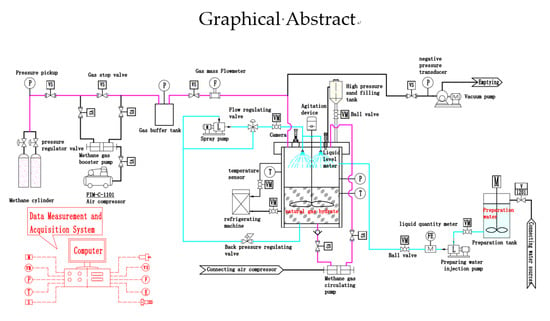
3.2. Experiment Steps
1. Experimental parameter setting
The speed of stirring is 100 tr/min, the discharge of methane circulation pump is 12 L/min, the discharge of spray pump is 10 L/min, the experiment water is tap water, the output of injection pump is 40 L/min, and the refrigeration temperature of refrigerator is set at 2.5 °C.
2. Experiment steps
• Preparation for the preparation experiment of marine non-diagenetic natural gas hydrate
① Equipment testing: Check all the equipment, instruments, instrument connection, and working status to ensure the complete and normal operation of the equipment connection. ② Preparation kettle cleaning: Open the preparation kettle cover, rinse the preparation kettle repeatedly with distilled water three times to ensure that the preparation kettle is clean, and then close the preparation kettle cover. ③ Vacuum pumping: Start the vacuum pump to extract the vacuum, and close the vacuum tube valve when the preset vacuum degree is reached. ④ Inject preparation water (the prepared water is 32% salt water, which is the same as the salinity of the South China Sea [
12]): Start the preparation water injection pump, inject 0.5 m
3 of preparation water into the preparation kettle, and shut off the injection pump to stop water injection after quantitative addition of the preparation water. ⑤ Open the sand filling valve and add 0.05 m
3 of sand to the preparation kettle. The added sand comes from the sample of hydrate in the South China Sea, and the particle size is about 40 μm.
• Preparation experiment of marine non-diagenetic natural gas hydrate
① Start the refrigeration unit of the preparation kettle, set the experimental temperature of hydrate formation at 2.5 °C, until the temperature of preparation water inside the preparation kettle is 2.5 °C, then start to inject methane gas. ② Open the methane valve, start the methane gas booster pump, inject methane gas into the preparation kettle; when the pressure in the preparation kettle is 7 MPa, stop the methane injection. ③ Start agitation device, that is, using agitation method to prepare hydrate (if spraying method is used to prepare natural gas hydrate experiment, then start to prepare water spray pump; if bubbling method is used to prepare natural gas hydrate experiment, then start methane circulation pump; if the three-in-one gas hydrate preparation experiment is carried out, the stirring device, the preparation water spray pump, and the methane gas circulation pump shall be opened simultaneously.) ④ Data acquisition starts when the pressure in the preparation kettle reaches 7 MPa and stops at 3.3 MPa. The reaction time, temperature, pressure, and resistivity when the pressure in the kettle decreases from 7 MPa to 3.3 MPa in the experiments of stirring, spraying, bubbling, and three-in-one methods are recorded, respectively, and the real time image acquisition and processing are carried out by preparing the windows on the top of the kettle cover. When 3.7 MPa methane gas is consumed under the four experimental conditions, the amount of hydrate produced is the same, but the reaction time is different under different experimental conditions, which means that the reaction rate is different under different experimental conditions. The longer the reaction time is under certain experimental conditions, the lower the hydrate formation rate is. ⑤ Finishing and cleaning of experimental equipment; end of experiment. ⑥ Experimental phenomenon analysis and data processing.
3.3. Experimental Phenomena and Data Processing
The data of image, temperature, pressure, resistivity, and reaction time in the experiment of gas hydrate preparation by stirring method, bubbling method, spraying method, and three-in-one method were recorded and processed, and the curves of temperature, pressure, and resistivity (as shown in Figures 5, 6, 8, 9, 11, 12, 14, and 15) in the preparation of natural gas hydrate by stirring, spraying, bubbling, and three-in-one methods and the experimental phenomena (as shown in Figures 4, 7, 10, and 13) in the process of preparation were obtained, respectively.
Figure 4 shows the experimental phenomena in the preparation of gas hydrate by stirring method. It can be seen that as the agitation continues, the amount of gas hydrate generated increases slowly and gradually covers the entire liquid surface. Figure shows the temperature and pressure curves during the preparation of gas hydrate by stirring method,
Figure 5a shows the first group of experiments, and
Figure 5b shows the second group of experiments. It can be seen from
Figure 5a that in the initial stage of the experiment, part of the methane gas is gradually dissolved into the preparation water with the progress of stirring after methane gas is injected into the preparation kettle because the temperature in the preparation kettle is relatively low, and the pressure in the preparation kettle is reduced accordingly, and the pressure decreases from 7 MPa to 6.5 MPa after lasting for 2 h, which is the dissolution stage of methane gas. With the reaction going on, there is a step change in temperature and pressure at 5.6 h, the pressure suddenly dropped and the temperature increased suddenly, which is the induction stage. Subsequently, the reaction period enters into rapid growth stage from the induction stage, the pressure dropped abruptly from 6.4MPa to 4.5 MPa, the temperature increased abruptly from 2.5 °C to 4 °C, and the rapid growth period ended at 15.8 h. Then the hydrate preparation enters a stable growth period, the experimental pressure drops to the end of the 3.3 MPa experiment, the cumulative preparation time is 98.3 h. It can be seen from
Figure 5b that in the initial stage of the experiment, part of the methane gas is gradually dissolved into the preparation water with the progress of stirring after methane gas is injected into the preparation kettle because the temperature in the preparation kettle is relatively low, and the pressure in the preparation kettle is reduced accordingly, and the pressure decreases from 7 MPa to 6.6 MPa after lasting for 1.9 h, which is the dissolution stage of methane gas. With the reaction going on, there is a step change in temperature and pressure at 5.65 h, the pressure suddenly dropped and the temperature increased suddenly, which is the induction stage. Subsequently, the reaction period enters into rapid growth stage from the induction stage, the pressure dropped abruptly from 6.3 MPa to 4.55 MPa, the temperature increased abruptly from 2.5 °C to 3.8 °C, and the rapid growth period ended at 15.85 h. Then the hydrate preparation enters a stable growth period, the experimental pressure drops to the end of the 3.3 MPa experiment, the cumulative preparation time is 98.75 h. The average cumulative preparation time of the two groups was 98.53 h.
