Modeling the Performance of a Zinc/Bromine Flow Battery
Abstract
:1. Introduction
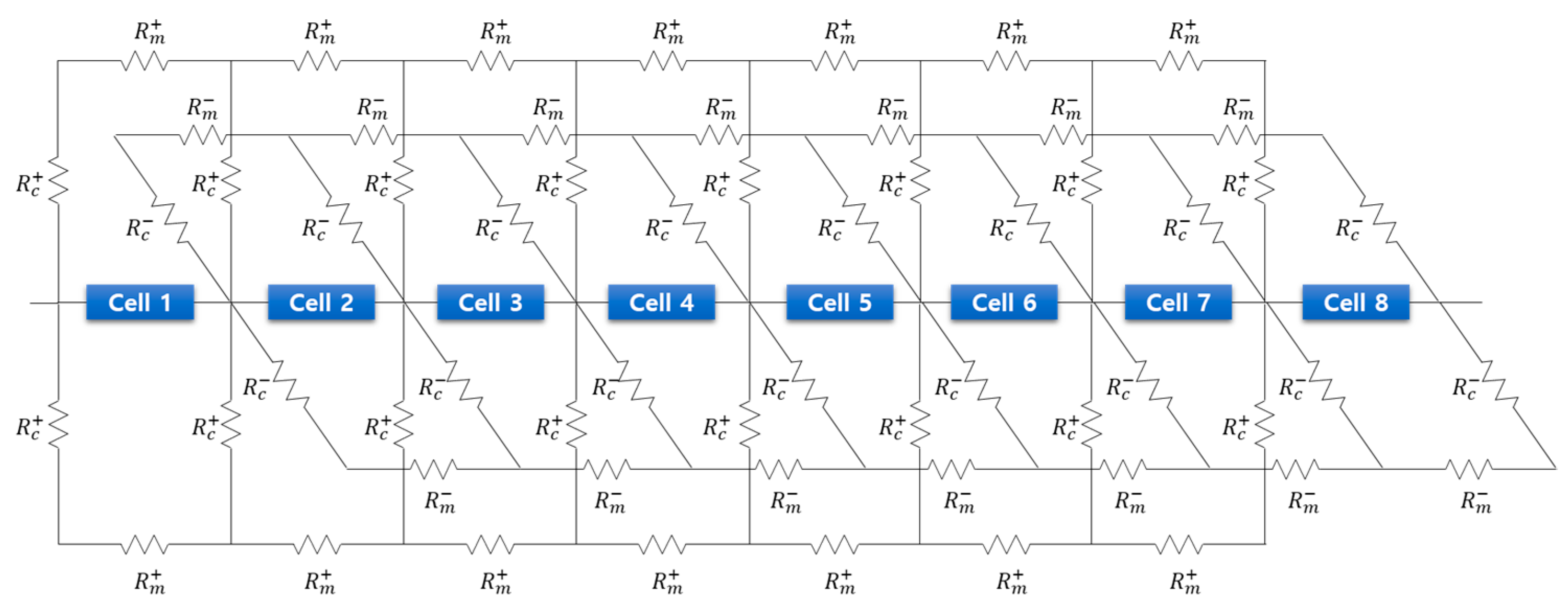
2. Mathematical Model
3. Experimental Section
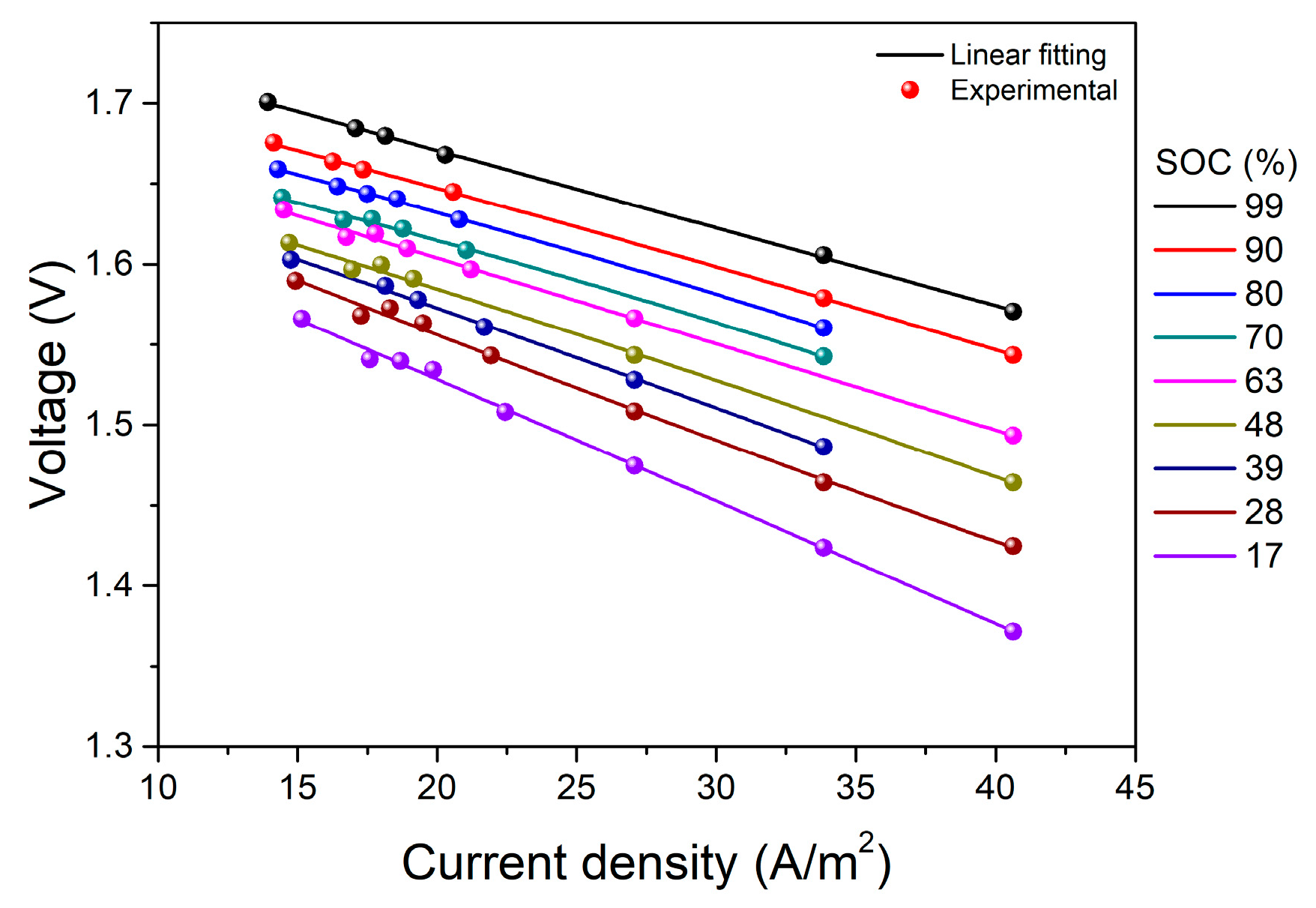
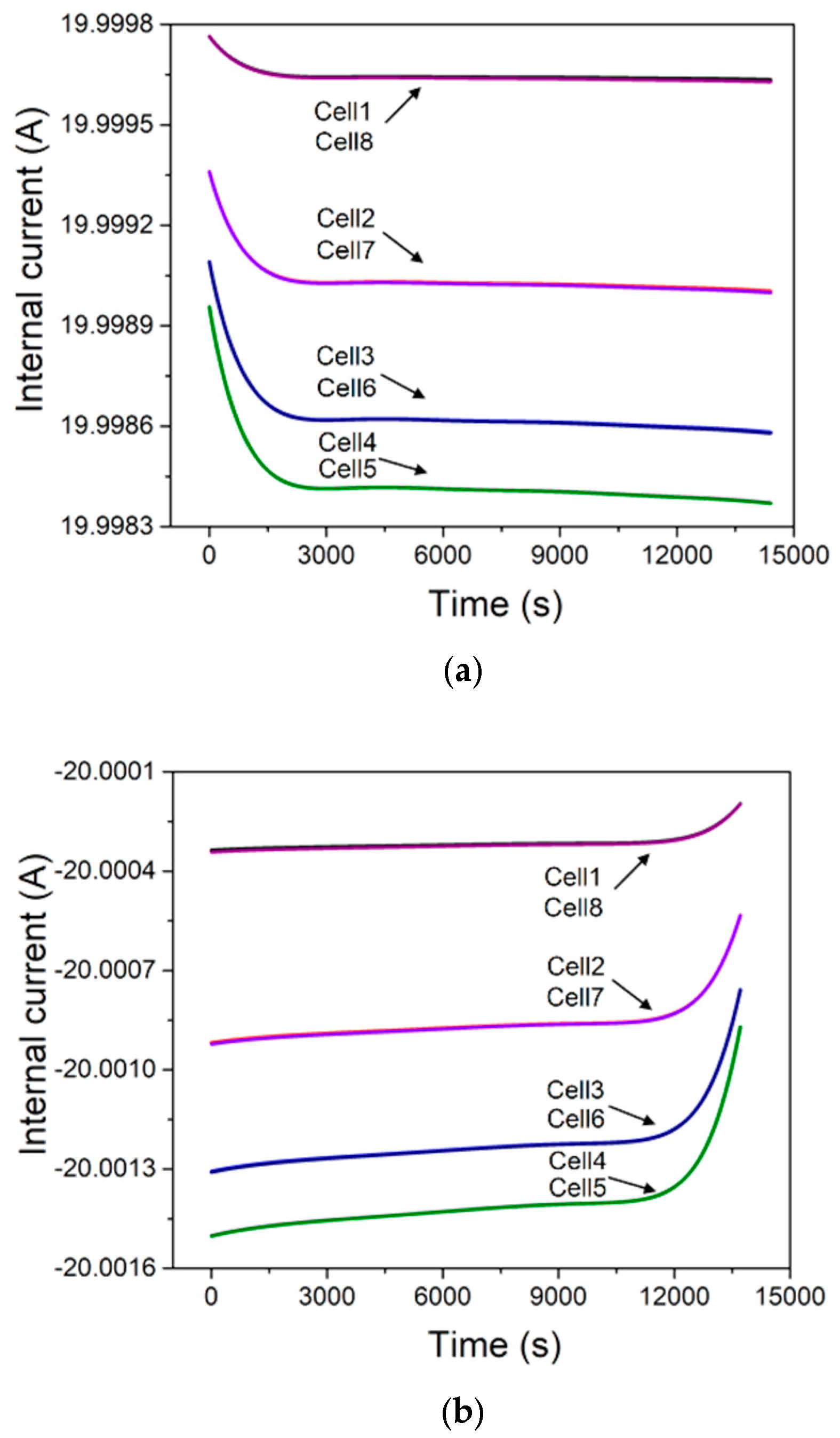
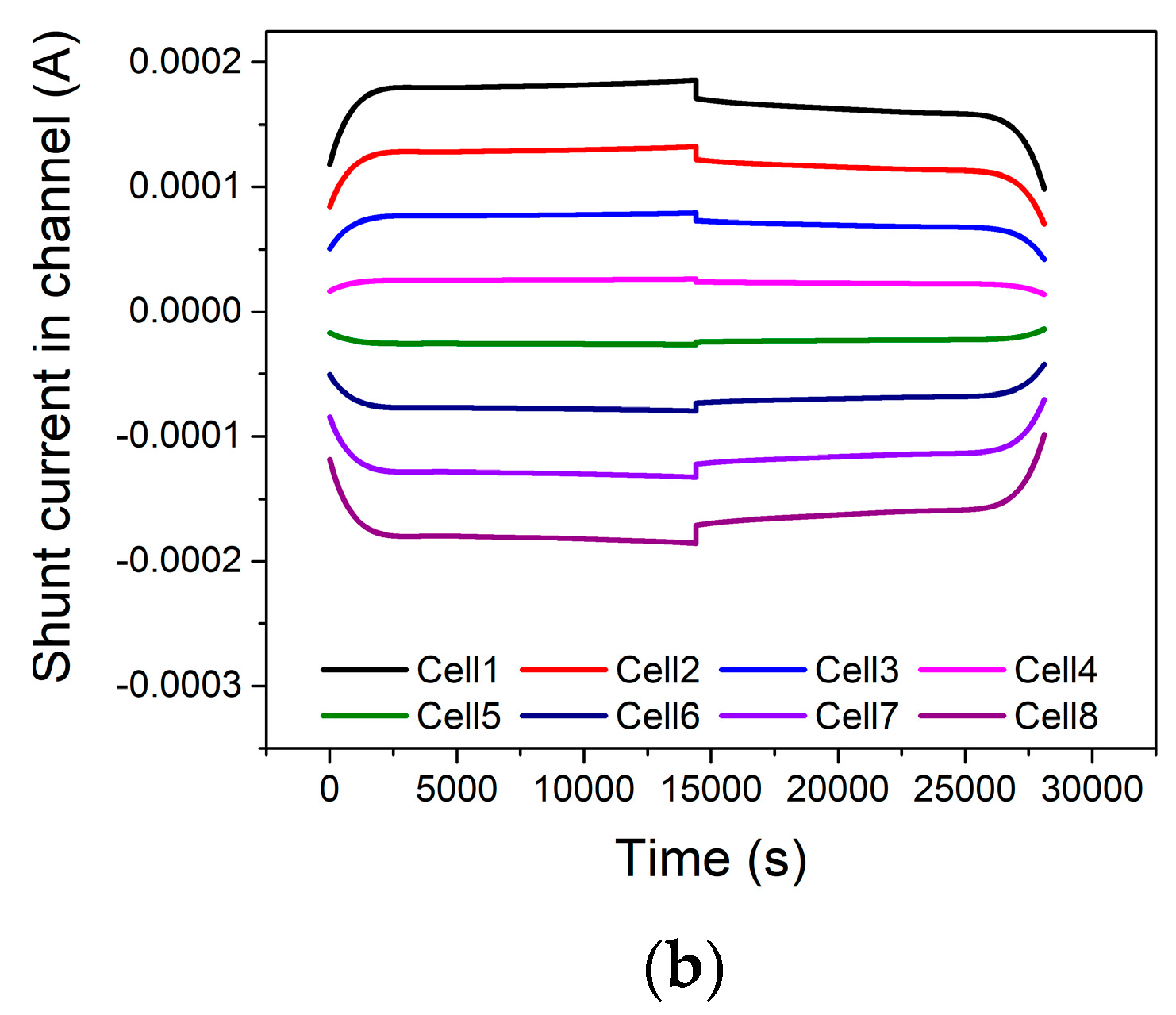
