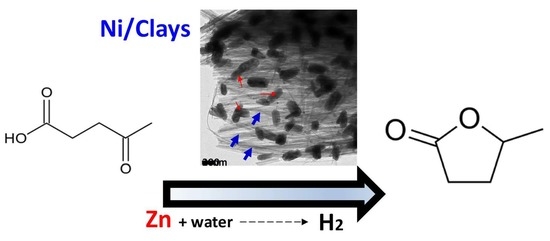
Ni Supported on Natural Clays as a Catalyst for the Transformation of Levulinic Acid into γ-Valerolactone without the Addition of Molecular Hydrogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.2. Characterization Techniques
2.3. Catalytic Tests and Analyses
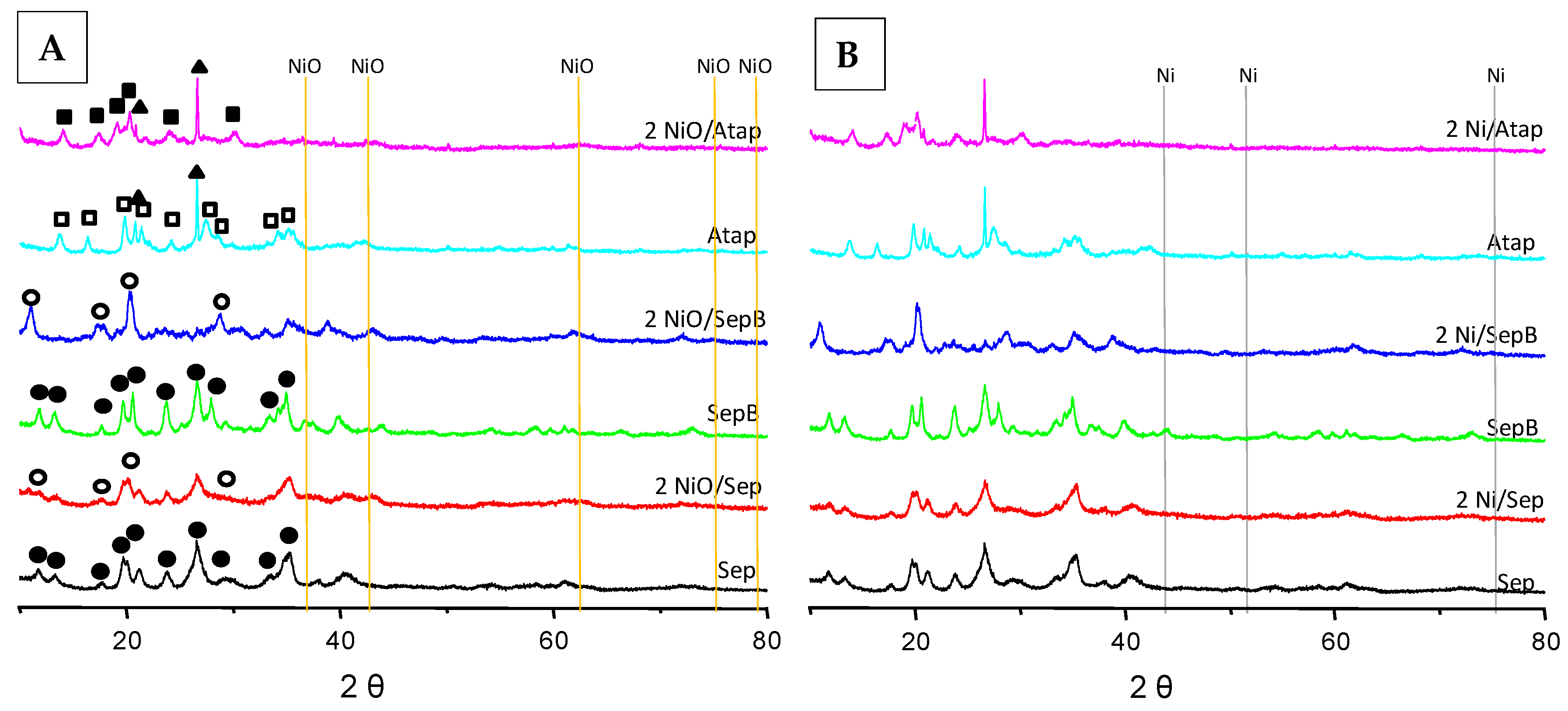
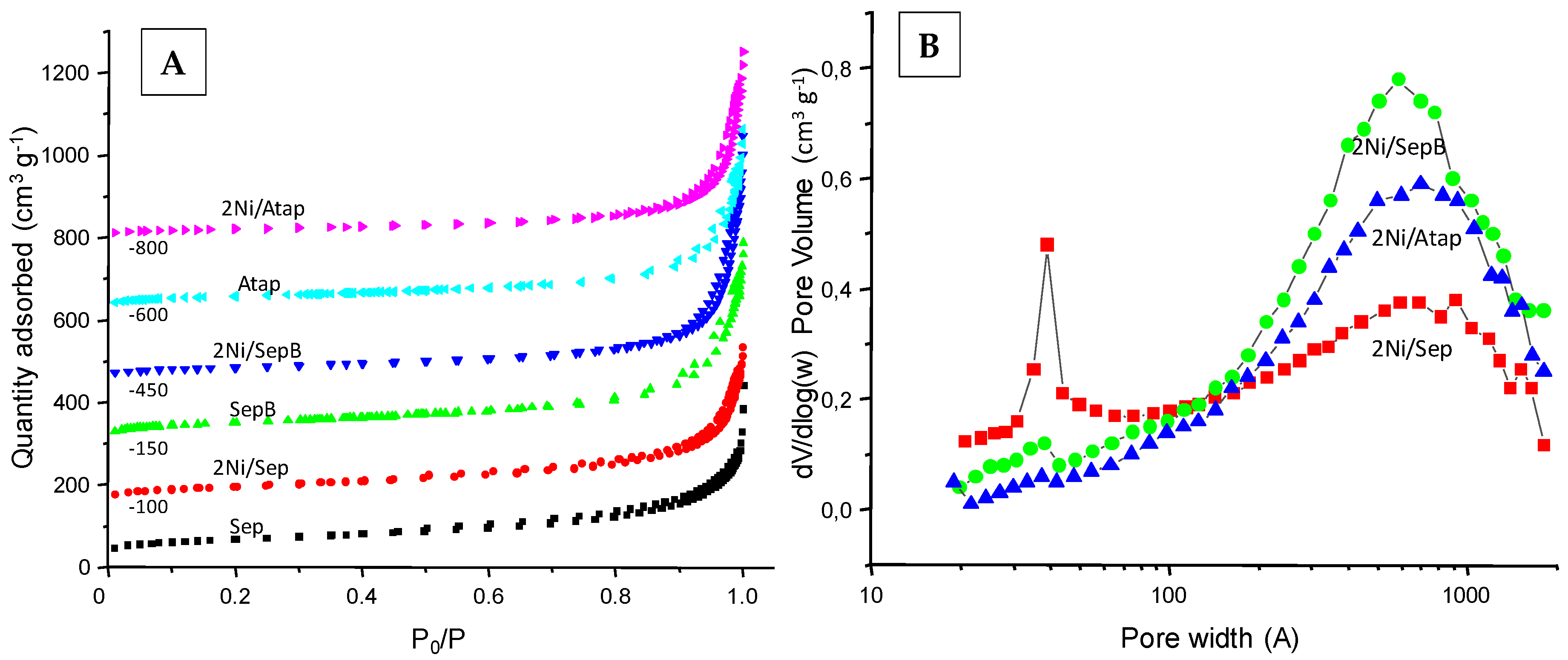
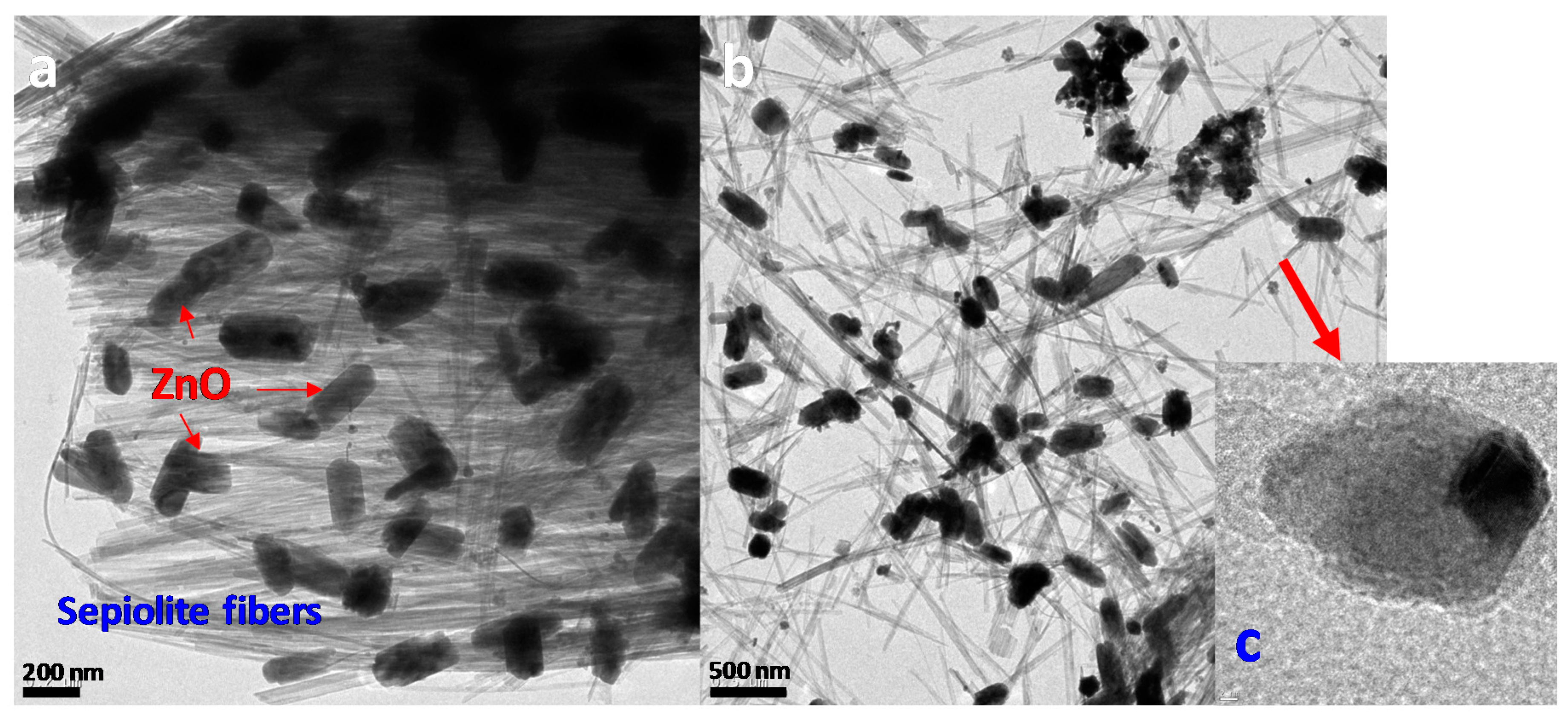
3. Results
Characterization Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mika, L.; Cséfalvay, E.; Németh, Á. Catalytic conversion of carbohydrates to initial platform chemicals: Chemistry and sustainability. Chem. Rev. 2017, 118, 505–613. [Google Scholar] [CrossRef]
- Escobar, J.; Lora, E.; Venturini, O.; Yáñez, E.; Castillo, E.; Almazan, O. Biofuels: Environment, technology and food security. Renew. Sustain. Energy Rev. 2009, 13, 1275–1287. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.; Sepulveda-Escribano, A. Transformations of biomass-derived platform molecules: From high added-value chemicals to fuels via aqueous-phase processing. Chem. Soc. Rev. 2011, 43, 5266–5281. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, J.; Lai, D.; Fu, Y.; Guo, Q. Catalytic conversion of biomass-derived carbohydrates into γ-valerolactone without using an external H2 supply. Angew. Chem. Int. Ed. 2009, 121, 6651–6654. [Google Scholar] [CrossRef]
- Alonso, D.; Wettstein, S.; Dumesic, J. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 2013, 15, 584–595. [Google Scholar] [CrossRef]
- Orlowski, I.; Douthwaite, M.; Iqbal, S.; Hayward, J.; Davies, T.; Bartley, J.; Miedziak, P.; Hirayama, J.; Morgan, D.; Willock, D.; et al. The hydrogenation of levulinic acid to γ-valerolactone over Cu–ZrO2 catalysts prepared by a pH-gradient methodology. J. Energy Chem. 2019, 36, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Gelosia, M.; Ingles, D.; Pompili, E.; D’Antonio, S.; Cavalaglio, G.; Petrozzi, A.; Coccia, V. Fractionation of Lignocellulosic Residues Coupling Steam Explosion and Organosolv Treatments Using Green Solvent γ-Valerolactone. Energies 2017, 10, 1264. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Horváth, I. Catalytic conversion of fructose to γ-valerolactone in γ-valerolactone. Acs Catal. 2012, 2, 2247–2249. [Google Scholar] [CrossRef]
- Zhang, Z. Synthesis of γ-valerolactone from carbohydrates and its applications. Chem. Sus. Chem. 2016, 9, 156–171. [Google Scholar] [CrossRef]
