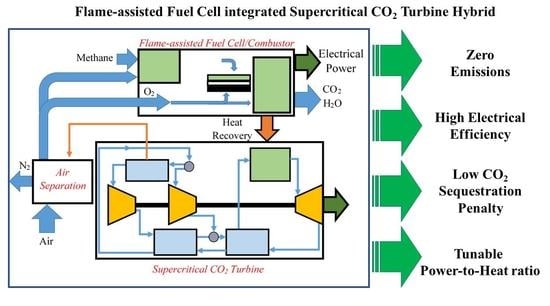
Hybrid Fuel Cell—Supercritical CO2 Brayton Cycle for CO2 Sequestration-Ready Combined Heat and Power
Abstract
:1. Introduction
2. Theory
2.1. Theoretical Basis: sCO2 Cycle, FFCs, Air Separation, and System Level
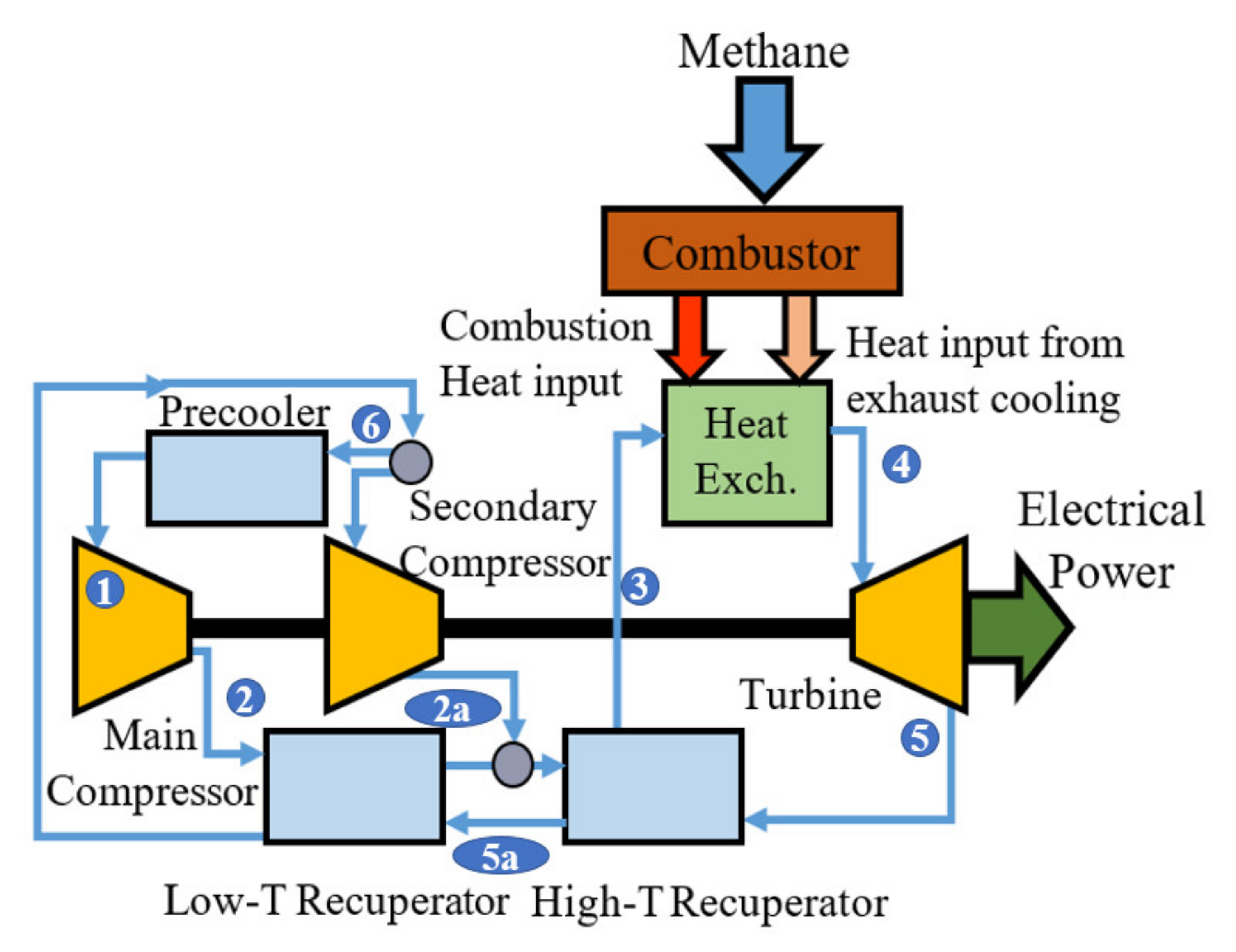
2.1.1. Standard sCO2 Brayton Cycle
2.1.2. Flame-Assisted Fuel Cells
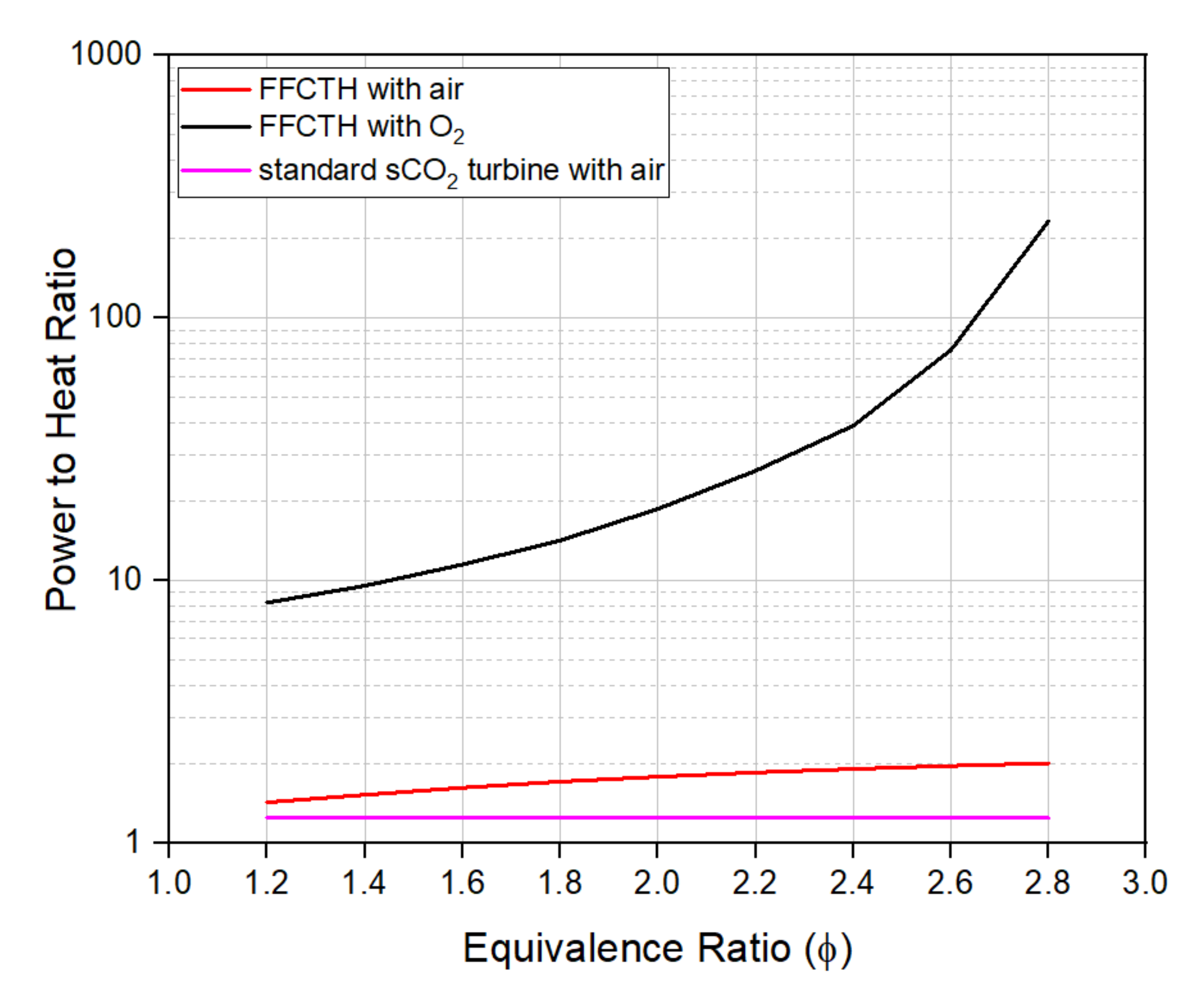
2.1.3. FFC sCO2 Turbine Hybrid with and without Carbon Sequestration
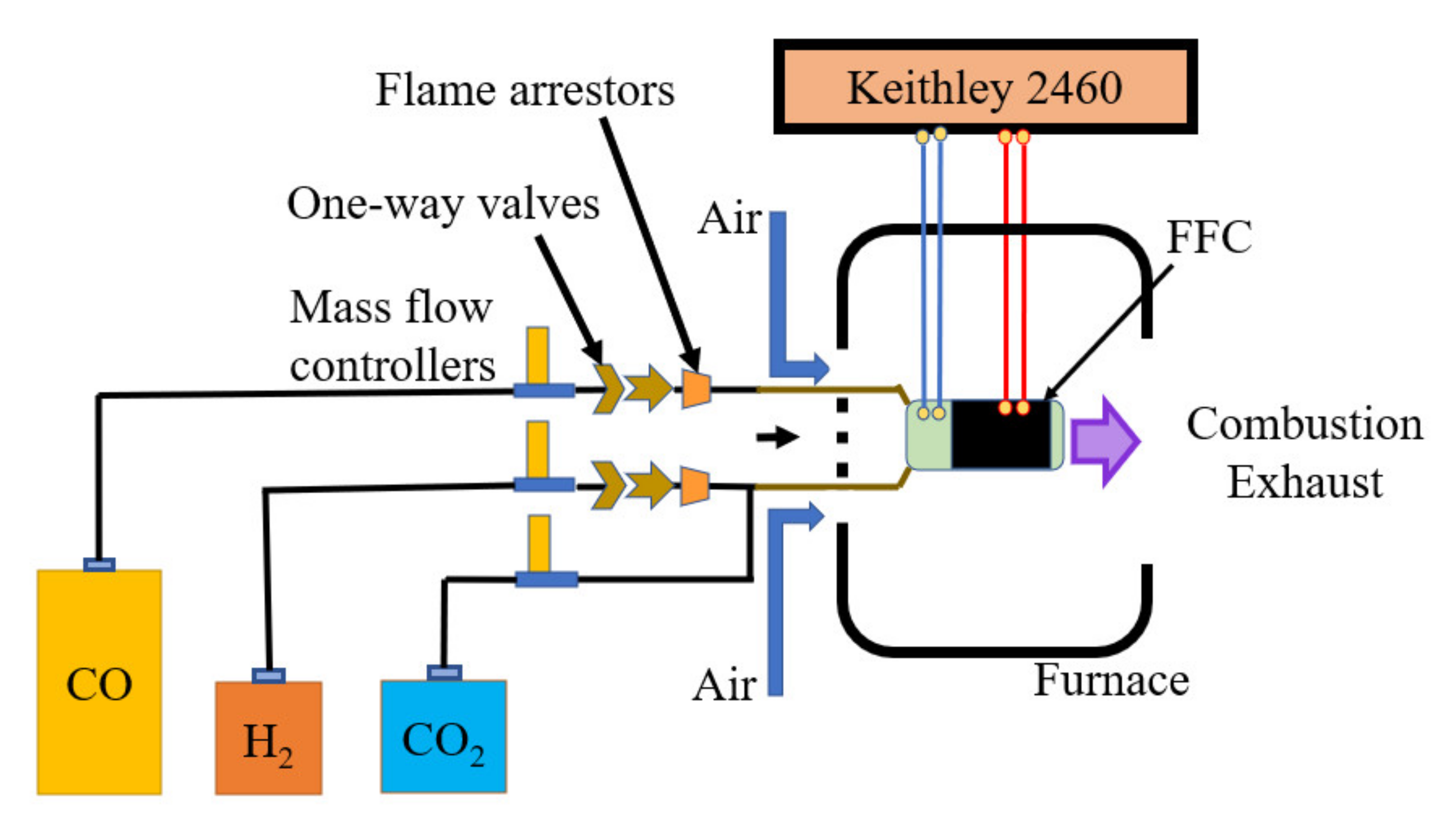
