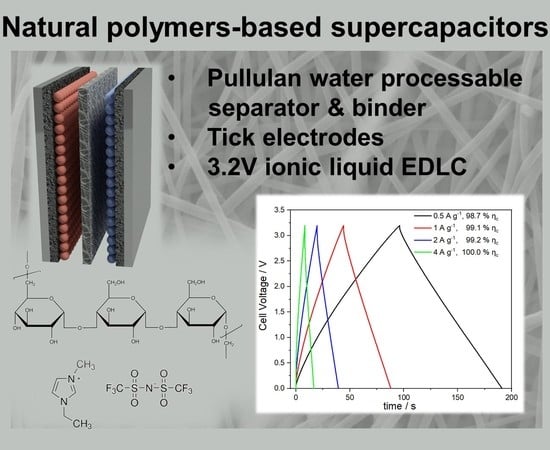
Natural Polymers for Green Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Electrospun Separator
2.3. Membrane Characterization
2.4. Preparation of the Electrodes
2.5. Supercapacitor Assembly
2.6. Supercapacitor Characterization
3. Results
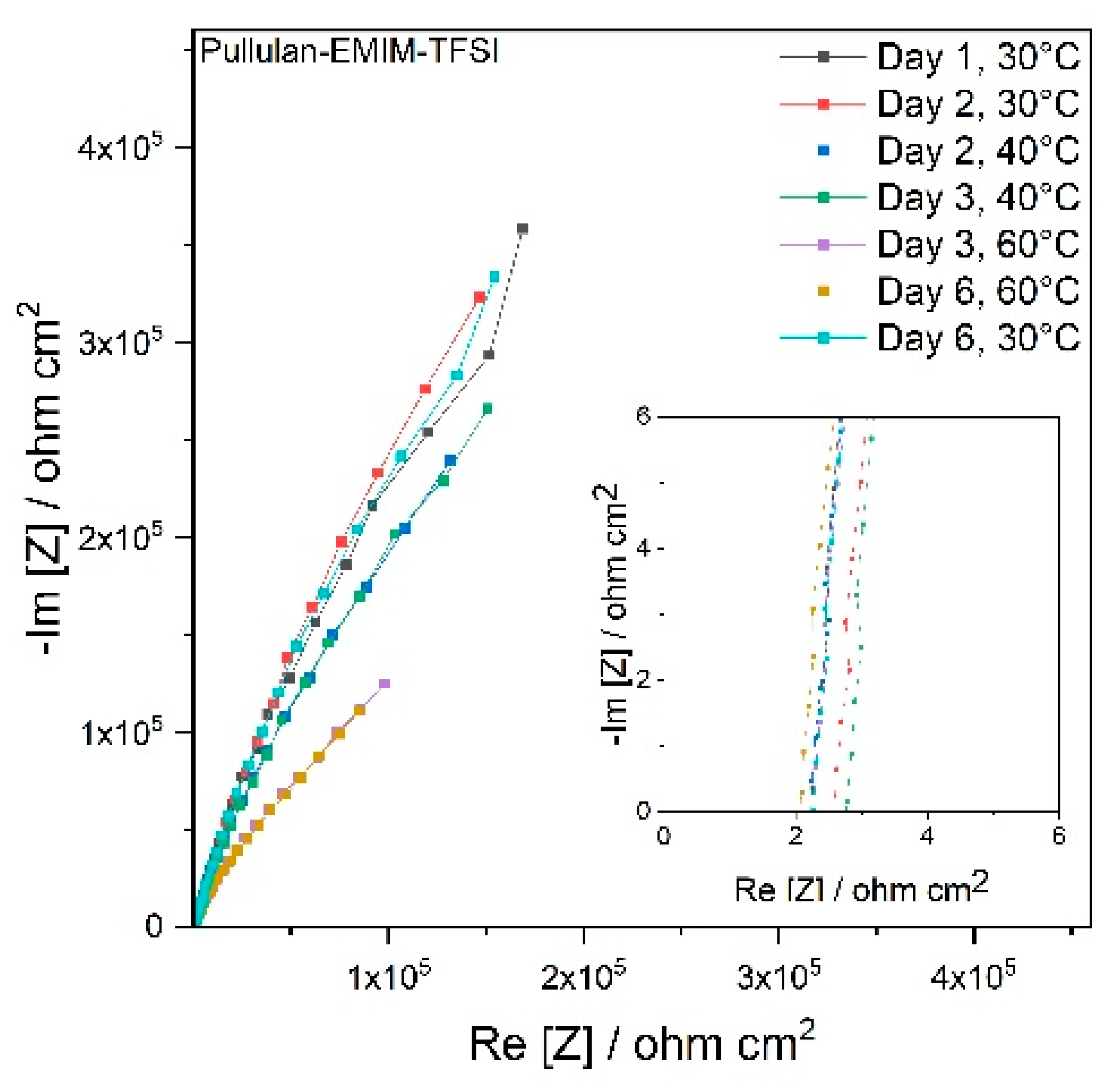
3.1. Electrospun Separator and Electrolyte Selection
3.2. Supercapacitor Testing
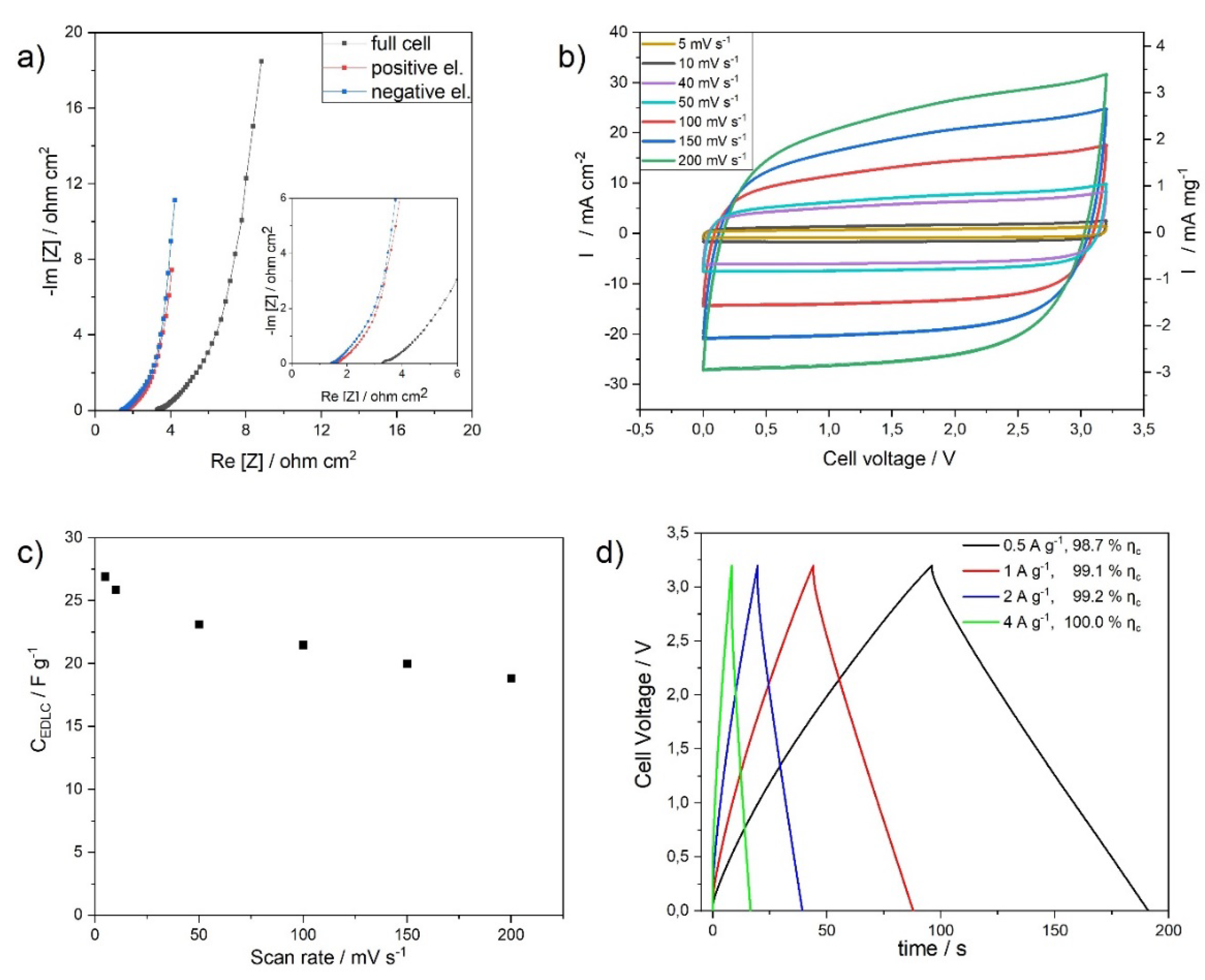
3.2.1. High Binder Low Mass Loading Electrodes (HBLME)
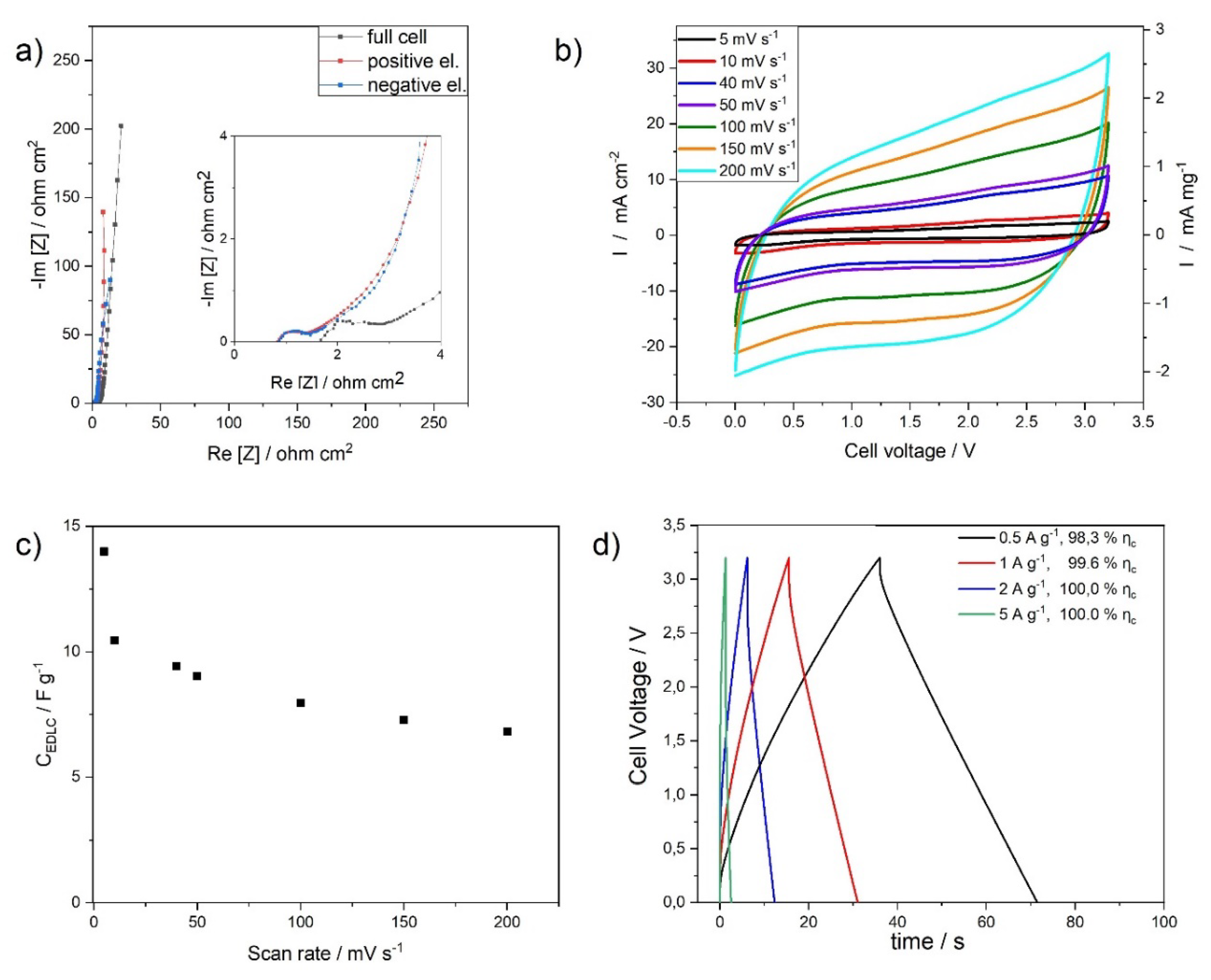
3.2.2. Lower Binder High Mass Loading Electrodes (LBHME)
3.2.3. Cycling Stability, Energy and Power of HBLME- and LBHME-EDLCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Z. Supercapacitor and supercapattery as emerging electrochemical energy stores. Int. Mater. Rev. 2017, 62, 173–202. [Google Scholar] [CrossRef] [Green Version]
- Siwach, P.; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Schütter, C.; Pohlmann, S.; Balducci, A. Industrial Requirements of Materials for Electrical Double Layer Capacitors: Impact on Current and Future Applications. Adv. Energy Mater. 2019, 9, 1900334. [Google Scholar] [CrossRef]
- DeVos, N.; Maton, C.; Stevens, C.v. Electrochemical Stability of Ionic Liquids: General Influences and Degradation Mechanisms. ChemElectroChem 2014, 1, 1258–1270. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Lazzari, M.; Mastragostino, M.; Soavi, F. Capacitance response of carbons in solvent-free ionic liquid electrolytes. Electrochem. Commun. 2007, 9, 1567–1572. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, M. A comprehensive review of lithium salts and beyond for rechargeable batteries: Progress and perspectives. Mater. Sci. Eng. R Rep. 2018, 134, 1–2110. [Google Scholar] [CrossRef]