- Horváth, I.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L. γ-Valerolactone—a sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Dutta, S.; Yu, I.K.M.; Tsang, D.C.W.; Ng, Y.H.; Ok, Y.S.; Sherwood, J.; Clark, J.H. Green synthesis of gamma-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts: A critical review. Chem. Eng. J. 2019, 372, 992–1006. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical and less classical solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Bereczky, A.; Lukács, K.; Farkas, M.; Dóbé, S. Effect of γ-Valerolactone Blending on Engine Performance, Combustion Characteristics and Exhaust Emissions in a Diesel Engine. Nat. Resour. 2014, 5, 177–191. [Google Scholar]
- Fábos, V.; Lui, M.Y.; Mui, Y.F.; Wong, Y.Y.; Mika, L.T.; Qi, L.; Cséfalvay, E.; Kovács, V.; Szűcs, T.; Horváth, I.T. Use of Gamma-Valerolactone as an Illuminating Liquid and Lighter Fluid. Acs Sust. Chem. Eng. 2015, 3, 1899–1904. [Google Scholar] [CrossRef] [Green Version]
- Tuck, C.O.; Pérez, E.; Horváth2, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Lange, J.P.; Price, R.; Ayoub, P.M.; Louis, J.; Petrus, L.; Clarke, L.; Gosselink, H. Valeric biofuels: A platform of cellulosic transportation fuels. Angew. Chem. Int. Edit. 2010, 49, 4479–4483. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Agulhon, P.; Grams, J.; Wąchała, M.; Wojciechowska, J.; Świerczyński, D.; Cacciaguerra, T.; Tanchoux, N.; Quignard, F. Synthesis of TiO2–ZrO2 Mixed Oxides via the Alginate Route: Application in the Ru Catalytic Hydrogenation of Levulinic Acid to Gamma-Valerolactone. Energies 2019, 12, 4706. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Wang, Y.; Delbecq, F.; Len, C. Continuous flow conversion of alkyl levulinates into γ-valerolactone in the presence of Ru/C as catalyst. Mol. Catal. 2019, 475, 110456. [Google Scholar] [CrossRef]
- Van Nguyen, C.; Matsagar, B.; Yeh, J.; Chiang, W.; Wu, K. MIL-53-NH2-derived carbon-Al2O3 composites supported Ru catalyst for effective hydrogenation of levulinic acid to γ-valerolactone under ambient conditions. Mol. Catal. 2019, 475, 110478. [Google Scholar] [CrossRef]
- Xiao, C.; Goh, T.; Qi, Z.; Goes, S.; Brashler, K.; Perez, C.; Huang, W. Conversion of levulinic acid to γ-valerolactone over few-layer graphene-supported ruthenium catalysts. Acs Catal. 2015, 6, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Sanchis, R.; García, T.; Dejoz, A.; Vázquez, I.; Llopis, F.; Solsona, B. Easy method for the transformation of levulinic acid into gamma-valerolactone using a nickel catalyst derived from nanocasted nickel oxide. Materials 2019, 12, 2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, M.; Zhang, C.; Liu, C.; Yang, R.; Dong, W. Construction of mesoporous Cu/ZrO2-Al2O3 as a ternary catalyst for efficient synthesis of γ-valerolactone from levulinic acid at low temperature. J. Catal. 2020, 381, 163–174. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, B.; Wu, H.; Liu, M.; Liu, H.; Zhang, J.; Yang, G.; Han, B. Highly efficient hydrogenation of levulinic acid into 2-methyltetrahydrofuran over Ni–Cu/Al2O3–ZrO2 bifunctional catalysts. Green Chem. 2019, 21, 606–613. [Google Scholar] [CrossRef]