3. Experimental
Fuel Cell Fabrication
4. Results and Discussion
4.1. Fuel Cell Performance
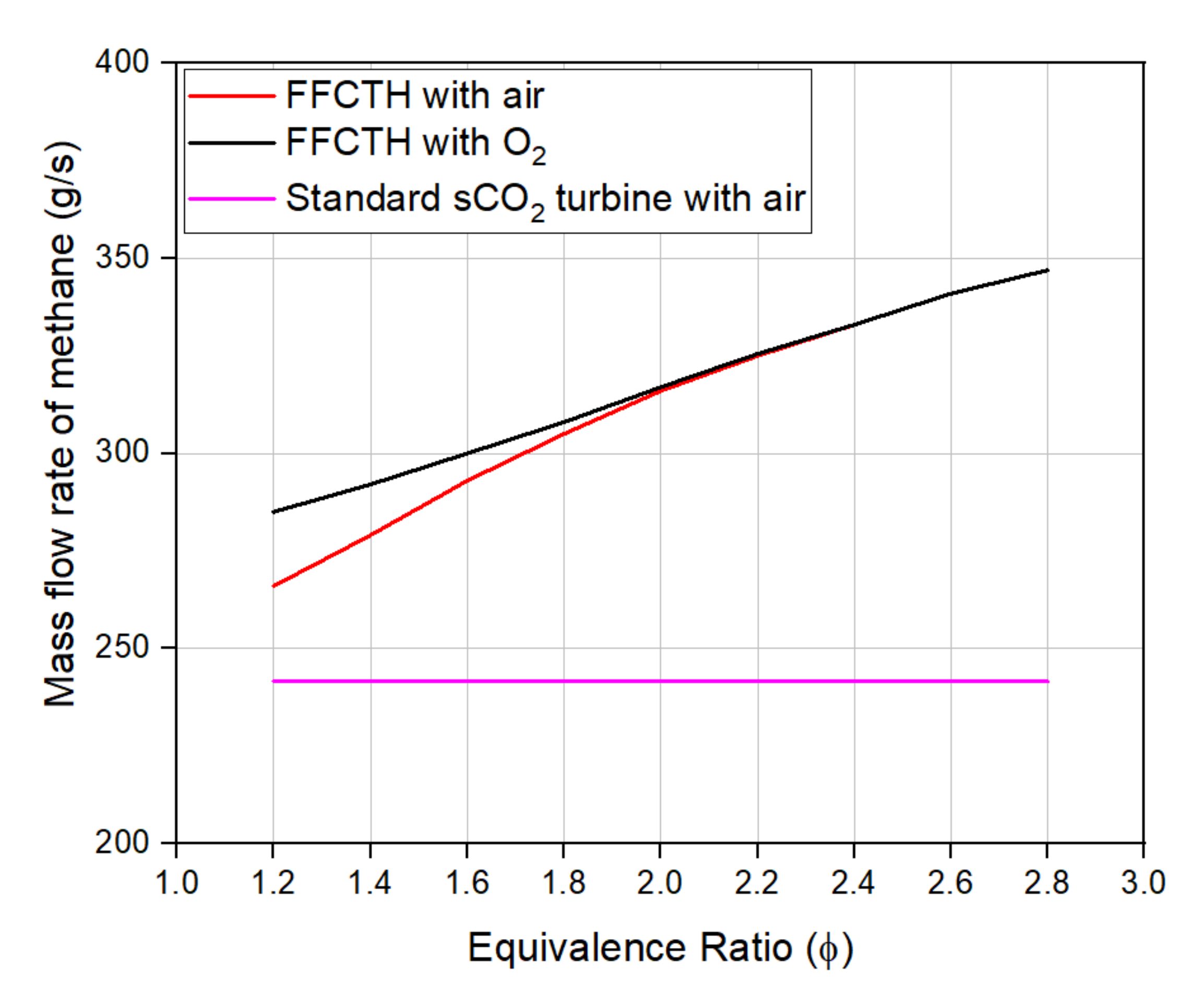
4.2. sCO2 Turbine Performance with and without an Integrated FFC Subsystem
5. Conclusions and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S. Energy Information Administration. Monthly Energy Review—January 2020; 2020; Volume 35. Available online: https://www.eia.gov/totalenergy/data/monthly/pdf/mer.pdf (accessed on 23 September 2020).
