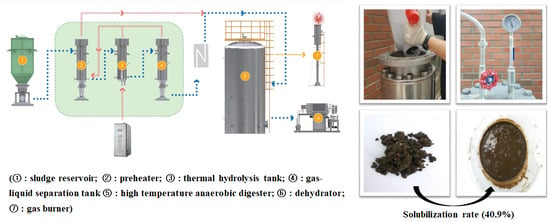
Improvement of Waste Dehydrated Sludge for Anaerobic Digestion through High-Temperature and High-Pressure Solubilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate Characteristics from Biochemical Methane Potential (BMP) Tests
2.2. Substrate Characteristics in Lab-Scale Experiments
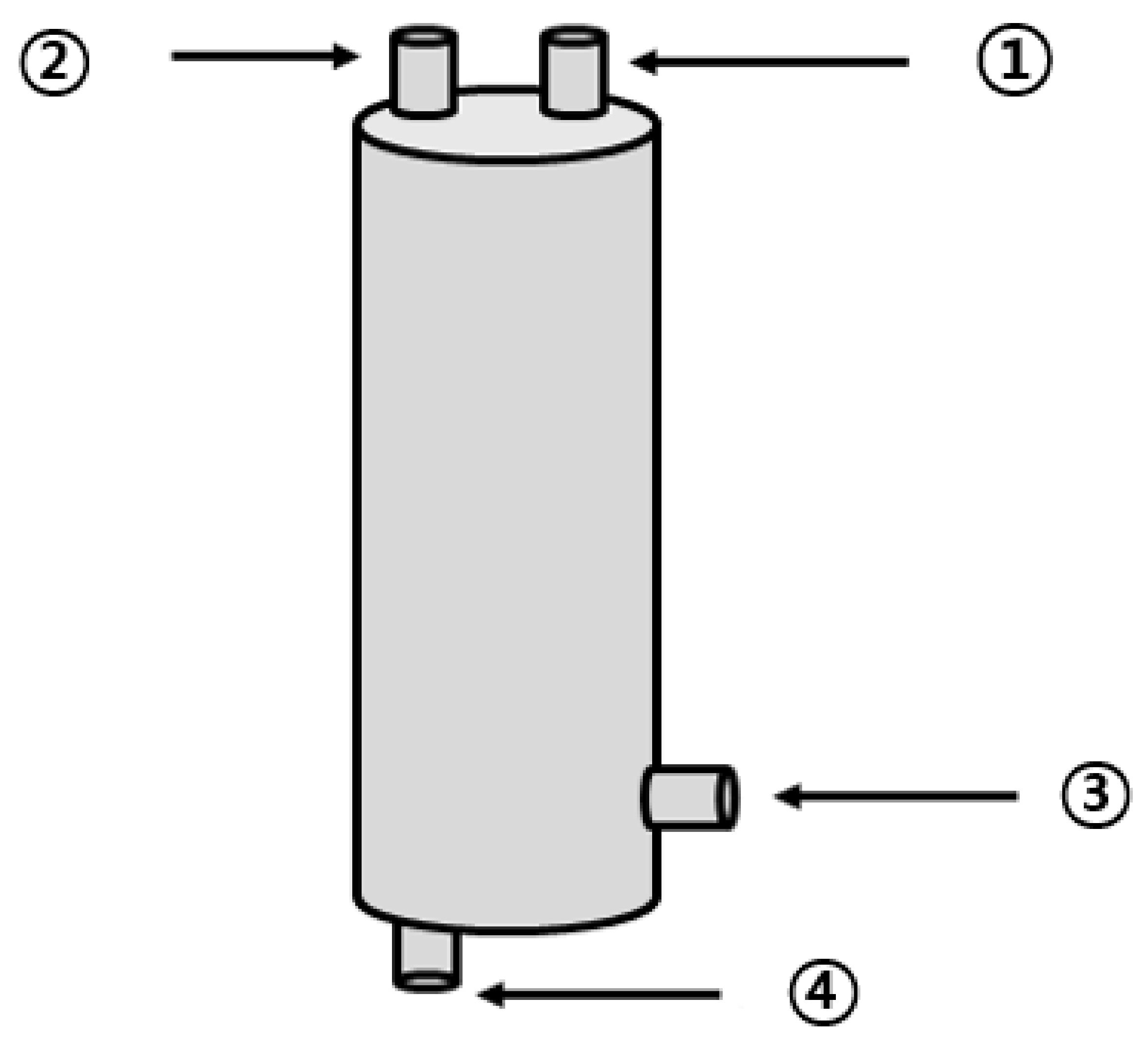
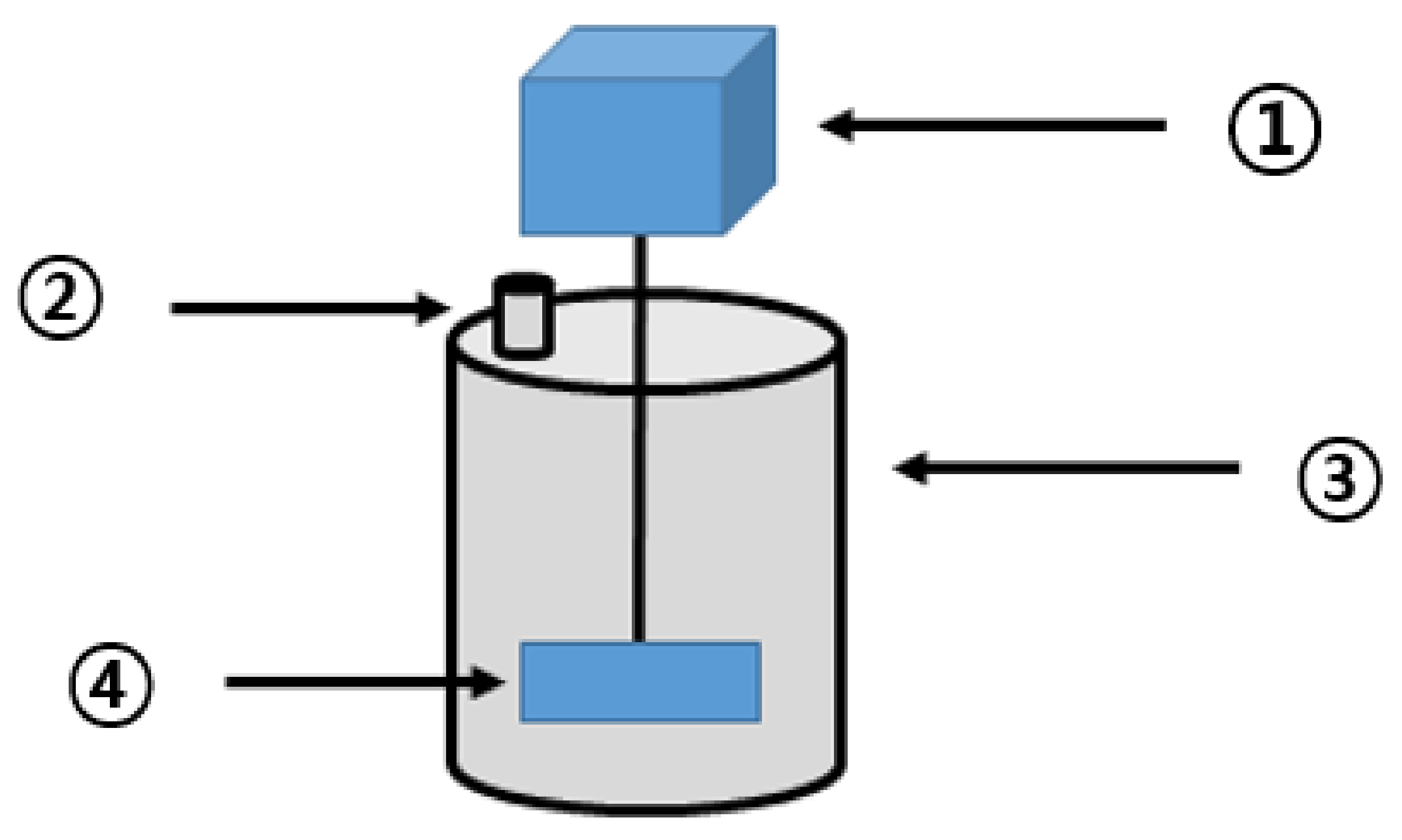
2.3. Configuration of the BMP Test Reactor
2.4. Operating Conditions in the BMP Test
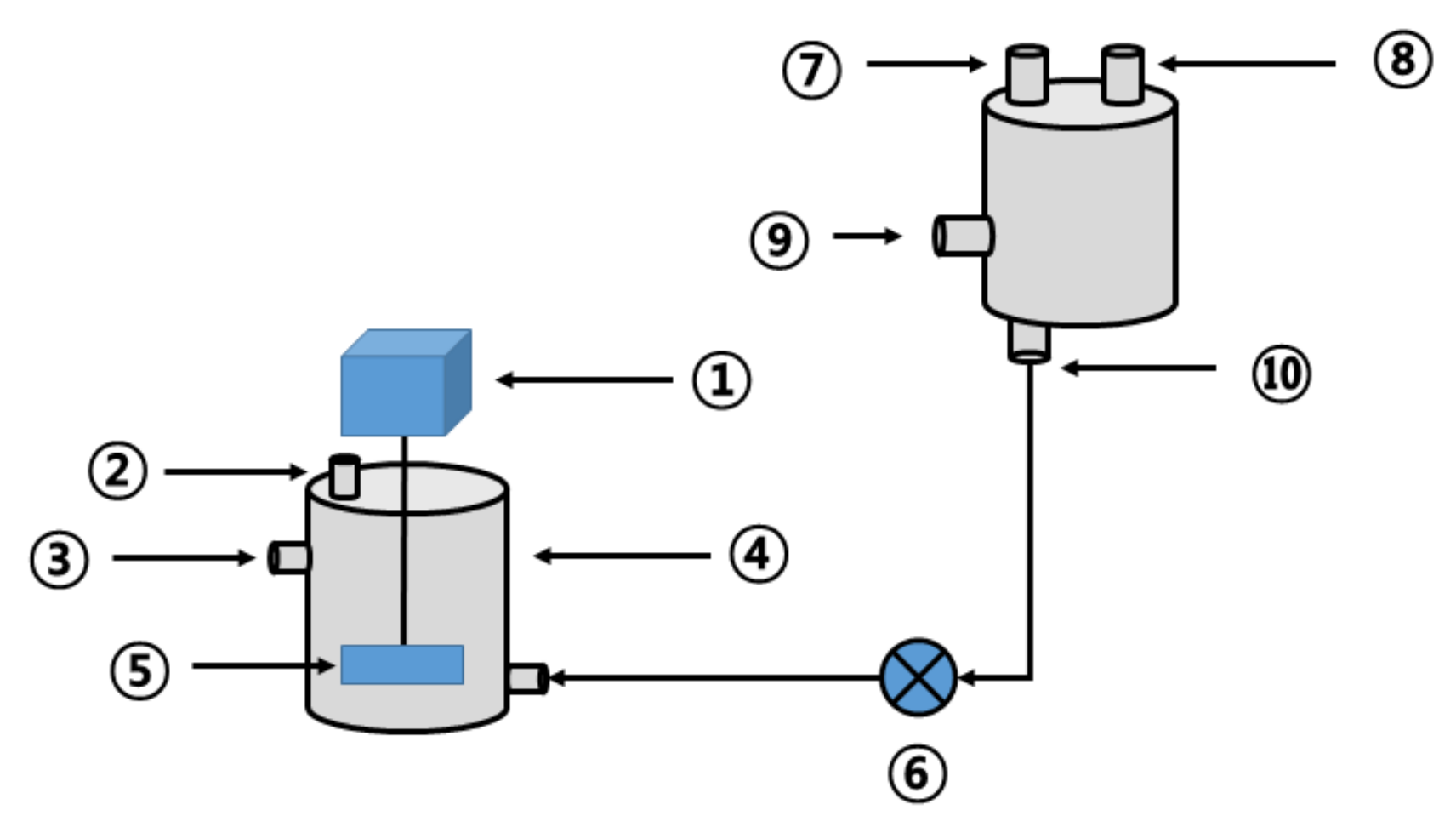
2.5. Lab-Scale Reactor Configuration
2.6. Lab-Scale Operating Conditions
2.7. Analysis Method
3. Results and Discussion
3.1. Results of the BMP Tests
3.1.1. Chanegs in the Physical Properties of Waste Hydrated Sludge Upon Thermal Hydrolysis
3.1.2. Waste Hydrated Sludge Element Composition after Thermal Hydrolysis
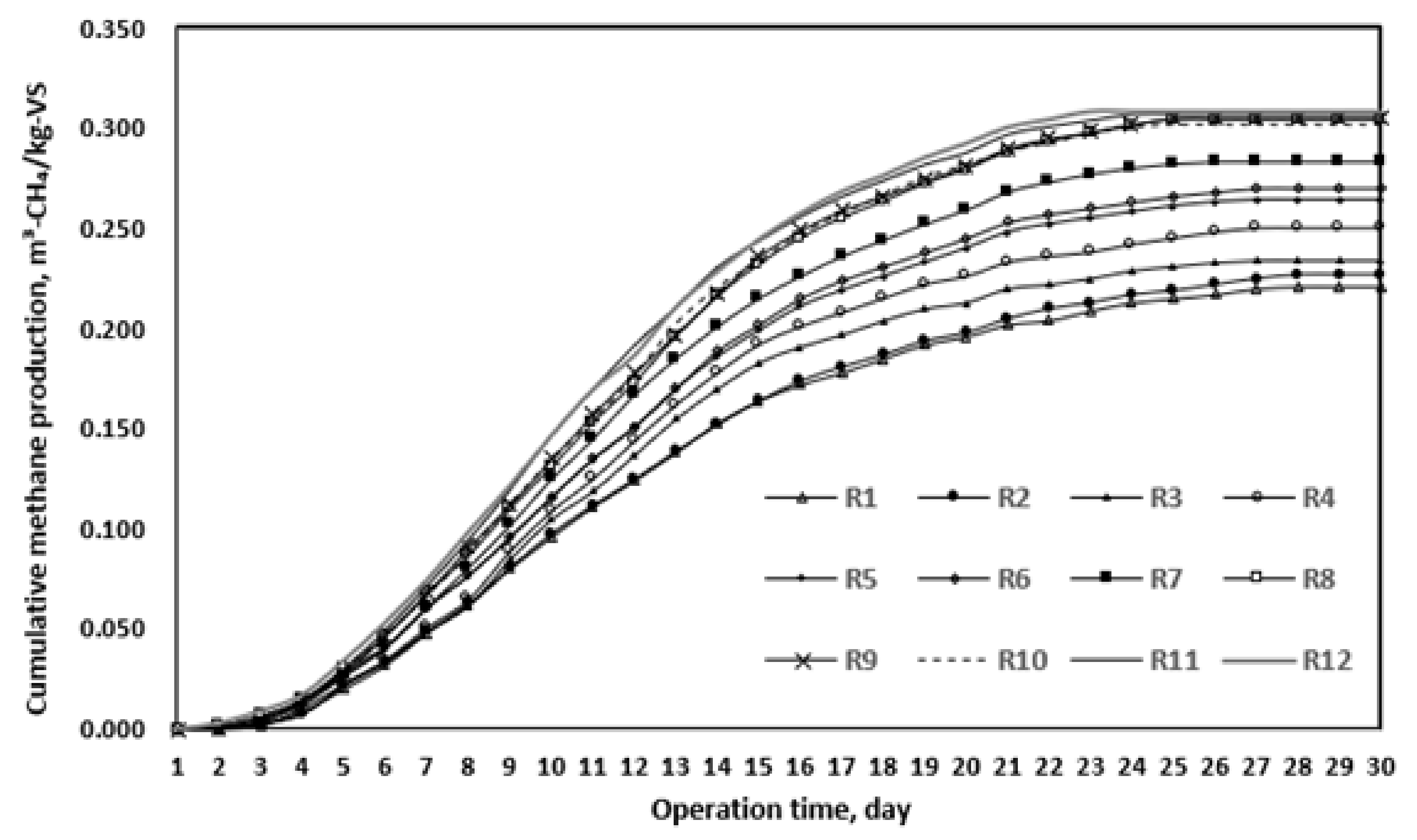
3.1.3. BMP Test Results after Thermal Hydrolysis
3.2. Results of Continuous Lab-Scale Test
3.2.1. Alkalinity and pH
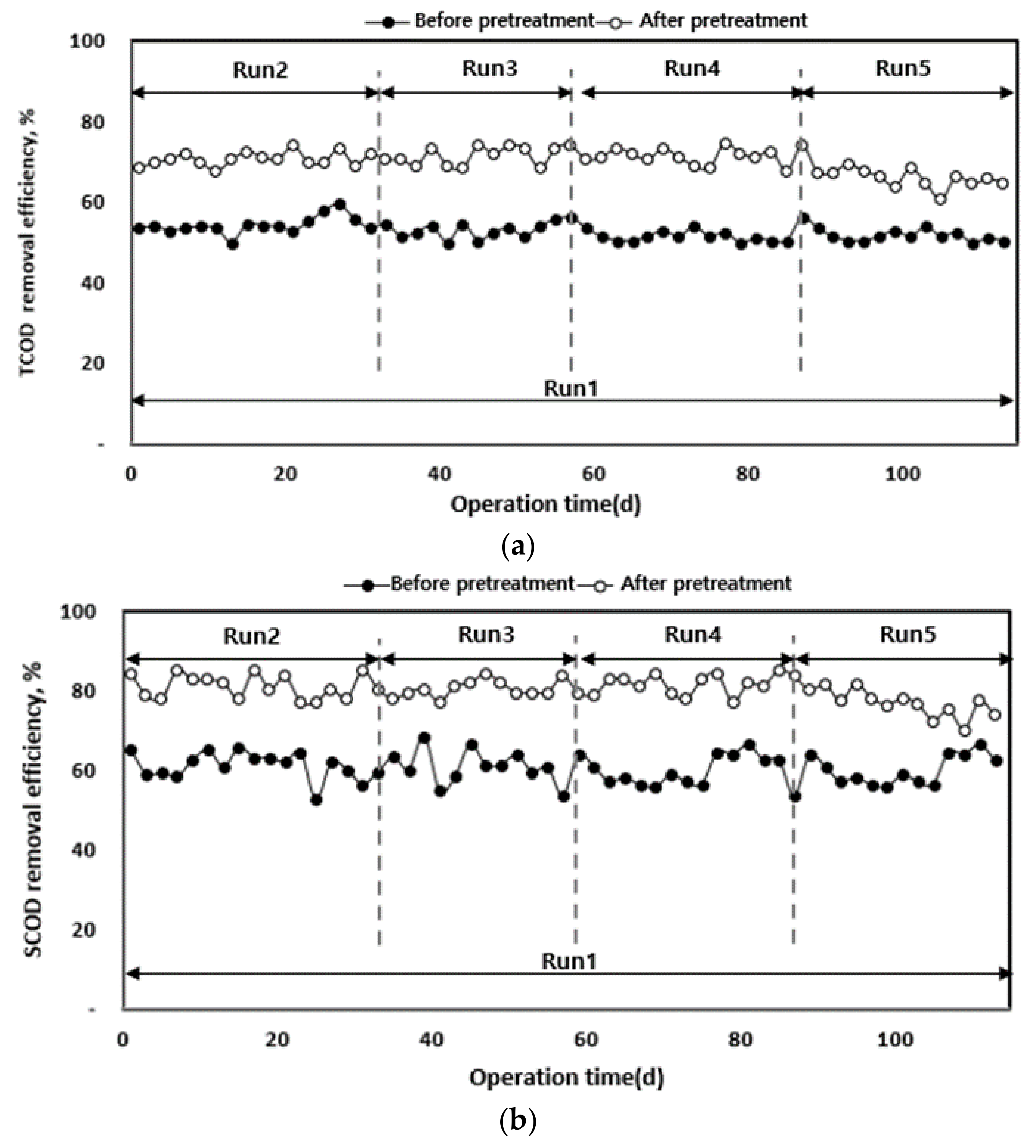
3.2.2. Organic Matter Removal
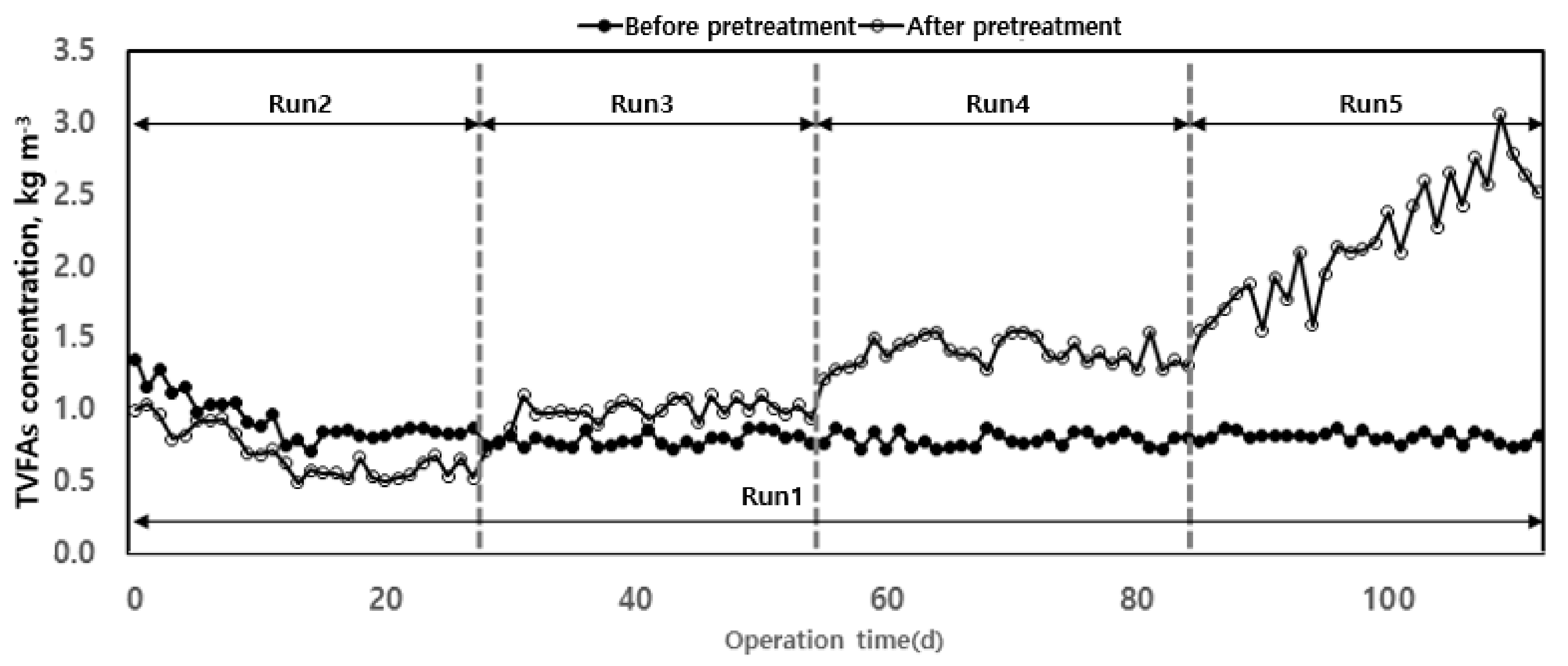
3.2.3. VFAs Changes
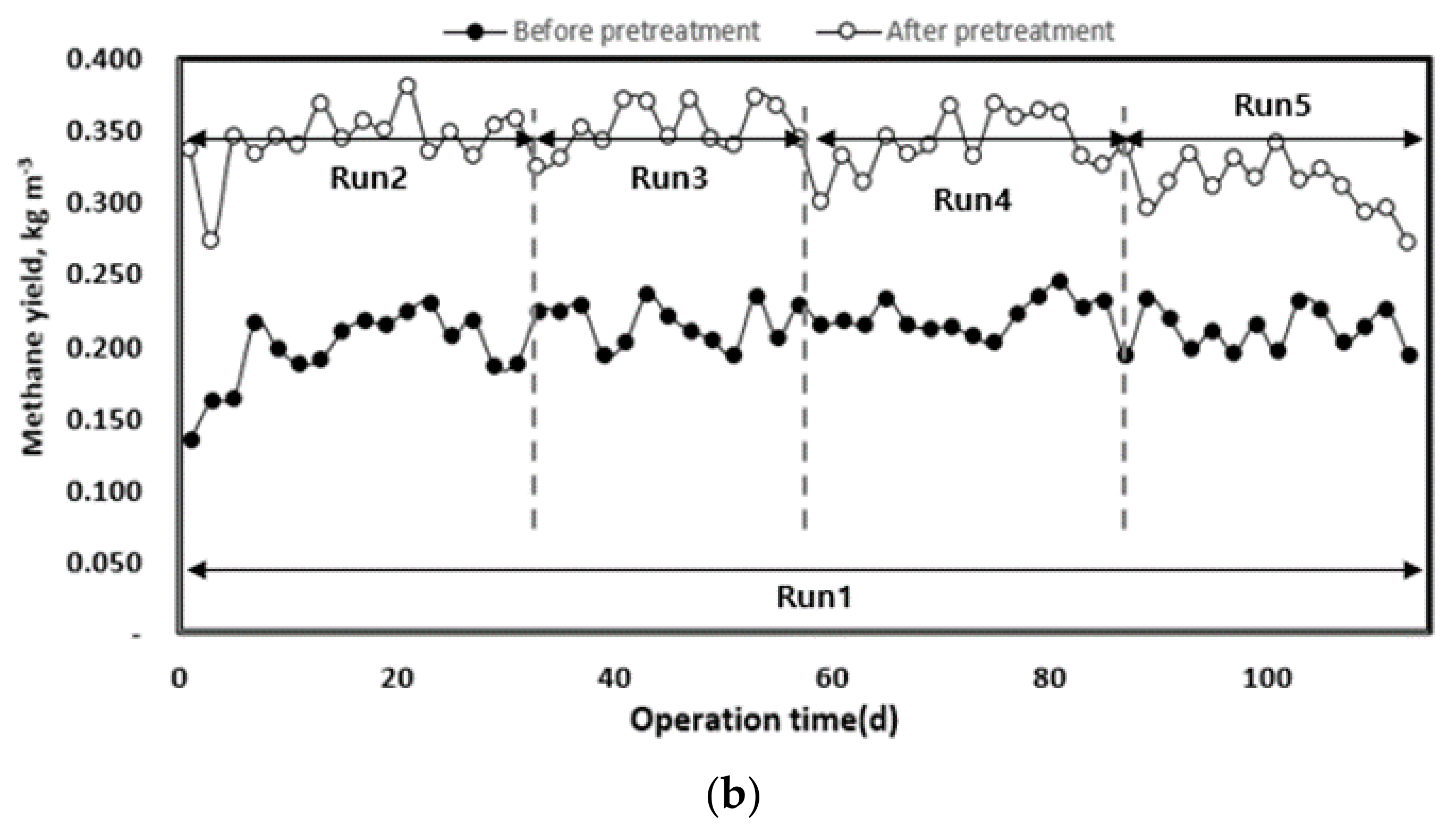
3.2.4. Methane Production and Yield
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tozlu, A.; Özahi, E.; Abuşoğlu, A. Waste to energy technologies for municipal solid waste management in Gaziantep. Renew. Sustain. Energy Rev. 2016, 54, 809–815. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Han, M.J.; Behera, S.K.; Park, H.S. Anaerobic co-digestion of food waste leachate and piggery wastewater for methane production: Statistical optimization of key process parameters. J. Chem. Technol. Biotechnol. 2012, 87, 1541–1550. [Google Scholar] [CrossRef]
