercial fertilizer (i.e., monoammonium phosphate (MAP)) supplanted by struvite precipitation.
- Scenario 2: Energy output corresponds to MAP avoidance energy plus avoided energy costs for WWTP removal of TN and TP in ACP, assuming ammonia (NH3) is not allowed to volatilize during struvite recovery (i.e., “closed system”).
- Scenario 3: Energy output corresponds to MAP avoidance energy plus avoided energy costs for WWTP removal of TN and TP, assuming NH3 is allowed to volatilize during struvite recovery (i.e., “open system”).
2.5. Economic Analysis of ACP Management via Struvite Precipitation
3. Results and Discussion
3.1. Characterization of Post-HTL ACP
3.2. Theoretical Recovery of Nitrogen and Phosphorus from ACP
3.3. Experimental Recovery of Nitrogen and Phosphorus from ACP
3.4. Energy Recovery Impacts of HTL Processing
3.5. Economic Benefit of Struvite Precipitation from ACP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhutto, A.W.; Qureshi, K.; Abro, R.; Harijan, K.; Zhao, Z.; Bazmi, A.A.; Abbas, T.; Yu, G. Progress in the production of biomass-to-liquid biofuels to decarbonize the transport sector-prospects and challenges. R. Soc. Chem. Adv. 2016, 6, 32140–32170. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2014, 178, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Bauer, S.; Reynolds, C.; Peng, S.; Colosi, L. Evaluating the water quality impacts of hydrothermal liquefaction: Assessment of carbon, nitrogen, and energy recovery impacts. Bioresour. Technol. Rep. 2018, 2, 115–120. [Google Scholar] [CrossRef]
- Jena, U.; Vaidyanathan, N.; Chinnasamy, S.; Das, K.C. Evaluation of microalgae cultivation using recovered aqueous co-product from thermochemical liquefaction of algal biomass. Bioresour. Technol. 2011, 102, 3380–3387. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Faus, R.; Powers, S.E.; Burken, J.G.; Alvarez, P.J. The water footprint of biofuels: A drink or drive issue? Environ. Sci. Technol. 2009, 43, 3005–3010. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.; Schideman, L.; Scott, J.; Rajagopalan, N.; Plewa, M.J. Chemical and biological characterization of wastewater generated from hydrothermal liquefaction of Spirulina. Environ. Sci. Technol. 2013, 47, 2131–2138. [Google Scholar] [CrossRef]
- Garcia Alba, L.; Torri, C.; Samorì, C.; Van der Spek, J.; Fabbri, D.; Kersten, S.R.A.; Brilman, D.W.F. Hydrothermal treatment (HTT) of microalgae: Evaluation of the process as conversion method in an algae biorefinery concept. Energy Fuels 2012, 26, 642–657. [Google Scholar] [CrossRef]
- Valdez, P.J.; Nelson, M.C.; Wang, H.Y.; Lin, X.N.; Savage, P.E. Hydrothermal liquefaction of Nannochloropsis sp.: Systematic study of process variables and analysis of the product. Biomass Bioenergy 2012, 46, 317–331. [Google Scholar] [CrossRef]
- Orfield, N.D.; Fang, A.J.; Valdez, P.J.; Nelson, M.C.; Savage, P.E.; Lin, X.N.; Keoleian, G.A. Life cycle design of an algal biorefinery featuring hydrothermal liquefaction: Effect of reaction conditions and an alternative pathway including microbial regrowth. ACS Sustain. Chem. Eng. 2014, 2, 867–874. [Google Scholar] [CrossRef]