- Lueken, R.; Klima, K.; Griffin, W.M.; Apt, J. The climate and health effects of a USA switch from coal to gas electricity generation. Energy 2016, 109, 1160–1166. [Google Scholar] [CrossRef]
- Bao, C.; Wang, Y.; Feng, D.; Jiang, Z.; Zhang, X. Macroscopic modeling of solid oxide fuel cell (SOFC) and model-based control of SOFC and gas turbine hybrid system. Prog. Energy Combust. Sci. 2018, 66, 83–140. [Google Scholar] [CrossRef]
- Levi, M. Climate consequences of natural gas as a bridge fuel. Clim. Chang. 2013, 118, 609–623. [Google Scholar] [CrossRef]
- De Gouw, J.A.; Parrish, D.D.; Frost, G.J.; Trainer, M. Reduced emissions of CO2, NOx, and SO2 from U.S. power plants owing to switch from coal to natural gas with combined cycle technology. Earth’s Future 2014, 2, 75–82. [Google Scholar] [CrossRef]
- Hayhoe, K.; Kheshgi, H.S.; Jain, A.K.; Wuebbles, D.J. Substitution of natural gas for coal: Climatic effects of utility sector emissions. Clim. Chang. 2002, 54, 107–139. [Google Scholar] [CrossRef]
- Venkatesh, A.; Jaramillo, P.; Griffin, W.M.; Matthews, H.S. Implications of changing natural gas prices in the United States electricity sector for SO2, NOX and life cycle GHG emissions. Environ. Res. Lett. 2012, 7, 034018. [Google Scholar] [CrossRef] [Green Version]
- Bell, M.L.; Dominici, F. Effect modification by community characteristics on the short-term effects of ozone exposure and mortality in 98 US communities. Am. J. Epidemiol. 2008, 167, 986–997. [Google Scholar] [CrossRef] [Green Version]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Walker, J.; Samet, J.M.; Zeger, S.L.; Dominici, F. Seasonal and Regional Short-term Effects of Fine Particles on Hospital Admissions in 202 US Counties, 1999–2005. Am. J. Epidemiol. 2008, 168, 1301–1310. [Google Scholar] [CrossRef] [Green Version]
- Laden, F.; Schwartz, J.; Speizer, F.E.; Dockery, D.W. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities Study. Am. J. Respir. Crit. Care Med. 2006, 173, 667–672. [Google Scholar] [CrossRef]
- Pope, C.A.; Ezzati, M.; Dockery, D.W. Fine-particulate air pollution and life expectancy in the United States. N. Engl. J. Med. 2009, 360, 376–386. [Google Scholar] [CrossRef] [Green Version]
- U.S. Environmental Protection Agency Office of Air and Radiation. Regulatory Impact Analysis for the Final Clean Air Interstate Rule; 2005. Available online: https://www.epa.gov/sites/production/files/2020-07/documents/transport_ria_final-clean-air-interstate-rule_2005-03.pdf (accessed on 23 September 2020).
- Interconnection P. Coal Capacity at Risk for Retirement in PJM: Potential Impacts of the Finalized EPA Cross State Air Pollution Rule and Proposed National Emissions Standards for Hazardous Air Pollutants. 2011. Available online: http://www.psc.ky.gov/PSCSCF/2011%20cases/2011-00401/Kentucky%20Power%20Responses%20to%20042312%20Order/AG/012712/AG%201-14%20Attachments/AG%201-14%20Attachment%207.pdf (accessed on 23 September 2020).
- Gaudernack, B.; Lynum, S. Natural gas utilisation without CO2 emissions. Energy Convers. Manag. 1997, 38, S165–S172. [Google Scholar] [CrossRef]
- O’Hayre, R.; Cha, S.-W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; Intergovernmental Panel on Climate Change, Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 9781119191766. [Google Scholar]
- Milcarek, R.J.; Garrett, M.J.; Welles, T.S.; Ahn, J. Performance investigation of a micro-tubular flame-assisted fuel cell stack with 3,000 rapid thermal cycles. J. Power Sources 2018, 394, 86–93. [Google Scholar] [CrossRef]
- Du, Y.; Finnerty, C.; Jiang, J. Thermal Stability of Portable Microtubular SOFCs and Stacks. J. Electrochem. Soc. 2008, 155, B972. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, H.; Cao, T.; Shi, Y.; Cai, N.; Ye, X.; Wang, S. Start-up and operation characteristics of a flame fuel cell unit. Appl. Energy 2016, 178, 415–421. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Ni, M.; Cai, N. A micro tri-generation system based on direct flame fuel cells for residential applications. Int. J. Hydrogen Energy 2014, 39, 5996–6005. [Google Scholar] [CrossRef]