- Zou, K.; Zhang, L. Implementing the London Dumping Convention in East Asia. Asia Pac. J. Ocean Law Policy 2017, 2, 247–267. [Google Scholar] [CrossRef]
- Polprasert, C.; Koottatep, T. Organic Waste Recycling; IWA Publishing: London, UK, 2007. [Google Scholar]
- Park, J.G.; Shin, W.B.; Shi, W.Q.; Jun, H.B. Changes of bacterial communities in an anaerobic digestion and a bio-electrochemical anaerobic digestion reactors according to organic load. Energies 2019, 12, 2958. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Ju, H.J. Feasibility of co-digestion of sewage sludge, swine waste, and food waste leachate. J. Korea Org. Resour. Recycl. Assoc. 2012, 20, 61–70. [Google Scholar]
- Mata-Alvarez, J.; Mace, S.; Llabres, P. Anaerobic digestion of organic solid wastes: An overview of research achievements and perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Cristancho, D.E.; Arellano, A.V. Study of the operational conditions for anaerobic digestion of urban solid wastes. Waste Manag. 2006, 26, 546–556. [Google Scholar]
- Lee, B.; Park, J.G.; Shin, W.B.; Tian, D.J.; Jun, H.B. Microbial communities change in an anaerobic digestion after application of microbial electrolysis cells. Bioresour. Technol. 2017, 234, 273–280. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Kim, J.; Park, C.; Kim, T.H.; Lee, M.; Kim, S.; Kim, S.W.; Lee, J. Effects of various pretreatments for enhanced anaerobic digestion with waste activated sludge. J. Biosci. Bioeng. 2003, 95, 271–275. [Google Scholar] [CrossRef]
- Valo, A.; Carrere, H.; Delgenes, J.P. Thermal, chemical and thermo-chemical pre-treatment of waste activated sludge for anaerobic digestion. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2004, 79, 1197–1203. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Betenbaugh, M.J.; Bouwer, E.J. The effects of alternative pretreatment strategies on anaerobic digestion and methane production from different algal strains. Bioresour. Technol. 