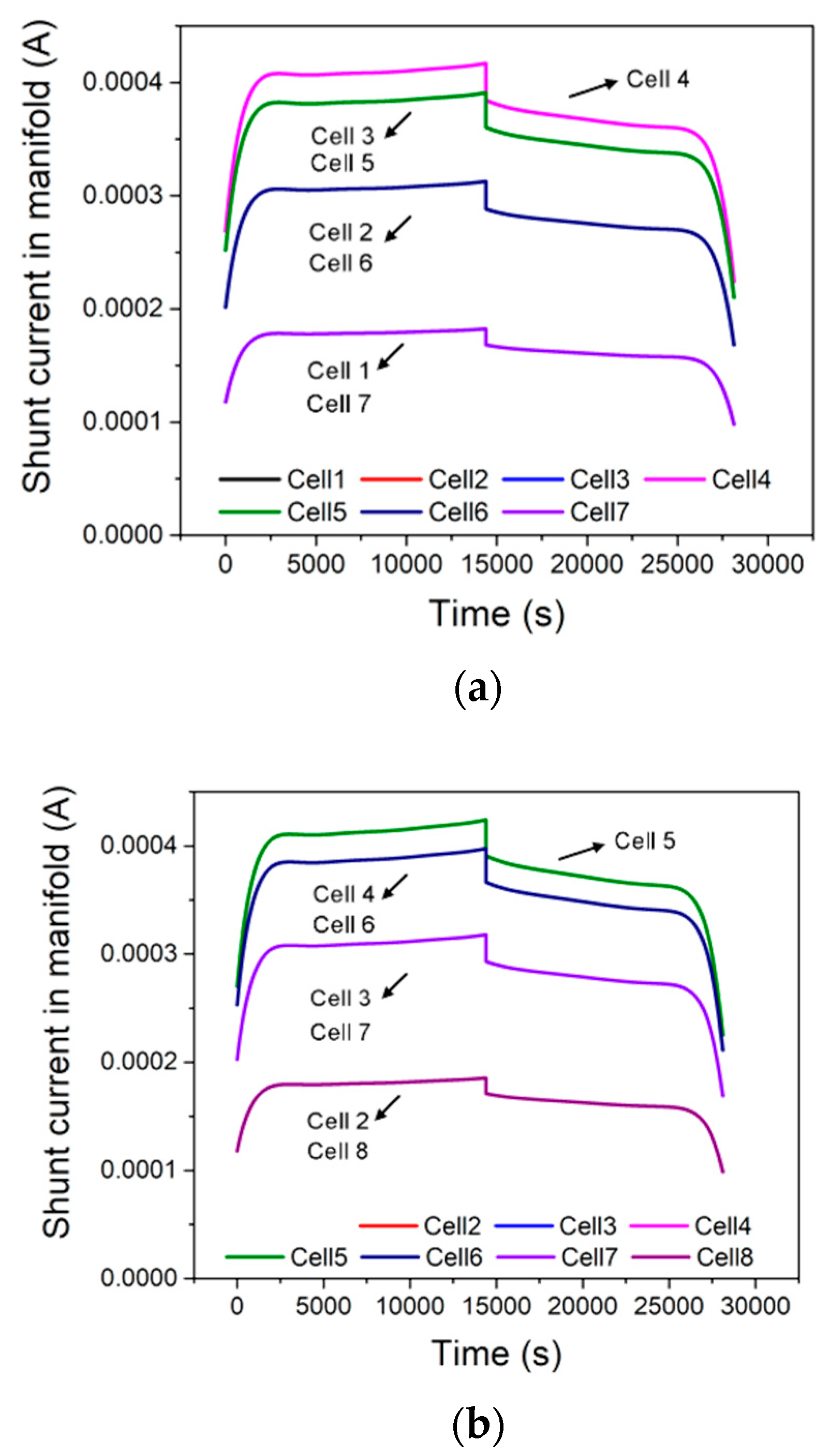
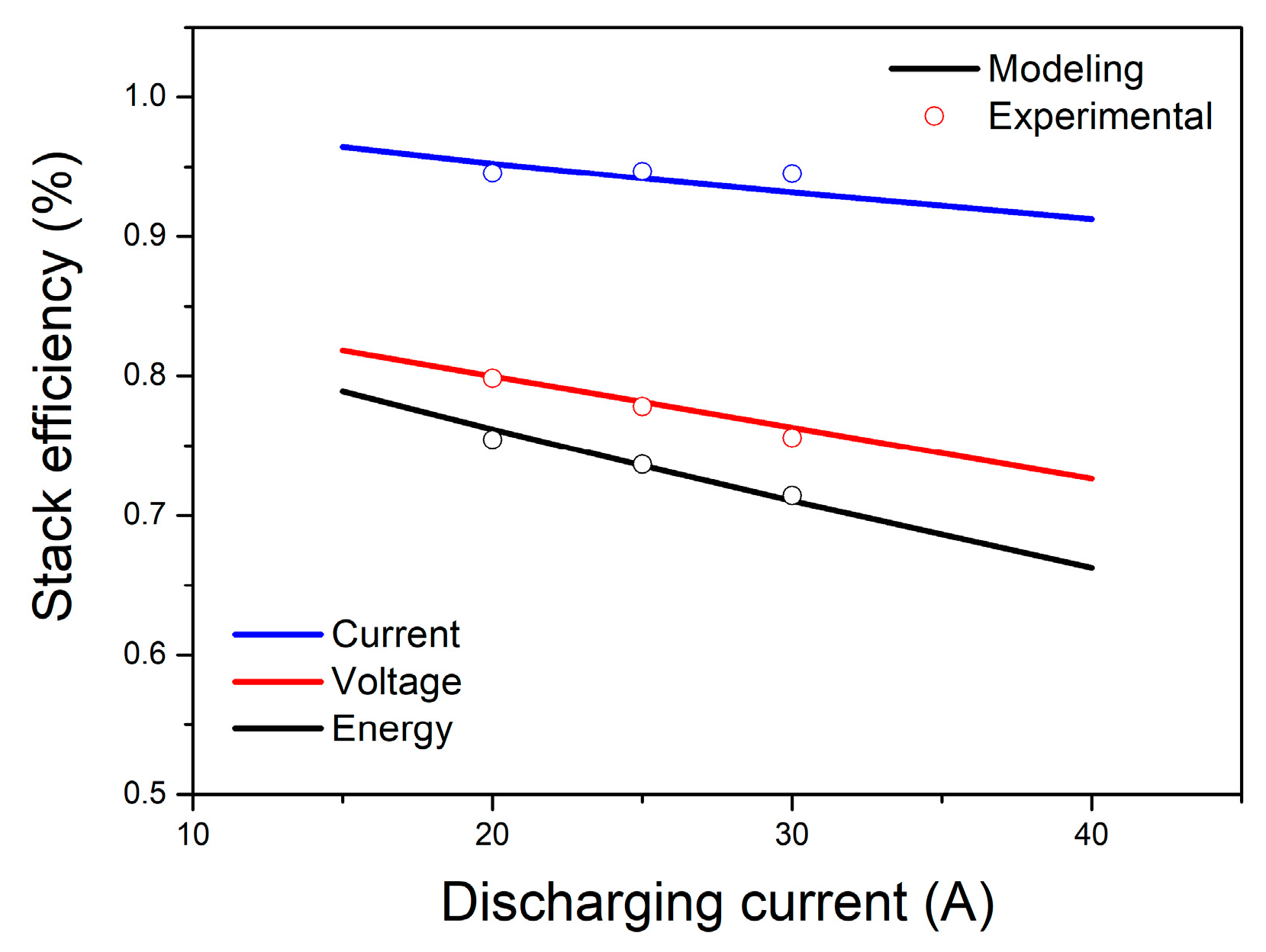
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Castillo, A.; Gayme, D.F. Grid-scale energy storage applications in renewable energy integration: A survey. Energ. Convers. Manag. 