- Kronemayer, H.; Barzan, D.; Horiuchi, M.; Suganuma, S.; Tokutake, Y.; Schulz, C.; Bessler, W.G. A direct-flame solid oxide fuel cell (DFFC) operated on methane, propane, and butane. J. Power Sources 2007, 166, 120–126. [Google Scholar] [CrossRef]
- Vogler, M.; Horiuchi, M.; Bessler, W.G. Modeling, simulation and optimization of a no-chamber solid oxide fuel cell operated with a flat-flame burner. J. Power Sources 2010, 195, 7067–7077. [Google Scholar] [CrossRef]
- Horiuchi, M.; Suganuma, S.; Watanabe, M. Electrochemical Power Generation Directly from Combustion Flame of Gases, Liquids, and Solids. J. Electrochem. Soc. 2004, 151, A1402. [Google Scholar] [CrossRef]
- Wang, K.; Milcarek, R.J.; Zeng, P.; Ahn, J. Flame-assisted fuel cells running methane. Int. J. Hydrogen Energy 2015, 40, 4659–4665. [Google Scholar] [CrossRef] [Green Version]
- Milcarek, R.J.; Garrett, M.J.; Wang, K.; Ahn, J. Micro-tubular flame-assisted fuel cells running methane. Int. J. Hydrogen Energy 2016, 41, 20670–20679. [Google Scholar] [CrossRef] [Green Version]
- Milcarek, R.J.; Wang, K.; Falkenstein-Smith, R.L.; Ahn, J. Micro-tubular flame-assisted fuel cells for micro-combined heat and power systems. J. Power Sources 2016, 306, 148–151. [Google Scholar] [CrossRef] [Green Version]
- Milcarek, R.J.; Ahn, J. Rich-burn, flame-assisted fuel cell, quick-mix, lean-burn (RFQL) combustor and power generation. J. Power Sources 2018, 381, 18–25. [Google Scholar] [CrossRef]
- Milcarek, R.J.; Garrett, M.J.; Baskaran, A.; Ahn, J. Combustion Characterization and Model Fuel Development for Micro-tubular Flame-assisted Fuel Cells. J. Vis. Exp. 2016, 116, e54638. [Google Scholar] [CrossRef] [PubMed]
- Milcarek, R.J.; Nakamura, H.; Tezuka, T.; Maruta, K.; Ahn, J. Microcombustion for micro-tubular flame-assisted fuel cell power and heat cogeneration. J. Power Sources 2019, 413, 191–197. [Google Scholar] [CrossRef]
- Ghotkar, R.; Milcarek, R.J. POWER2019-1852 Integration of Flame-assisted Fuel Cells with a Gas Turbine running Jet-A as fuel. In Proceedings of the ASME 2019 Power Conference collocated the ASME 2019 Nuclear Forum (POWER2019), Salt Lake City, UT, USA, 15–18 July 2019; pp. 1–9. [Google Scholar]
- Milcarek, R.J.; Ahn, J. Micro-tubular flame-assisted fuel cells running methane, propane and butane: On soot, efficiency and power density. Energy 2019, 169, 776–782. [Google Scholar] [CrossRef]
- Milcarek, R.J.; DeBiase, V.P.; Ahn, J. Investigation of startup, performance and cycling of a residential furnace integrated with micro-tubular flame-assisted fuel cells for micro-combined heat and power. Energy 2020, 196, 117148. [Google Scholar] [CrossRef]
- Ghotkar, R.; Milcarek, R.J. Investigation of flame-assisted fuel cells integrated with an auxiliary power unit gas turbine. Energy 2020, 204, 117979. [Google Scholar] [CrossRef]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Gunning, P.J.; Hills, C.D.; Carey, P.J. Accelerated carbonation treatment of industrial wastes. Waste Manag. 2010, 30, 1081–1090. [Google Scholar] [CrossRef]
- Eloneva, S.; Teir, S.; Salminen, J.; Fogelholm, C.J.; Zevenhoven, R. Fixation of CO2 by carbonating calcium derived from blast furnace slag. Energy 2008, 33, 1461–1467. [Google Scholar] [CrossRef]
- Cormos, C.C. Integrated assessment of IGCC power generation technology with carbon capture and storage (CCS). Energy 2012, 42, 434–445. [Google Scholar] [CrossRef]
- Tola, V.; Pettinau, A. Power generation plants with carbon capture and storage: A techno-economic comparison between coal combustion and gasification technologies. Appl. Energy 2014, 113, 1461–1474. [Google Scholar] [CrossRef]
- Ermanoski, I.; Stechel, E.B. Thermally-driven adsorption/desorption cycle for oxygen pumping in thermochemical fuel production. Sol. Energy 2020, 198, 578–585. [Google Scholar] [CrossRef]
- Bray, J.M.; Schneider, W.F. Potential energy surfaces for oxygen adsorption, dissociation, and diffusion at the Pt(321) surface. Langmuir 2011, 27, 8177–8186. [Google Scholar] [CrossRef]
- Rochau, G.E.; Pasch, J.J.; Cannon, G.; Carlson, M.; Fleming, D.; Kruizenga, A.; Sharpe, R.; Wilson, M. Supercritical CO2 Brayton Cycles. 2014. Available online: https://www.osti.gov/servlets/purl/1221819 (accessed on 23 September 2020).