- Ruschhaupt, P.; Varzi, A.; Passerini, S. Natural Polymers as Green Binders for High-Loading Supercapacitor Electrodes. ChemSusChem 2020, 13, 763–770. [Google Scholar] [CrossRef]
- Arbizzani, C.; Yu, Y.; Li, J.; Xiao, J.; Xia, Y.; Yang, Y.; Santato, C.; Raccichini, R.; Passerini, S. Good practice guide for papers on supercapacitors and related hybrid capacitors for the Journal of Power Sources. J. Power Sources 2020, 450, 227636. [Google Scholar] [CrossRef]
- Stojanovska, E.; Kilic, A. Carbon nanofibers as thick electrodes for aqueous supercapacitors. J. Energy Storage 2019, 26, 100981. [Google Scholar] [CrossRef]
- Krause, A.; Balducci, A. High voltage electrochemical double layer capacitor containing mixtures of ionic liquids and organic carbonate as electrolytes. Electrochem. Commun. 2011, 13, 814–817. [Google Scholar] [CrossRef]
- Krummacher, J.; Balducci, A. Al(TFSI)3 as a Conducting Salt for High-Voltage Electrochemical Double-Layer Capacitors. Chem. Mater. 2018, 30, 4857–4863. [Google Scholar] [CrossRef]
- Krummacher, J.; Hess, L.H.; Balducci, A. Al(TFSI) 3 in Acetonitrile as Electrolytes for Electrochemical Double Layer Capacitors. J. Electrochem. Soc. 2019, 166, A1763–A1768. [Google Scholar] [CrossRef]
- Lazzari, M.; Soavi, F.; Mastragostino, M. Mesoporous carbon design for ionic liquid-based, double-layer supercapacitors. Fuel Cells 2010, 10, 840–847. [Google Scholar] [CrossRef] [Green Version]