2014, 155, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Mudhoo, A. (Ed.) Biogas Production: Pretreatment Methods in Anaerobic Digestion; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Michaud, S.; Bernet, N.; Buffière, P.; Roustan, M.; Moletta, R. Methane yield as a monitoring parameter for the start-up of anaerobic fixed film reactors. Water Res. 2002, 36, 1385–1391. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.G.; Shin, W.B.; Kim, B.S.; Byun, B.S.; Jun, H.B. Maximizing biogas production by pre-treatment and by optimizing the mixture ratio of co-digestion with organic wastes. Environ. Eng. Res. 2019, 24, 662–669. [Google Scholar] [CrossRef] [Green Version]
- Kavitha, S.; Jayashree, C.; Kumar, S.A.; Yeom, I.T.; Banu, J.R. The enhancement of anaerobic biodegradability of waste activated sludge by surfactant mediated biological pretreatment. Bioresour. Technol. 2014, 168, 159–166. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, H.; Chen, S.; Dichtl, N.; Dai, X.; Li, N. Effects of thermal hydrolysis on organic matter solubilization and anaerobic digestion of high solid sludge. Chem. Eng. J. 2015, 264, 174–180. [Google Scholar] [CrossRef]
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.J.; Delgenès, J.P.; Steyer, J.P.; Ferrer, I. Pre-treatment methods to improve sludge anaerobic degradability: A review. J. Hazard. Mater. 2010, 183, 1–15. [Google Scholar] [CrossRef]
- Mahmood, T.; Elliott, A. A review of secondary sludge reduction technologies for the pulp and paper industry. Water Res. 2006, 40, 2093–2112. [Google Scholar] [CrossRef]