- Xu, Q.; Li, X.; Pan, T.; Yu, C.; Deng, J.; Guo, Q.; Fu, Y. Supported copper catalysts for highly efficient hydrogenation of biomass-derived levulinic acid and γ-valerolactone. Green Chem. 2016, 18, 1287–1294. [Google Scholar] [CrossRef]
- Yuan, J.; Li, S.; Yu, L.; Liu, Y.; Cao, Y.; He, H.; Fan, K. Copper-based catalysts for the efficient conversion of carbohydrate biomass into γ-valerolactone in the absence of externally added hydrogen. Energy Environ. Sci. 2013, 6, 3308–3313. [Google Scholar] [CrossRef]
- Tanwongwan, W.; Eiad-ua, A.; Kraithong, W.; Viriya-empikul, N.; Suttisintong, K.; Klamchuen, A.; Kasamechonchung, P.; Khemthong, P.; Faungnawakij, K.; Kuboon, S. Simultaneous activation of copper mixed metal oxide catalysts in alcohols for gamma-valerolactone production from methyl levulinate. Appl. Catal. A Gen. 2019, 579, 91–98. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Y.; Guo, Q.; Fu, Y. RANEY ® Ni catalyzed transfer hydrogenation of levulinate esters to γ-valerolactone at room temperature. Chem. Commun. 2013, 49, 5328–5330. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, K.; Alshammari, A.; Sohail, M.; Jagadeesh, R. Levulinic acid derived reusable cobalt-nanoparticles-catalyzed sustainable synthesis of γ-valerolactone. Acs. Sustain. Chem. Eng. 2019, 7, 14756–14764. [Google Scholar] [CrossRef]
- Fang, C.; Kuboon, S.; Khemthong, P.; Butburee, T.; Chakthranont, P.; Itthibenchapong, V.; Kasamechonchung, P.; Witoon, T.; Faungnawakij, K. Highly dispersed Ni Cu nanoparticles on SBA-15 for selective hydrogenation of methyl levulinate to γ-valerolactone. Int. J. Hydrog. Energ. 2019, 3, 272–283. [Google Scholar] [CrossRef]
- Li, C.; Ni, X.; Di, X.; Liang, C. Aqueous phase hydrogenation of levulinic acid to γ-valerolactone on supported Ru catalysts prepared by microwave-assisted thermolytic method. J. Fuel Chem. Technol. 2018, 46, 161–170. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Li, J.; Li, M.; Liu, C.; Yang, R.; Dong, W. Highly selective synthesis of γ-valerolactone from levulinic acid at mild conditions catalyzed by boron oxide doped Cu/ZrO2 catalysts. Appl. Catal. A Gen. 2019, 587, 117244. [Google Scholar] [CrossRef]
- Jones, D.; Iqbal, S.; Ishikawa, S.; Reece, C.; Thomas, L.; Miedziak, P.; Morgan, D.; Edwards, J.; Bartley, J.; Willock, D.; et al. The conversion of levulinic acid into γ-valerolactone using Cu–ZrO2 catalysts. Catal. Sci. Technol. 2016, 6, 6022. [Google Scholar] [CrossRef]