- Chmiola, J.; Largeot, C.; Taberna, P.L.; Simon, P.; Gogotsi, Y. Desolvation of ions in subnanometer pores and its effect on capacitance and double-layer theory. Angew. Chem. Int. Ed. 2008, 47, 3392–3395. [Google Scholar] [CrossRef]
- Varzi, A.; Passerini, S. Enabling high areal capacitance in electrochemical double layer capacitors by means of the environmentally friendly starch binder. J. Power Sources 2015, 300, 216–222. [Google Scholar] [CrossRef]
- Ahmed, S.; Nelson, P.A.; Gallagher, K.G.; Dees, D.W. Energy impact of cathode drying and solvent recovery during lithium-ion battery manufacturing. J. Power Sources 2016, 322, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Mauger, A.; Julien, C.; Paolella, A.; Armand, M.; Zaghib, K. Recent Progress on Organic Electrodes Materials for Rechargeable Batteries and Supercapacitors. Materials 2019, 12, 1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage-the transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef] [Green Version]
- Yamagata, M.; Ikebe, S.; Soeda, K.; Ishikawa, M. Ultrahigh-performance nonaqueous electric double-layer capacitors using an activated carbon composite electrode with alginate. RSC Adv. 2013, 3, 1037–1040. [Google Scholar] [CrossRef]
- Böckenfeld, N.; Jeong, S.S.; Winter, M.; Passerini, S.; Balducci, A. Natural, cheap and environmentally friendly binder for supercapacitors. J. Power Sources 2013, 221, 14–20. [Google Scholar] [CrossRef]
- Dühnen, S.; Betz, J.; Kolek, M.; Schmuch, R.; Winter, M.; Placket, T. Toward Green Battery Cells: Perspective on Materials and Technologies. Small Methods 2020, 2000039. [Google Scholar] [CrossRef]
- Bonnefoi, L.; Simon, P.; Fauvarque, J.F.; Sarrazin, C.; Sarrau, J.F.; Dugast, A. Electrode compositions for carbon power supercapacitors. J. Power Sources 1999, 80, 149–155. [Google Scholar] [CrossRef]