2014, 87, 885–894. [Google Scholar] [CrossRef]
- Kuntz, M.T.; Dawe, J. Renewable. Rechargeable. Remarkable. Mech. Eng. 2005, 127, 35–39. [Google Scholar] [CrossRef]
- Lim, H.S.; Lackner, A.M.; Knechtli, R.C. Zinc-bromine secondary battery. J. Electrochem. Soc. 1977, 124, 1154–1157. [Google Scholar] [CrossRef]
- Lai, Q.; Zhang, H.; Li, X.; Zhang, L.; Cheng, Y. A novel single flow zinc-bromine battery with improved energy density. J. Power Sources 2013, 235, 1–4. [Google Scholar] [CrossRef]
- Khor, A.; Leung, P.; Mohamed, M.R.; Flox, C.; Xu, Q.; An, L.; Wills, R.G.A.; Morante, J.R.; Shah, A.A. Review of zinc-based hybrid flow batteries: From fundamentals to applications. Mater. Today Energy 2018, 8, 80–108. [Google Scholar] [CrossRef]
- Rose, D.M.; Ferreira, S.R. Initial Test Results from the RedFlow 5 kW, 10 kWh Zinc-Bromide Module, Phase 1; Report 2012-1352; Sandia National Laboratories: Albuquerque, NM, USA, 2012.
- Vionx Energy. Available online: https://www.vionxenergy.com (accessed on 8 March 2019).
- Redflow. Available online: https://redflow.com (accessed on 8 March 2019).