- Seo, Y.; Huh, C.; Lee, S.; Chang, D. Comparison of CO2 liquefaction pressures for ship-based carbon capture and storage (CCS) chain. Int. J. Greenh. Gas Control 2016, 52, 1–12. [Google Scholar] [CrossRef]
- Milcarek, R.J.; Garrett, M.J.; Ahn, J. Micro-tubular flame-assisted fuel cells. J. Fluid Sci. Technol. 2017, 12, JFST0021. [Google Scholar] [CrossRef] [Green Version]
- McBride, B.J.; Gordon, S.; McBride, B.J. Computer Program for Calculation of Complex Chemical Equilibrium Compositions and Applications. NASA Ref. Publ. 1311 1994, 184. Available online: https://web.stanford.edu/~cantwell/AA284A_Course_Material/AA284A_Resources/NASA_Glenn_Reports/McBride%20and%20Gordon,%20Computer%20Program%20for%20Calculation%20of%20Complex%20Chemical%20Equilibrium%20NASA%20RP%201311%201996%20Part%20II.pdf (accessed on 23 September 2020).
- Speight, J.G. Natural Gas; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9781933762142. [Google Scholar]
- Milcarek, R.J.; Wang, K.; Garrett, M.J.; Ahn, J. Performance Investigation of Dual Layer Yttria-Stabilized Zirconia–Samaria-Doped Ceria Electrolyte for Intermediate Temperature Solid Oxide Fuel Cells. J. Electrochem. Energy Convers. Storage 2016, 13, 011002. [Google Scholar] [CrossRef]
- Zeng, H.; Gong, S.; Shi, Y.; Wang, Y.; Cai, N. Micro-tubular solid oxide fuel cell stack operated with catalytically enhanced porous media fuel-rich combustor. Energy 2019, 179, 154–162. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Bell, I.H.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP; Version 9.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2013; Volume 135. [Google Scholar]
- Park, S.K.; Kim, T.S.; Sohn, J.L.; Lee, Y.D. An integrated power generation system combining solid oxide fuel cell and oxy-fuel combustion for high performance and CO2 capture. Appl. Energy 2011, 88, 1187–1196. [Google Scholar] [CrossRef]







| Φ | CO (mL·min−1) | H2 (mL·min−1) | CO2 (mL·min−1) | H2O (mL·min−1) | N2 (mL·min−1) |
|---|---|---|---|---|---|
| 1.20 | 0.05 | 0.03 | 0.05 | 0.18 | 0.66 |
| 1.40 | 0.08 | 0.06 | 0.04 | 0.17 | 0.63 |
| 1.60 | 0.10 | 0.09 | 0.03 | 0.16 | 0.60 |
| 1.80 | 0.12 | 0.13 | 0.02 | 0.15 | 0.57 |
| 2.00 | 0.13 | 0.17 | 0.02 | 0.13 | 0.55 |
| 2.20 | 0.14 | 0.20 | 0.02 | 0.11 | 0.53 |
| 2.40 | 0.15 | 0.23 | 0.01 | 0.09 | 0.51 |
| 2.60 | 0.16 | 0.26 | 0.01 | 0.08 | 0.49 |
| 2.80 | 0.17 | 0.29 | 0.01 | 0.06 | 0.47 |
| Φ | CO (mL·min−1) | H2 (mL·min−1) | CO2 (mL·min−1) | Total (mL·min−1) |
|---|---|---|---|---|
| 1.20 | 0.2672 | 0.1560 | 0.0848 | 0.4715 |
| 1.40 | 0.2855 | 0.2000 | 0.0695 | 0.4333 |
| 1.60 | 0.2981 | 0.2503 | 0.0555 | 0.3899 |