- Gai, C.; Zhang, Y.; Chen, W.T.; Zhou, Y.; Schideman, L.; Zhang, P.; Tommaso, G.; Kuo, C.T.; Dong, Y. Characterization of aqueous phase from the hydrothermal liquefaction of Chlorella pyrenoidosa. Bioresour. Technol. 2015, 184, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Tommaso, G.; Chen, W.; Li, P.; Schideman, L.; Zhang, Y. Chemical characterization and anaerobic biodegradability of hydrothermal liquefaction aqueous products from mixed-culture wastewater algae. Bioresour. Technol. 2015, 178, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus recovery as struvite from farm, municipal and industrial waste: Feedstock suitability, methods and pre-treatment. Waste Manag. 2016, 49, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Yetilmezsoy, K.; Fatih, I.; Emel, K.; Havva Melda, A. Feasibility of struvite recovery process for fertilizer industry: A study of financial and economic analysis. J. Clean. Prod. 2017, 152, 88–102. [Google Scholar] [CrossRef]
- Ishii, S.K.L.; Boyer, T.H. Life cycle comparison of centralized wastewater treatment and urine source separation with struvite precipitation: Focus on urine nutrient management. Water Res. 2015, 79, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Capdevielle, A.; Sýkorová, E.; Biscans, B.; Béline, F.; Daumer, M. Optimization of struvite precipitation in synthetic biologically treated swine wastewater—Determination of the optimal process parameters. J. Hazard. Mater. 2013, 244–245, 357–369. [Google Scholar] [CrossRef]
- Lahr, R.; Goetsch, H.; Haig, S.; Noe-Hays, A.; Love, N.; Aga, D.; Bott, C.; Foxman, B.; Jimenez, J.; Luo, T.; et al. Urine bacterial community convergence through fertilizer production: Storage, pasteurization, and struvite precipitation. Environ. Sci. Technol. 2016, 50, 11619–11626. [Google Scholar] [CrossRef]
- Rahman, M.M.; Liu, Y.; Kwag, J.H.; Ra, C. Recovery of struvite from animal wastewater and its nutrient leaching loss in soil. J. Hazard. Mater. 2011, 186, 2026–2030. [Google Scholar] [CrossRef]
- Yetilmezsoy, K.; Sapci-Zengin, Z. Recovery of ammonium nitrogen from the effluent of UASB treating poultry manure wastewater by MAP precipitation as a slow release fertilizer. J. Hazard. Mater. 2009, 166, 260–269. [Google Scholar] [CrossRef]
- Çelen, I.; Buchanan, J.R.; Burns, R.T.; Robinson, R.B.; Raman, D.R. Using chemical equilibrium model to predict amendments required to precipitate phosphorus as struvite in liquid swine manure. Water Res. 2007, 41, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Waste Water, 22nd ed.; American Public Health Association: Washington, DC, USA, 2013; Available online: http://www.standardmethods.org/PDF/22nd_Ed_Errata_12_16_13.pdf (accessed on 10 February 2016).
- Xu, D.; Savage, P.E. Characterization of biocrudes recovered with and without solvent after hydrothermal liquefaction of algae. Algal Res. Part A 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Method 300.1: Determination of Inorganic Anions in Drinking Water by Ion Chromatography; USEPA, Office of Water: Washington, DC, USA, 1997. [Google Scholar]
- Gustafsson, J.P. Visual MINTEQ Ver. 3.1. KTH, Sweden. 2013. Available online: https://vminteq.lwr.kth.se/ (accessed on 20 March 2017).