- Guerrero-Torres, A.; Jiménez-Gómez, C.; Cecilia, J.; García-Sancho, C.; Franco, F.; Quirante-Sánchez, J.; Maireles-Torres, P. Ni supported on sepiolite catalysts for the hydrogenation of furfural to value-added chemicals: Influence of the synthesis method on the catalytic performance. Top. Catal. 2019, 62, 535–550. [Google Scholar] [CrossRef]
- Jing, Y.; Guo, Y.; Xia, Q.; Liu, X.; Wang, Y. Catalytic production of value-added chemicals and liquid fuels from lignocellulosic biomass. Chemicals 2019, 5, 2520–2546. [Google Scholar] [CrossRef]
- Upare, P.; Lee, J.; Hwang, D.; Halligudi, S.; Hwang, Y.; Chang, J. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Putrakumar, B.; Nagaraju, N.; Kumar, V.; Chary, K. Hydrogenation of levulinic acid to γ-valerolactone over copper catalysts supported on γ-Al2O3. Catal. Today 2015, 250, 209–217. [Google Scholar] [CrossRef]
- Manzer, L. Catalytic synthesis of α-methylene-γ-valerolactone: A biomass-derived acrylic monomer. Appl. Catal. A Gen. 2004, 272, 249–256. [Google Scholar] [CrossRef]
- Lange, J.; Vestering, J.; Haan, R. Towards ‘bio-based’ nylon: Conversion of γ-valerolactone to methyl pentenoate under catalytic distillation conditions. Chem. Commun. 2007, 33, 3488–3490. [Google Scholar] [CrossRef]
- Feng, J.; Gu, X.; Xue, Y.; Han, Y.; Lu, X. Production of γ-valerolactone from levulinic acid over a Ru/C catalyst using formic acid as the sole hydrogen source. Sci. Total Environ. 2018, 633, 426–432. [Google Scholar] [CrossRef]
- Du, X.; He, L.; Zhao, S.; Liu, Y.; Cao, Y.; He, H.; Fan, K. Hydrogen-independent reductive transformation of carbohydrate biomass into γ-valerolactone and pyrrolidone derivatives with supported gold catalysts. Angew. Chem. Int. Ed. 2011, 50, 7815–7819. [Google Scholar] [CrossRef]
- Son, P.; Nishimura, S.; Ebitani, K. Production of γ-valerolactone from biomass-derived compounds using formic acid as a hydrogen source over supported metal catalysts in water solvent. Rsc Adv. 2014, 4, 10525–10530. [Google Scholar] [CrossRef]
- Damma, D.; Smirniotis, P. Recent advances in iron-based high-temperature water-gas shift catalysis for hydrogen production. Curr. Opin. Chem. Eng. 2018, 21, 103–110. [Google Scholar] [CrossRef]
- Mastuli, M.; Kamarulzaman, N.; Kasim, M.; Sivasangar, S.; Saiman, M.; Taufiq-Yap, Y. Catalytic gasification of oil palm frond biomass in supercritical water using MgO supported Ni, Cu and Zn oxides as catalysts for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 11215–11228. [Google Scholar] [CrossRef]