- Berlouis, L.; Bryans, D.; Zafar, J.; Tuohy, P.; Kang, T.H.; Kim, D.S.; Kim, D.S.; Shaw, M.; Atkinson, P.; Peacock, A. Testing of a Prototype 25 kW/50 kWh Zn-Br2 Battery at the Power Networks Demonstration Centre and Integrated to a Community Wind Turbine. In Proceedings of the International Flow Battery Forum (IFBF), Lausanne, Switzerland, July 2018. [Google Scholar]
- Wang, C.; Li, X.; Xi, X.; Zhou, W.; Lai, Q.; Zhang, H. Bimodal highly ordered mesostructure carbon with high activity for Br2/Br− redox couple in bromine based batteries. Nano Energy 2016, 21, 217–227. [Google Scholar] [CrossRef]
- Yang, H.S.; Park, J.H.; Ra, H.W.; Jin, C.S.; Yang, J.H. Critical rate of electrolyte circulation for preventing zinc dendrite formation in a zinc-bromine redox flow battery. J. Power Source 2016, 325, 446–452. [Google Scholar] [CrossRef]
- Banik, S.J.; Akolkar, R. Suppressing dendrite growth during zinc electrodeposition by PEG-200 additive. J. Electrochem. Soc. 2013, 160, D519–D523. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, T.S. Fundamental models for flow batteries. Prog. Energ. Combust. 2015, 49, 40–58. [Google Scholar] [CrossRef]
- Arenas, L.F.; Ponce de Leon, C.; Walsh, F.C. Engineering aspects of the design, construction and performance of modular redox flow batteries for energy storage. J. Energy Storage 2017, 11, 119–153. [Google Scholar] [CrossRef] [Green Version]
- Newman, J.; Thomas-Alyea, K.E. Electrochemical Systems, 3rd ed.; John Wiley & Sons: Hoboken NJ, USA, 2004. [Google Scholar]
- Wei, Z.; Tseng, K.J.; Wai, N.; Lim, T.M.; Skyllas-Kazacos, M. Adaptive estimation of state of charge and capacity with online identified battery model for vanadium redox flow battery. J. Power Source 2016, 332, 389–398. [Google Scholar] [CrossRef]
- Wei, Z.; Lim, T.M.; Skyllas-Kazacos, M.; Wai, N.; Tseng, K.J. Online state of charge and model parameter co-estimation based on a novel multi-timescale estimator for vanadium redox flow battery. Appl. Energy 2016, 172, 169–179. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, J.; Xiong, R.; Dong, G.; Pou, J.; Tseng, K.J. Online estimation of power capacity with noise effect attenuation for lithium-ion battery. IEEE Trans. Ind. Electron. 2019, 66, 5724–5735. [Google Scholar] [CrossRef]
- Evans, T.I.; White, R.E. A review of mathematical modeling of the zinc/bromine flow cell and battery. J. Electrochem. Soc. 1987, 134, 2725–2733. [Google Scholar] [CrossRef]
- Lee, J.; Selman, J.R. Effects of separator and terminal on the current distribution in parallel-plate electrochemical flow reactors. J. Electrochem. Soc. 1982, 129, 1670–1678. [Google Scholar] [CrossRef]
- Lee, J.; Selman, J.R. Electrode corrosion in a compartmented flow cell by diffusion from the counterelectrode. J. Electrochem. Soc. 1983, 130, 1237–1242. [Google Scholar] [CrossRef]
- Van Zee, J.; White, R.E.; Grimes, P.; Bellows, R. A simple model of Exxon’s Zn/Br2 battery. In Electrochemical Cell Design; White, R.E., Ed.; Springer: Boston, MA, USA, 1984; pp. 293–309. [Google Scholar]
- Mader, M.J.; White, R.E. A mathematical model of a Zn/Br2 cell on charge. J. Electrochem. Soc. 1986, 133, 1297–1307. [Google Scholar] [CrossRef]
- Evans, T.I.; White, R.E. A mathematical model of a zinc/bromine flow cell. J. Electrochem. Soc. 1987, 134, 866–874. [Google Scholar] [CrossRef]
- Simpson, G.D.; White, R.E. An algebraic model for a zinc/bromine flow cell. J. Electrochem. Soc. 1989, 136, 2137–2144. [Google Scholar] [CrossRef]