| 1.80 | 0.3061 | 0.3053 | 0.0435 | 0.3418 |
| 2.00 | 0.3110 | 0.3614 | 0.0341 | 0.2921 |
| 2.20 | 0.3141 | 0.4142 | 0.0272 | 0.2444 |
| 2.40 | 0.3163 | 0.4612 | 0.0222 | 0.2010 |
| 2.60 | 0.3182 | 0.5020 | 0.0185 | 0.1625 |
| 2.80 | 0.3201 | 0.5369 | 0.0158 | 0.1289 |
| Φ | CO (mL·min−1) | H2 (mL·min−1) | CO2 (mL·min−1) | Total (mL·min−1) |
|---|---|---|---|---|
| 1.20 | 3.33 | 1.94 | 7.18 | 12.45 |
| 1.40 | 3.81 | 2.68 | 6.87 | 13.36 |
| 1.60 | 4.22 | 3.55 | 6.40 | 14.17 |
| 1.80 | 4.55 | 4.55 | 5.78 | 14.88 |
| 2.00 | 4.82 | 5.59 | 5.06 | 15.47 |
| 2.20 | 5.00 | 6.61 | 4.33 | 15.94 |
| 2.40 | 5.15 | 7.51 | 3.63 | 16.29 |
| 2.60 | 5.25 | 8.28 | 2.96 | 16.49 |
| 2.80 | 5.32 | 8.93 | 2.38 | 16.63 |
| State Point | Temperature (K) | Pressure (MPa) |
|---|---|---|
| 1 | 300 | 7.5 |
| 2 | 318 | 20 |
| 2a | 414 | 20 |
| 3 | 733 | 20 |
| 4 | 913 | 20 |
| 5 | 780 | 7.5 |
| 5a | 452 | 7.5 |
| 6 | 331 | 7.5 |
| Property | Value |
|---|---|
| Power generated by standard supercritical CO2 turbine cycle | 6 MW |
| Efficiency of the standard supercritical CO2 turbine cycle (based on states 1–6) | 53.14% |
| Compression ratio | 2.66 |
| Φ | CO | H2 | CO2 | H2O |
|---|---|---|---|---|
| 1.20 | 0.17 | 0.09 | 0.17 | 0.57 |
| 1.40 | 0.23 | 0.16 | 0.11 | 0.50 |
| 1.60 | 0.26 | 0.24 | 0.08 | 0.42 |
| 1.80 | 0.28 | 0.31 | 0.06 | 0.35 |
| 2.00 | 0.29 | 0.38 | 0.04 | 0.29 |
| 2.20 | 0.30 | 0.43 | 0.03 | 0.24 |
| 2.40 | 0.31 | 0.47 | 0.03 | 0.19 |
| 2.60 | 0.31 | 0.51 | 0.02 | 0.16 |
| 2.80 | 0.31 | 0.54 | 0.02 | 0.12 |
| Type of Power Generation Technique | Base Case Electrical Efficiency | Electrical Efficiency with CO2 Sequestration | Reference |
|---|---|---|---|
| Gas turbine | 53.67 | 46.75 | [36] |
| Gas turbine | 43.88 | 35.26 | [37] |
| Gas turbine +SOFC | Maximum of 69.5 | Maximum of 65.7 | [48] |
| sCO2 turbine | 53.14 | 51.7 | This work |
| sCO2 turbine + FFC | Maximum of 60 | Maximum of 59.3 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghotkar, R.; Stechel, E.B.; Ermanoski, I.; Milcarek, R.J. Hybrid Fuel Cell—Supercritical CO2 Brayton Cycle for CO2 Sequestration-Ready Combined Heat and Power. Energies 2020, 13, 5043. https://doi.org/10.3390/en13195043
Ghotkar R, Stechel EB, Ermanoski I, Milcarek RJ. Hybrid Fuel Cell—Supercritical CO2 Brayton Cycle for CO2 Sequestration-Ready Combined Heat and Power. Energies. 2020; 13(19):5043. https://doi.org/10.3390/en13195043
Chicago/Turabian StyleGhotkar, Rhushikesh, Ellen B. Stechel, Ivan Ermanoski, and Ryan J. Milcarek. 2020. "Hybrid Fuel Cell—Supercritical CO2 Brayton Cycle for CO2 Sequestration-Ready Combined Heat and Power" Energies 13, no. 19: 5043. https://doi.org/10.3390/en13195043