- Jin, F.; Zeng, X.; Liu, J.; Jin, Y.; Wang, L.; Zhong, H.; Yao, G.; Huo, Z. Highly efficient and autocatalytic H2O dissociation for CO2 reduction into formic acid with zinc. Sci. Rep. 2015, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Li, Q.; Liu, J.; Yao, G.; Wang, J.; Zeng, X.; Huo, Z.; Jin, F. New method for highly efficient conversion of biomass-derived levulinic acid to γ-valerolactone in water without precious metal catalysts. Acs Sustain. Chem. Eng. 2017, 5, 6517–6523. [Google Scholar] [CrossRef]
- Steinfeld, A.; Brack, M.; Meier, A.; Weidenkaff, A.; Wuillemin, D. A solar chemical reactor for co-production of zinc and synthesis gas. Energy 1998, 23, 803–814. [Google Scholar] [CrossRef]
- Osinga, T.; Olalde, G.; Steinfeld, A. Solar carbothermal reduction of ZnO: Shrinking packed-bed reactor modeling and experimental validation. Ind. Eng. Chem. Res. 2004, 43, 7981–7988. [Google Scholar] [CrossRef]
- Haueter, P.; Moeller, S.; Palumbo, R.; Steinfeld, A. The production of zinc by thermal dissociation of zinc oxide—Solar chemical reactor design. J. Sol. Energy. 1999, 67, 161–167. [Google Scholar] [CrossRef]
- Yadav, D.; Banerjee, R. A comparative life cycle energy and carbon emission analysis of the solar carbothermal and hydrometallurgy routes for zinc production. Appl. Energy. 2018, 229, 577–602. [Google Scholar] [CrossRef]
- Sun, M.; Xia, J.; Wang, H.; Liu, X.; Xia, Q.; Wang, Y. An efficient NixZryO catalyst for hydrogenation of bio-derived methyl levulinate to γ-valerolactone in water under low hydrogen pressure. Appl. Catal. B Environ. 2018, 227, 488–498. [Google Scholar] [CrossRef]
- Geboers, J.; Wang, X.; de Carvalho, A.B.; Rinaldi, R. Densification of biorefinery schemes by H-transfer with Raney Ni and 2-propanol: A case study of a potential avenue for valorization of alkyl levulinates to alkyl γ-hydroxypentanoates and γ-valerolactone. J. Mol. Catal. A Chem. 2014, 388–389, 106–115. [Google Scholar] [CrossRef]
- Sakakibara, K.; Endo, K.; Osawa, T. Facile synthesis of γ-valerolactone by transfer hydrogenation of methyl levulinate and levulinic acid over Ni/ZrO2. Catal. Commun. 2019, 125, 52–55. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, G.; Zhang, X.; Li, F. Facile Fabrication of Composition-Tuned Ru–Ni Bimetallics in Ordered Mesoporous Carbon for Levulinic Acid Hydrogenation. ACS Catal. 2014, 4, 1419–1425. [Google Scholar] [CrossRef]
- Song, S.; Yao, S.; Cao, J.; Di, L.; Wu, G.; Guan, N.; Li, L. Heterostructured Ni/NiO composite as a robust catalyst for the hydrogenation of levulinic acid to γ-valerolactone. Appl. Catal. B Environ. 2017, 217, 115–124. [Google Scholar] [CrossRef]
- Jiang, K.; Sheng, D.; Zhang, Z.; Fu, J.; Hou, Z.; Lu, X. Hydrogenation of levulinic acid to γ-valerolactone in dioxane over mixed MgO–Al2O3 supported Ni catalyst. Catal Today. 2016, 274, 55–59. [Google Scholar] [CrossRef]