- Cano, R.; Nielfa, A.; Fdz-Polanco, M. Thermal hydrolysis integration in the anaerobic digestion process of different solid wastes: Energy and economic feasibility study. Bioresour. Technol. 2014, 168, 14–22. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.L. Sludge: A waste or renewable source for energy and resources recovery. Renew. Sustain. Energy Rev. 2013, 25, 708–728. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.A.; Novak, J.T. Hydrolysis of macromolecular components of primary and secondary wastewater sludge by thermal hydrolytic pre-treatment. Water Res. 2009, 43, 4489–4498. [Google Scholar] [CrossRef] [PubMed]
- Buswell, A.M.; Mueller, H.F. Mechanism of methane fermentation. Ind. Eng. Chem. 1952, 44, 550–552. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Blazek, J.V.; Morreels, C.L., Jr.; Krovetz, L.J. Pressure wave transmission along the human aorta: Changes with age and in arterial degenerative disease. Circ. Res. 1968, 23, 567–579. [Google Scholar] [CrossRef] [Green Version]
- Boyle, E.A.; Sclater, F.; Edmond, J.M. On the marine geochemistry of cadmium. Nature 1976, 263, 42. [Google Scholar] [CrossRef]
- Kayhanian, M.; Tchobanoglous, G. Innovative two-stage process for the recovery of energy and compost from the organic fraction of municipal solid waste (MSW). Water Sci. Technol. 1993, 27, 133–143. [Google Scholar] [CrossRef]
- Connaughton, S.; Collins, G.; O’Flaherty, V. Psychrophilic and mesophilic anaerobic digestion of brewery effluent: A comparative study. Water Res. 2006, 40, 2503–2510. [Google Scholar] [CrossRef]
- Schieder, D.; Schneider, R.; Bischof, F. Thermal hydrolysis (TDH) as a pretreatment method for the digestion of organic waste. Water Sci. Technol. 2000, 41, 181–187. [Google Scholar] [CrossRef]
- Strong, P.J.; McDonald, B.; Gapes, D.J. Combined thermochemical and fermentative destruction of municipal biosolids: A comparison between thermal hydrolysis and wet oxidative pre-treatment. Bioresour. Technol. 2011, 102, 5520–5527. [Google Scholar] [CrossRef]