- Uysal, A.; Yilzmazel, Y.D.; Demirer, G.N. The determination of fertilizer quality of the formed struvite from effluent of a sewage sludge anaerobic digester. J. Hazard. Mater. 2012, 181, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Tchobanoglous, G.; Burton, F.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill Higher Education: New York, NY, USA, 2003. [Google Scholar]
- Connelly, E.B.; Colosi, L.M.; Clarens, A.F.; Lambert, J.H. Life cycle assessment of biofuels from algae hydrothermal liquefaction: The upstream and downstream factors affecting regulatory compliance. Energy Fuels 2015, 29, 1653–1661. [Google Scholar] [CrossRef]
- Vardon, D.; Sharma, B.; Blazina, G.; Rajagopalan, K.; Strathmann, T. Thermochemical conversion of raw and defatted algal biomass via hydrothermal liquefaction and slow pyrolysis. Bioresour. Technol. 2012, 109, 178–187. [Google Scholar] [CrossRef]
- Sawayama, S.; Minowa, T.; Yokoyama, S.-Y. Possibility of energy production and CO2 mitigation by thermochemical liquefaction of microalgae. Biomass Bioenergy 1999, 17, 33–39. [Google Scholar] [CrossRef]
- Jaffer, Y.; Clark, T.A.; Pearce, P.; Parsons, S.A. Potential phosphorus recovery by struvite formation. Water Res. 2002, 36, 1834–1842. [Google Scholar] [CrossRef]
- Tao, W.; Fattah, K.P.; Huchzermeier, M.P. Struvite recovery from anaerobically digested dairy manure: A review of application potential and hindrances. J. Environ. Manag. 2016, 169, 46–57. [Google Scholar] [CrossRef]
- Ali, M.I.; Schneider, P.A.; Hudson, N. Thermodynamics and solution chemistry of struvite. J. Indian Inst. Sci. 2005, 85, 141–149. [Google Scholar]
- Jia, G.; Zhang, H.; Krampe, J.; Muster, T.; Gao, B.; Zhu, N.; Jin, B. Applying a chemical equilibrium model for optimizing struvite precipitation for ammonium recovery from anaerobic digester effluent. J. Clean. Prod. 2017, 147, 297–305. [Google Scholar] [CrossRef]
- Molinos-Senante, M.; Hernández-Sancho, F.; Sala-Garrido, R.; Garrido-Baserba, M. Economic feasibility study for phosphorus recovery processes. Ambio 2010, 40, 408–416. [Google Scholar] [CrossRef]


| Waste Feedstock | PH | TN (mM as N) | NH4+ (mM as N) | NH4−N/TN-N Ratio | TP (mM as P) | OP (mM as P) | OP-P/TP-P Ratio | NH4−N/OP-P Ratio | MG2+ (mM) | CA2+ (mM) | K+ (mM) | NA+ (mM) | CL− (mM) | NO3− (mM) | SO42− (mM) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dairy Manure | 4.4 | 75 | 23 | 0.30 | 15.4 | 1.3 | 0.08 | 17.9 | 2.2 | 7.5 | 7.7 | 6.1 | 8.8 | ND | 0.3 |
| Pre-Digested Sludge | 8.4 | 232 | 70 | 0.30 | 25.8 | 3.6 | 0.14 | 19.3 | 0.1 | 0.5 | 6.9 | 3.0 | 2.1 | ND | 2.4 |
| Digested Sludge | 8.6 | 156 | 150 | 0.96 | 7.1 | 1.0 | 0.14 | 150.0 | 0.2 | 0.8 | 7.6 | 4.3 | 2.8 | ND | 3.6 |
| Brewing Yeast | 8.3 | 175 | 98 | 0.56 | 70.8 | 24.3 | 0.34 | 4.0 | 0.2 | 0.5 | 33.9 | 1.7 | 2.7 | ND | 3.1 |
| Spent Grains | 5.3 | 146 | 50 | 0.34 | 33.5 | 11.4 | 0.34 | 4.4 | 1.2 | 0.4 | 0.3 | 1.3 | 0.1 | 0.04 | 0.6 |
| White Lees | 6.4 | 7 | 2 | 0.32 | 1.5 | 1.7 | 1.18 | 1.3 | 0.2 | 0.2 | 81.2 | 0.4 | 0.2 | 0.02 | 0.4 |
| Red Lees | 8.8 | 135 | 80 | 0.59 | 117.2 | 22.2 | 0.19 | 3.6 | 0.1 | 0.5 | 182.0 | 0.7 | 0.3 | ND | ND |