- Gundekari, S.; Srinivasan, K. In situ generated Ni(0)@boehmite from NiAl-LDH: An efficient catalyst for selective hydrogenation of biomass derived levulinic acid to γ-valerolactone. Catal. Commun. 2017, 102, 40–43. [Google Scholar] [CrossRef]
- Yi, Z.; Hu, D.; Xu, H.; Wu, Z.; Zhang, M.; Yan, K. Metal regulating the highly selective synthesis of gamma-valerolactone and valeric biofuels from biomass-derived levulinic acid. Fuel 2020, 259, 116208. [Google Scholar] [CrossRef]
- Upare, P.P.; Jeong, M.; Hwang, Y.K.; Kim, D.H.; Kim, Y.D.; Hwang, D.W.; Lee, U.-H.; Chang, J. Nickel-promoted copper–silica nanocomposite catalysts for hydrogenation of levulinic acid to lactones using formic acid as a hydrogen feeder. Appl. Catal. A Gen. 2015, 491, 127–135. [Google Scholar] [CrossRef]
- Peddakasu, G.B.; Velisoju, V.K.; Kandula, M.; Gutta, N.; VR Chary, K.; Akula, V. Role of group V elements on the hydrogenation activity of Ni/TiO2 catalyst for the vapour phase conversion of levulinic acid to γ-valerolactone. Catal. Today 2019, 325, 68–72. [Google Scholar] [CrossRef]
- Gupta, S.S.R.; Kantam, M.L. Selective hydrogenation of levulinic acid into γ-valerolactone over Cu/Ni hydrotalcite-derived catalyst. Catal. Today 2018, 309, 189–194. [Google Scholar] [CrossRef]
- Sun, D.; Ohkubo, A.; Asami, K.; Katori, T.; Yamada, Y.; Sato, S. Vapor-phase hydrogenation of levulinic acid and methyl levulinate to γ-valerolactone over non-noble metal-based catalysts. Mol. Catal. 2017, 437, 105–113. [Google Scholar] [CrossRef]
- Hengst, K.; Schubert, M.; Carvalho, H.W.P.; Lu, C.; Kleist, W.; Grunwaldt, J. Synthesis of γ-valerolactone by hydrogenation of levulinic acid over supported nickel catalysts. Appl. Catal. A Gen. 2015, 502, 18–26. [Google Scholar] [CrossRef]
- Popova, M.; Djinović, P.; Ristić, A.; Lazarova, H.; Dražić, G.; Pintar, A.; Balu, A.M.; Novak Tušar, N. Vapor-Phase Hydrogenation of Levulinic Acid to γ-Valerolactone Over Bi-Functional Ni/HZSM-5 Catalyst. Front. Chem. 2018, 6, 285. [Google Scholar] [CrossRef] [PubMed]
- Gundekari, S.; Gundekari, S.; Srinivasan, K.; Srinivasan, K. Screening of Solvents, Hydrogen Source, and Investigation of Reaction Mechanism for the Hydrocyclisation of Levulinic Acid to γ-Valerolactone Using Ni/SiO2–Al2O3 Catalyst. Catal. Lett. 2019, 149, 215–227. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, X.; Liu, C.; Han, Y.; Ji, N. Synthesis of γ-valerolactone from different biomass-derived feedstocks: Recent advances on reaction mechanisms and catalytic systems. Renew. Sustain. Energy Rev. 2019, 112, 140–157. [Google Scholar] [CrossRef]