- Agdag, O.N.; Sponza, D.T. Effect of alkalinity on the performance of a simulated landfill bioreactor digesting organic solid wastes. Chemosphere 2005, 59, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Siegert, I.; Banks, C. The effect of volatile fatty acid additions on the anaerobic digestion of cellulose and glucose in batch reactors. Process Biochem. 2005, 40, 3412–3418. [Google Scholar] [CrossRef]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.; Mahmood, T. Pretreatment technologies for advancing anaerobic digestion of pulp and paper biotreatment residues. Water Res. 2007, 41, 4273–4286. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Gadhamshetty, V.; Nitayavardhana, S.; Khanal, S.K. Automatic process control in anaerobic digestion technology: A critical review. Bioresour. Technol. 2015, 193, 513–522. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Kardos, L.; Juhász, A.; Palkó, G.Y.; Oláh, J.; Barkács, K.; Zaray, G. Comparing of mesophilic and thermophilic anaerobic fermented sewage sludge based on chemical and biochemical tests. Appl. Ecol. Environ. Res. 2011, 9, 293–302. [Google Scholar] [CrossRef]
- Kong, X.; Wei, Y.; Xu, S.; Liu, J.; Li, H.; Liu, Y.; Yu, S. Inhibiting excessive acidification using zero-valent iron in anaerobic digestion of food waste at high organic load rates. Bioresour. Technol. 2016, 211, 65–71. [Google Scholar] [CrossRef]


| Description | Specification |
|---|---|
| Total chemical oxygen demand as chromium (TCODCr), g m−3 | 43,350 |
| Soluble chemical oxygen demand as chromium (SCODCr), g m−3 | 8600 |
| Total solid (TS), g m−3 | 119,753 |
| Volatile solid (VS), g m−3 | 99,489 |
| VS/TS ratio | 0.83 |
| Nitrogen, % | 6.24 |
| Carbon, % | 43.92 |
| Hydrogen, % | 6.48 |