- Phillips, D.M.; Drummy, L.F.; Conrady, D.G.; Fox, D.M.; Naik, R.R.; Stone, M.O.; Trulove, P.C.; de Long, H.C.; Mantz, R.A. Dissolution and regeneration of Bombyx mori silk fibroin using ionic liquids. J. Am. Chem. Soc. 2004, 126, 14350–14351. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Recham, N.; Armand, M.; Tarascon, J.M. Novel low temperature approaches for the eco-efficient synthesis of electrode materials for secondary Li-ion batteries. Comptes Rendus Chim. 2010, 13, 106–116. [Google Scholar] [CrossRef]
- Heinze, T.; Dorn, S.; Schöbitz, M.; Liebert, T.; Köhler, S.; Meister, F. Interactions of Ionic Liquids with Polysaccharides—2: Cellulose. Macromol. Symp. 2008, 262, 8–22. [Google Scholar] [CrossRef]
- Varzi, A.; Balducci, A.; Passerini, S. Natural Cellulose: A Green Alternative Binder for High Voltage Electrochemical Double Layer Capacitors Containing Ionic Liquid-Based Electrolytes. J. Electrochem. Soc. 2014, 161, A368–A375. [Google Scholar] [CrossRef]
- Poli, F.; Momodu, D.; Spina, G.E.; Terella, A.; Mutuma, B.K.; Focarete, M.L.; Manyala, N.; Soavi, F. Pullulan-ionic liquid-based supercapacitor: A novel, smart combination of components for an easy-to-dispose device. Electrochim. Acta 2020, 338, 135872. [Google Scholar] [CrossRef]
- Arbizzani, C.; Lazzari, M.; Soavi, F.; Mastragostino, M.; Conte, M. ILHYPOS Ionic Liquid-Based Supercapacitors. ECS Trans. 2010, 25, 25–30. [Google Scholar]
- Li, Y.; Li, Q.; Tan, Z. A review of electrospun nanofiber-based separators for rechargeable lithium-ion batteries. J. Power Sources 2019, 443, 227262. [Google Scholar] [CrossRef]