- Solsona, B.; López Nieto, J.M.; Agouram, S.; Soriano, M.D.; Dejoz, A.; Vázquez, M.I.; Concepción, P. Optimizing Both Catalyst Preparation and Catalytic Behaviour for the Oxidative Dehydrogenation of Ethane of Ni–Sn–O Catalysts. Top. Catal. 2016, 59, 1564–1572. [Google Scholar] [CrossRef]
- Solsona, B.; Concepción, P.; Demicol, B.; Hernández, S.; Delgado, J.J.; Calvino, J.J.; López Nieto, J.M. Selective oxidative dehydrogenation of ethane over SnO2-promoted NiO catalysts. J. Catal. 2012, 295, 104–114. [Google Scholar] [CrossRef]
- Post, J.E.; Bish, D.L.; Heaney, P.J. Synchrotron powder X-ray diffraction study of the structure and dehydration behavior of sepiolite. Am. Mineral. 2007, 92, 91–97. [Google Scholar] [CrossRef]
- Preisinger, A.A. Sepiolite and related compounds: Its stability and application. Clays Clay Miner. 1961, 10, 365–371. [Google Scholar] [CrossRef]
- Ruiz, R.; Del Moral, J.C.; Pesquera, C.; Benito, I.; González, F. Reversible folding in sepiolite: Study by thermal and textural análisis. Thermochim. Acta 1996, 279, 103–110. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Wang, F.; Tang, M.; Liang, J.; Ren, C. Study on pore distribution and formation rule of sepiolite mineral nanomaterials. J. Nanomater. 2012, 2012, 382603. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Liu, Y.; Lu, Y.; Chai, J.; Sun, L. Catalytic application of layered double hydroxide-derived catalysts for the conversion of biomass-derived molecules. Catal. Sci. Technol. 2017, 7, 1622–1645. [Google Scholar] [CrossRef]
- Li, W.; Fan, G.; Yang, L.; Li, F. Highly Efficient Vapor-Phase Hydrogenation of Biomass-Derived Levulinic Acid Over Structured Nanowall-Like Nickel-Based Catalyst. Chem. Cat. Chem. 2016, 8, 2724–2733. [Google Scholar] [CrossRef]







| Catalyst | Substrate | Solvent | H2 Source | T (°C) | Time (h) | GVL Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Ni-Zr-O | ML | Water | Molecular H2 | 200 | 3 | 98 | [50] |
| 170 | 3 | 97 | |||||
| 150 | 3 | 95 | |||||
| Ni-Cu/SBA-15 | ML | 2-propanol | 2-propanol | 170 | 3 | 87 | [30] |
| RANEY Ni | ML | 2-propanol | 2-propanol | 120 | 1 | 94 | [51] |
| RANEY Ni | EL | 2-Propanol | 2-propanol | 80 | 2 | 99 | [28] |
| Ni/ZrO2 | ML | 2-propanol | 2-propanol | 100 | 20 | 94 | [52] |
| LA | 2-propanol | 2-propanol | 120 | 20 | 86 | ||
| Ru−Ni/Meso-C | LA | H2 | Molecular H2 | 150 | 2 | 96 | [53] |
| Ni/NiO | LA | Dioxane | Molecular H2 | 120 | 4,5 | 99 | [54] |
| Ni/Mg2Al2O5 | LA | Dioxane | Molecular H2 | 160 | 1 | 99 | [55] |
| Ni/Al-LDH | LA | Water | Molecular H2 | 200 | 6 | 100 | [56] |
| Ni/HZSM-5 | LA | Dioxane | Molecular H2 | 220 | 10 | 93 | [57] |
| Ni-Cu/SiO2 | LA | N2 | Formic acid | 285 | - | 98 | [58] |
| 10NiNb/TiO2 | LA | Water | Molecular H2 | 275 | - | 25 | [59] |