| Sulphur, % | 0.64 |
| Oxygen, % | 12.99 |
| C/N ratio | 7.04 |
| Parameter | Before Solubilization | After Solubilization | ||
|---|---|---|---|---|
| Range | Average | Range | Average | |
| pH | 6.52–7.90 | 7.19 ± 0.36 | 5.65–6.63 | 6.08 ± 0.21 |
| Alkalinity, g m−3 as CaCO3 | 2203–4373 | 3455 ± 540 | 2044–3860 | 3124 ± 467 |
| TCODCr, g m−3 | 37,840–54,120 | 45,147 ± 2944 | 37,600–53,500 | 44,628 ± 3655 |
| SCODCr, g m−3 | 6700–13,500 | 9599 ± 1635 | 18,110–34,640 | 28,144 ± 1096 |
| NH4+-N, g m−3 | 948–1411 | 1174 ± 104 | 1120–1567 | 1395 ± 92 |
| TS, g m−3 | 38,130–52,950 | 44,199 ± 3926 | 37,240–49,460 | 42,278 ± 3243 |
| VS, g m−3 | 23,170–31,640 | 27,146 ± 2698 | 21,060–29,260 | 25,353 ± 2300 |
| Parameter | Pressure (Bar) | Time (Min) | Temperature (°C) |
|---|---|---|---|
| R1 | 5 | 20 | 110 |
| R2 | 5 | 30 | 110 |
| R3 | 5 | 40 | 110 |
| R4 | 6 | 20 | 141 |
| R5 | 6 | 30 | 141 |
| R6 | 6 | 40 | 141 |
| R7 | 7 | 20 | 165 |
| R8 | 7 | 30 | 165 |
| R9 | 7 | 40 | 165 |
| R10 | 8 | 20 | 187 |
| R11 | 8 | 30 | 187 |
| R12 | 8 | 40 | 187 |
| Parameter | Lab-Scale Reactor | |
|---|---|---|
| Reactor 1 | Reactor 2 | |
| Material | SUS 304 | |
| Size, mm | D280 × H410, 25 L | |
| Effective volume, L | 15 | |
| Stirring speed, rpm | 40 | |
| Substrate | - | Pre-treatment |
| Parameter | Lab-Scale Operating Conditions | ||||
|---|---|---|---|---|---|
| Run 1 | Run 2 | Run 3 | Run 4 | Run 5 | |
| Operation time | 113 d | 28 d | 27 d | 30 d | 28 d |
| Thermal solubilization conditions | 165 °C, 20 min | ||||
| Anaerobic digestive conditions | 55 °C | ||||
| Injection amount | 0.6 m3 d−1 | 0.6 m3 d−1 | 0.9 m3 d−1 | 1.2 m3 d−1 | 1.5 m3 d−1 |
| Thermal energy supply method | Boiler | Solubilized Heat Energy Recycling | |||
| OLR (COD-based) | 2.0 kg (m3 d)−1 | 2.0 kg (m3 d)−1 | 3.0 kg (m3 d)−1 | 4.0 kg (m3 d)−1 | 5.0 kg (m3 d)−1 |