- Yoo, H.D.; Jang, J.H.; Ryu, J.H.; Park, Y.; Oh, S.M. Impedance analysis of porous carbon electrodes to predict rate capability of electric double-layer capacitors. J. Power Sources 2014, 267, 411–420. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]



| Name | Composition | Mass Loading Range * |
|---|---|---|
| HBLME | 70% BP10/10% CB/20% binder | 3.6–4.6 mg cm−2 |
| LBHME | 85% BP10/5% CB/10% binder | 6.3–7.5 mg cm−2 |
| Conductivity (mS cm−1) | σ (30°) | σ (40°) | σ (60°) |
|---|---|---|---|
| EmimTFSI | 12.60 | 15.10 | 25.70 |
| 0.5 m LiTFSI in TEGDME | 2.05 | 2.63 | 4.82 |
| PYR14 TFSI | 3.01 | 3.90 | 6.30 |
| Electrode Label | HBLME-EDLC | LBHME-EDLC |
|---|---|---|
| Mass loading (mg cm−2) | 9.3 | 13.8 |
| ESR (ohm) | 5.9 | 7.9 |
| Capacitance * (F g−1) | 15.9 | 6.2 |
| Areal capacitance (mF cm−2) | 148.0 | 85.5 |
| Specific energy ** (Wh kg−1) | 19.6 | 7.2 |
| Specific power *** (kW kg−1) | 4.6 | 3.7 |
| Areal energy density (µWh cm−2) | 182.3 | 99.4 |
| Areal power density (mW cm−2) | 42.8 | 51.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spina, G.E.; Poli, F.; Brilloni, A.; Marchese, D.; Soavi, F. Natural Polymers for Green Supercapacitors. Energies 2020, 13, 3115. https://doi.org/10.3390/en13123115
Spina GE, Poli F, Brilloni A, Marchese D, Soavi F. Natural Polymers for Green Supercapacitors. Energies. 2020; 13(12):3115. https://doi.org/10.3390/en13123115
Chicago/Turabian StyleSpina, Giovanni Emanuele, Federico Poli, Alessandro Brilloni, Daniele Marchese, and Francesca Soavi. 2020. "Natural Polymers for Green Supercapacitors" Energies 13, no. 12: 3115. https://doi.org/10.3390/en13123115