| Cu/Ni/Mg/Al | LA | Dioxane | Molecular H2 | 140 | 3 | 100 | [60] |
| Ni/SiO2 | LA | H2 | Molecular H2 | 250 | 0.3 | 89 | [61] |
| Ni/Al2O3 | LA | H2 | Molecular H2 | 200 | 4 | 92 | [62] |
| Ni/HZSM-5 | LA | H2 | Molecular H2 | 320 | 0.5 | 99 | [63] |
| Ni/SiO2–Al2O3 | LA | THF | Molecular H2 | 200 | 0.5 | 100 | [64] |
| Isopropyl alcohol | Isopropyl alcohol | 0.25 | 99 | ||||
| Water | Formic acid | 10 | 70 |
| Sample | SBET (m2·g−1) | VT (cm3·g−1) |
|---|---|---|
| Sep | 242 | 0.392 |
| 2Ni/Sep | 202 | 0.447 |
| SepB | 381 | 0.619 |
| 2Ni/SepB | 121 | 0.579 |
| Atap | 216 | 0.499 |
| 2Ni/Atap | 75 | 0.440 |
| Additional | GVL Yields | molGVL kg−1catalyst h−1 | molGVL kg−1Ni h−1 | ||||
|---|---|---|---|---|---|---|---|
| Catalyst | H2 Source | 180 °C | 120 °C | 180 °C | 120 °C | 180 °C | 120 °C |
| 2 Ni/Sep | No | 14.6 | 2.31 | 0.510 | 0.082 | 25.8 | 4.08 |
| 2 Ni/SepB | No | 12.0 | 5.05 | 0.428 | 0.180 | 21.3 | 8.93 |
| 2 Ni/Atap | No | 12.9 | 1.01 | 0.451 | 0.040 | 22.7 | 1.77 |
| 2 Ni/Sep | Zn added | 85.2 | 72.6 | 3.01 | 2.57 | 151 | 128 |
| 2 Ni/SepB | Zn added | >98 | 82.0 | 3.54 | 2.90 | 177 | 145 |
| 2 Ni/Atap | Zn added | >98 | 82.7 | 3.54 | 2.92 | 177 | 146 |
| 2 Ni/Sep | Formic acid | 5.39 | 6.50 | 0.187 | 0.233 | 9.52 | 11.5 |
| 2 Ni/SepB | Formic acid | 3.02 | 4.54 | 0.111 | 0.156 | 5.41 | 8.02 |
| 2 Ni/Atap | Formic acid | 4.02 | 6.26 | 0.142 | 0.219 | 7.09 | 11.1 |
| Experiment/Sample | Reaction Temperature (°C) | ZnO/Zn | Zn2SiO4/Zn |
|---|---|---|---|
| Zn alone | 180 | 0.71 | - |
| 2Ni/SepB + Zn | 120 | 98 | 0 |
| 2Ni/SepB + Zn | 180 | 8.9 | 4.0 |
| 2Ni/Atap + Zn | 120 | 5.2 | 2.2 |
| 2Ni/Atap + Zn | 180 | 1.5 | 3.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, A.; Sanchis, R.; Llopis, F.J.; Vázquez, I.; Pico, M.P.; López, M.L.; Álvarez-Serrano, I.; Solsona, B. Ni Supported on Natural Clays as a Catalyst for the Transformation of Levulinic Acid into γ-Valerolactone without the Addition of Molecular Hydrogen. Energies 2020, 13, 3448. https://doi.org/10.3390/en13133448
García A, Sanchis R, Llopis FJ, Vázquez I, Pico MP, López ML, Álvarez-Serrano I, Solsona B. Ni Supported on Natural Clays as a Catalyst for the Transformation of Levulinic Acid into γ-Valerolactone without the Addition of Molecular Hydrogen. Energies. 2020; 13(13):3448. https://doi.org/10.3390/en13133448
Chicago/Turabian StyleGarcía, Adrián, Rut Sanchis, Francisco J. Llopis, Isabel Vázquez, María Pilar Pico, María Luisa López, Inmaculada Álvarez-Serrano, and Benjamín Solsona. 2020. "Ni Supported on Natural Clays as a Catalyst for the Transformation of Levulinic Acid into γ-Valerolactone without the Addition of Molecular Hydrogen" Energies 13, no. 13: 3448. https://doi.org/10.3390/en13133448