| Parameter | pH | TCODCr (g m−3) | SCODCr (g m−3) | TS (g m−3) | VS (g m−3) | SCOD/TCOD (%) | VS/TS Ratio |
|---|---|---|---|---|---|---|---|
| Blank | 7.2 | 43,350 | 8600 | 119,753 | 99,489 | 19.8 | 0.83 |
| R1 | 7.4 | 45,300 | 11,283 | 117,358 | 98,494 | 24.9 | 0.84 |
| R2 | 7.3 | 45,579 | 12,443 | 116,185 | 99,479 | 27.3 | 0.86 |
| R3 | 7.4 | 44,740 | 13,272 | 115,023 | 100,474 | 29.7 | 0.87 |
| R4 | 7.5 | 45,047 | 15,777 | 113,872 | 98,465 | 35 | 0.87 |
| R5 | 7.4 | 47,001 | 18,456 | 117,641 | 99,449 | 39.3 | 0.85 |
| R6 | 7.5 | 42,640 | 18,241 | 119,144 | 96,485 | 42.8 | 0.81 |
| R7 | 7.5 | 46,260 | 26,673 | 116,912 | 96,467 | 57.7 | 0.83 |
| R8 | 7.6 | 46,007 | 27,457 | 123,379 | 98,369 | 59.7 | 0.80 |
| R9 | 7.6 | 41,787 | 25,607 | 114,736 | 96,447 | 61.3 | 0.84 |
| R10 | 7.6 | 45,321 | 28,345 | 120,152 | 100,228 | 62.5 | 0.83 |
| R11 | 7.7 | 48,858 | 30,686 | 123,368 | 98,390 | 62.8 | 0.80 |
| R12 | 7.6 | 44,498 | 28,527 | 119,068 | 99,326 | 64.1 | 0.83 |
| SCOD (g m−3) | 110 (°C) | 141 (°C) | 165 (°C) | 187 (°C) | Standard Deviation |
|---|---|---|---|---|---|
| 10 min | 11,283 | 15,777 | 26,673 | 28,345 | 7192.2 |
| 20 min | 12,443 | 18,456 | 27,457 | 30,686 | 7225.8 |
| 30 min | 13,272 | 18,241 | 25,607 | 28,527 | 6011.2 |
| Standard deviation | 815.7 | 1215.4 | 758.2 | 1063.3 | - |
| Parameter. | C (%) | H (%) | O (%) | N (%) | S (%) | CH4 Yield (m3 (kg VS)−1) |
|---|---|---|---|---|---|---|
| Blank | 43.9 | 6.5 | 23 | 6.2 | 0.6 | 0.594 |
| R1 | 43.6 | 6.1 | 22.6 | 6.4 | 0.5 | 0.584 |
| R2 | 42.7 | 6.2 | 23.1 | 6.3 | 0.6 | 0.579 |
| R3 | 44 | 6.2 | 22.4 | 6.3 | 0.6 | 0.593 |
| R4 | 43.2 | 6 | 22.4 | 6.5 | 0.4 | 0.580 |
| R5 | 44.5 | 6 | 22.6 | 6.3 | 0.5 | 0.587 |
| R6 | 44.5 | 6.6 | 23.2 | 6.1 | 0.4 | 0.599 |
| R7 | 43.8 | 6.6 | 23.2 | 6 | 0.6 | 0.598 |