| Waste Feedstock | Optimal pH | NaOH Consumed (mM) | MgCl2 Consumed (mM) | Struvite Recovered (mM) | OP Removed (mM) | NH4+/NH3 Removed (mM) | Residual NH4+/NH3 (%) | Residual TN (%) | Residual OP (%) | Residual TP (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Dairy Manure | 8.0 | 1.40 | 0 | 0 | 1.27 | 0 | 100 | 100 | 0 | 75 |
| Pre-Digested Sludge | 10.5 | 1.00 | 3.29 | 3.28 | 3.59 | 3.28 | 95 | 98 | >1 | 57 |
| Digested Sludge | 10.5 | 1.50 | 0.41 | 0.48 | 0.97 | 0.48 | 100 | 100 | 2 | 58 |
| Brewing Yeast | 10.5 | 1.14 | 23.9 | 23.9 | 24.2 | 23.9 | 76 | 82 | >1 | 5 |
| Spent Grains | 10.5 | 1.35 | 9.88 | 11.1 | 11.3 | 11.1 | 78 | 90 | 1 | 3 |
| White Lees | 9.0 | 0.09 | 3.70 | 0.56 | 0.69 | 0.56 | 75 | 90 | 34 | 36 |
| Red Lees | 10.5 | 1.12 | 21.8 | 21.8 | 22.1 | 21.8 | 73 | 79 | >1 | 42 |
| Waste Feedstock | Initial Concentration (mg/L) | Recovered as Precipitates (mg/L) | Remaining in ACP (mg/L) | Mass Difference (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PO43−-P | NH4+-N | Mg2+ | PO43−-P | NH4+-N | Mg2+ | PO43−-P | NH4+-N | Mg2+ | PO43−-P | NH4+-N | Mg2+ | |
| Pre-Digested Sludge | 112 | 975 | 121 | 106 | 38 | 73 | 5.6 | 620 | 40 | 0.8 | 32.5 | 6.4 |
| Digested Sludge | 31 | 2100 | 36 | 29 | 13 | 25 | 0.0 | 1528 | 11 | 5.2 | 26.6 | 1.0 |
| Brewing Yeast | 753 | 1370 | 875 | 751 | 285 | 503 | 1.6 | 568 | 285 | 0.0 | 37.8 | 9.9 |
| Spent Grains | 352 | 700 | 409 | 324 | 122 | 218 | 0.0 | 422 | 190 | 8.1 | 22.3 | 0.2 |
| White Lees | 53 | 31 | 62 | 30 | 13 | 13 | 18.0 | 10 | 31 | 10.3 | 27.4 | 11.5 |
| Red Lees | 687 | 1115 | 798 | 646 | 266 | 521 | 0.0 | 587 | 204 | 6.0 | 23.6 | 9.1 |
| Average | 331 | 1048 | 385 | 314 | 123 | 227 | 4.2 | 622 | 127 | 5.1 | 28.4 | 8.0 |
| Author/Year | Feedstock/Scenario | Original EROI | Revised EROI with ACP Management a | Revised EROI with Struvite Precipitation b |
|---|---|---|---|---|
| Connelly et al., 2015 [28] | Algae, “CO2 from ethanol” | 1.3 | 1.1 | 1.2 |
| Algae, “CO2 from natural wells” | 1.2 | 1.0 | N/A c | |
| Sawayama et al., 1999 [30] | B. braunii (algae) | 6.7 | 3.7 | 3.9 |
| D. tertiolecta (algae) | 2.9 | 2.0 | 2.1 | |
| Japanese oak | 1.8 | 1.3 | 1.3 | |
| Japanese larch bark | 0.9 | 0.8 | 0.8 | |
| Sewage sludge | 2.9 | 2.0 | 2.0 | |
| Barley silage | 2.3 | 1.7 | 1.7 | |
| Kitchen garbage | 0.7 | 0.6 | 0.6 | |
| Vardon et al., 2012 [29] | Scenedesmus (algae), 80% moisture | 2.3 | 1.3 | 1.4 |
| Defatted Scenedesmus, 80% moisture | 1.8 | 1.1 | 1.1 | |
| Spirulina (algae), 80% moisture | 1.6 | 0.9 | 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, S.K.; Cheng, F.; Colosi, L.M. Evaluating the Impacts of ACP Management on the Energy Performance of Hydrothermal Liquefaction via Nutrient Recovery. Energies 2019, 12, 729. https://doi.org/10.3390/en12040729
Bauer SK, Cheng F, Colosi LM. Evaluating the Impacts of ACP Management on the Energy Performance of Hydrothermal Liquefaction via Nutrient Recovery. Energies. 2019; 12(4):729. https://doi.org/10.3390/en12040729
Chicago/Turabian StyleBauer, Sarah K., Fangwei Cheng, and Lisa M. Colosi. 2019. "Evaluating the Impacts of ACP Management on the Energy Performance of Hydrothermal Liquefaction via Nutrient Recovery" Energies 12, no. 4: 729. https://doi.org/10.3390/en12040729