- Kalu, E.E.; White, R.E. Zn/Br2 cell: Effects of plated zinc and complexing organic phase. AIChE J. 1991, 37, 1164–1174. [Google Scholar] [CrossRef]
- Manla, E.; Nasiri, A.; Rentel, C.H.; Hughes, M. Modeling of zinc bromide energy storage for vehicular applications. IEEE Trans. Ind. Electron. 2010, 57, 624–632. [Google Scholar] [CrossRef]
- Knehr, K.W.; Biswas, S.; Steingart, D.A. Quantification of the voltage losses in the minimal architecture zinc-bromine battery using GITT and EIS. J. Electrochem. Soc. 2017, 164, A3101–A3108. [Google Scholar] [CrossRef]
- Kim, U.S.; Shin, C.B.; Kim, C.S. Effect of electrode configuration on the thermal behavior of a lithium-polymer battery. J. Power Sources 2008, 180, 909–916. [Google Scholar] [CrossRef]
- Kim, U.S.; Shin, C.B.; Kim, C.S. Modeling for the scale-up of a lithium-ion polymer battery. J. Power Sources 2009, 189, 841–846. [Google Scholar] [CrossRef]
- Kim, U.S.; Yi, J.; Shin, C.B.; Han, T.; Park, S. Modeling for the thermal behavior of a lithium-ion battery during charge. J. Power Sources 2011, 196, 5115–5121. [Google Scholar] [CrossRef]
- Kim, U.S.; Yi, J.; Shin, C.B.; Han, T.; Park, S. Modeling the dependence of the discharge behavior of a lithium-ion battery on the environmental temperature. J. Electrochem. Soc. 2011, 158, A611–A618. [Google Scholar]
- Tiedemann, W.; Newman, J. Current and potential distribution in lead-acid battery plates. In Battery Design and Optimization; Gross, S., Ed.; The Electrochemical Society, Inc.: Pennington, NJ, USA, 1979; pp. 39–49. [Google Scholar]
- Newman, J.; Tiedemann, W. Potential and current distribution in electrochemical cells—Interpretation of the half-cell voltage measurements as a function of reference-electrode location. J. Electrochem. Soc. 1993, 140, 1961–1968. [Google Scholar] [CrossRef]
- Gu, H. Mathematical analysis of a Zn/NiOOH cell. J. Electrochem. Soc. 1983, 130, 1459–1464. [Google Scholar] [CrossRef]
- Kaminski, E.A.; Savinell, R.F. A technique for calculating shunt leakage and cell currents in bipolar stacks having divided or undivided cells. J. Electrochem. Soc. 1983, 130, 1103–1107. [Google Scholar] [CrossRef]
- White, R.E.; Walton, C.W.; Burney, H.S.; Beaver, R.N. Predicting shunt currents in stacks of bipolar plate cells. J. Electrochem. Soc. 1986, 133, 485–492. [Google Scholar] [CrossRef]





| Parameter | Value |
|---|---|
| a0 (V) | 0.75 |
| a1 (V) | 15.45 |
| a2 (V) | −107.49 |
| a3 (V) | 417.26 |
| a4 (V) | −1003.76 |
| a5 (V) | 1566.64 |
| a6 (V) | −1597.93 |
| a7 (V) | 1032.84 |
| a8 (V) | −385.50 |
| a9 (V) | 63.53 |
| b0 (Sm−2) | 47.46 |
| b1 (Sm−2) | −343.44 |
| b2 (Sm−2) | 11091.86 |
| b3 (Sm−2) | −56547.87 |
| b4 (Sm−2) | 131795.34 |
| b5 (Sm−2) | −158962.42 |
| b6 (Sm−2) | 96327.06 |
| b7 (Sm−2) | −23197.91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, B.; Lee, D.; Yi, J.; Shin, C.B.; Kim, D.J.; Choi, E.M.; Kang, T.H. Modeling the Performance of a Zinc/Bromine Flow Battery. Energies 2019, 12, 1159. https://doi.org/10.3390/en12061159
Koo B, Lee D, Yi J, Shin CB, Kim DJ, Choi EM, Kang TH. Modeling the Performance of a Zinc/Bromine Flow Battery. Energies. 2019; 12(6):1159. https://doi.org/10.3390/en12061159
Chicago/Turabian StyleKoo, Boram, Dongcheul Lee, Jaeshin Yi, Chee Burm Shin, Dong Joo Kim, Eun Mi Choi, and Tae Hyuk Kang. 2019. "Modeling the Performance of a Zinc/Bromine Flow Battery" Energies 12, no. 6: 1159. https://doi.org/10.3390/en12061159