| R8 | 44.4 | 6.5 | 22.8 | 6.3 | 0.5 | 0.598 |
| R9 | 42.7 | 6.7 | 22.4 | 6.5 | 0.7 | 0.584 |
| R10 | 43.2 | 6.7 | 22.8 | 6.3 | 0.6 | 0.598 |
| R11 | 44.7 | 6.8 | 22.6 | 6.4 | 0.4 | 0.609 |
| R12 | 42.8 | 6.9 | 23.8 | 6.6 | 0.5 | 0.586 |
| Parameter | Cumulative Methane Yield (m3 (kg VS)−1) | Theoretical Methane Yield (m3 (kg VS)−1) | Biodegradability (%) | Maximum Rate Operation Time (d) | K (d−1) |
|---|---|---|---|---|---|
| blank | 0.167 | 0.5941 | 28.1 | 20 | 0.0713 |
| R1 | 0.221 | 0.5847 | 37.8 | 19 | 0.0802 |
| R2 | 0.2497 | 0.579 | 43.1 | 18 | 0.0834 |
| R3 | 0.2574 | 0.5931 | 43.4 | 18 | 0.0857 |
| R4 | 0.2825 | 0.5809 | 48.6 | 17 | 0.0884 |
| R5 | 0.2983 | 0.5873 | 50.8 | 16 | 0.0897 |
| R6 | 0.3051 | 0.5998 | 50.9 | 16 | 0.0914 |
| R7 | 0.3679 | 0.5983 | 61.5 | 15 | 0.0978 |
| R8 | 0.3709 | 0.5984 | 62 | 15 | 0.0979 |
| R9 | 0.363 | 0.5843 | 62.1 | 15 | 0.0983 |
| R10 | 0.3715 | 0.5988 | 62 | 15 | 0.0984 |
| R11 | 0.3776 | 0.6087 | 62 | 15 | 0.0986 |
| R12 | 0.3784 | 0.5863 | 64.5 | 15 | 0.0987 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, E.-H.; Park, J.-G.; Lee, B.; Shi, W.-Q.; Jun, H.-B. Improvement of Waste Dehydrated Sludge for Anaerobic Digestion through High-Temperature and High-Pressure Solubilization. Energies 2020, 13, 88. https://doi.org/10.3390/en13010088
Hong E-H, Park J-G, Lee B, Shi W-Q, Jun H-B. Improvement of Waste Dehydrated Sludge for Anaerobic Digestion through High-Temperature and High-Pressure Solubilization. Energies. 2020; 13(1):88. https://doi.org/10.3390/en13010088
Chicago/Turabian StyleHong, Eui-Hwan, Jun-Gyu Park, Beom Lee, Wei-Qi Shi, and Hang-Bae Jun. 2020. "Improvement of Waste Dehydrated Sludge for Anaerobic Digestion through High-Temperature and High-Pressure Solubilization" Energies 13, no. 1: 88. https://doi.org/10.3390/en13010088