Figure 6 shows the curve of resistivity change during preparation of natural gas hydrate by stirring method,
Figure 6a shows the first group of experiments, and
Figure 6b shows the second group of experiments. It can be seen that at the early stage of the experiment, part of the methane gas gradually dissolved into the preparation water with the proceeding of agitation after methane gas was injected into the preparation kettle. The measured resistivity increased with the continuous increase of methane gas solubility. During the rapid induction period of hydrate, the change of resistivity was small. After entering the rapid growth period of hydrate, the resistivity increased rapidly with the reaction time because of the rapid rate of hydrate preparation. After entering the stable growth period of hydrate, the hydrate preparation rate decreased and the resistivity increased with the increase of reaction time, but the increase rate was relatively slow.
Figure 7 shows the experimental phenomenon in the preparation of gas hydrate by spray method. It can be seen that as the spray continued, the amount of gas hydrate generated increased slowly and gradually covered the whole impeller (the impeller was used as a reference in order to observe the experimental phenomena conveniently; the impeller was located at the liquid surface).
Figure 8 shows the temperature and pressure curves during the preparation of gas hydrate by spray method,
Figure 8a shows the first group of experiments, and
Figure 8b shows the second group of experiments. It can be seen from
Figure 8a that in the initial stage of the experiment, part of the methane gas was gradually dissolved into the preparation water with the progress of spray after methane gas was injected into the preparation kettle because the temperature in the preparation kettle was relatively low, and the pressure in the preparation kettle was reduced accordingly, and the pressure decreased from 7 MPa to 6.5 MPa after lasting for 1.9 h. As the experiment went on, the temperature and pressure had a step change at the same time. There was a sudden drop in pressure and a sudden increase in temperature. The pressure dropped sharply from 6.5 MPa to 4.52 MPa, and the temperature rose abruptly from 2.5 °C to 4.1 °C. The rapid growth period ended after the experiment was carried out for 7.94 h. In the experimental process of preparing hydrate by spray method, the induction period was so short that it is difficult to observe from the diagram. Subsequently, the hydrate preparation entered a stable growth period, the experimental pressure dropped to 3.3 MPa and the experiment ended, and the cumulative preparation time was 68.74 h. It can be seen from
Figure 8b that in the initial stage of the experiment, part of the methane gas was gradually dissolved into the preparation water with the progress of spray after methane gas was injected into the preparation kettle, because the temperature in the preparation kettle was relatively low, and the pressure in the preparation kettle was reduced accordingly, and the pressure decreased from 7 MPa to 6.53 MPa after lasting for 2.05 h. As the experiment went on, the temperature and pressure had a step change at the same time. There was a sudden drop in pressure and a sudden increase in temperature. The pressure dropped sharply from 6.2 MPa to 4.3 MPa, and the temperature rose abruptly from 2.4 °C to 4 °C. The rapid growth period ended after the experiment was carried out for 7.99 h. In the experimental process of preparing hydrate by spray method, the induction period was so short that it is difficult to observe from the diagram. Subsequently, the hydrate preparation entered a stable growth period, the experimental pressure dropped to 3.3 MPa and the experiment ended, and the cumulative preparation time was 69 h. The average cumulative preparation time of the two groups was 68.87 h.
Figure 9 shows the curve of resistivity change during preparation of natural gas hydrate by spray method,
Figure 9a shows the first group of experiments, and
Figure 9b shows the second group of experiments. It can be seen that at the early stage of the experiment, part of the methane gas gradually dissolved into the preparation water with the proceeding of spray after methane gas was injected into the preparation kettle. The measured resistivity increased with the continuous increase of methane gas solubility. During the rapid induction period of hydrate, the change of resistivity was small. After entering the rapid growth period of hydrate, the resistivity increased rapidly with the reaction time because of the rapid rate of hydrate preparation. After entering the stable growth period of hydrate, the hydrate preparation rate decreased and the resistivity increased with the increase of reaction time, but the increase rate was relatively slow.
Figure 10 shows the experimental phenomenon in the preparation of gas hydrate by bubbling method. It can be seen that as the bubbling continued, the amount of gas hydrate generated increased slowly and gradually covered the whole liquid surface.
Figure 11 shows the temperature and pressure curves during the preparation of gas hydrate by bubbling method,
Figure 11a shows the first group of experiments, and
Figure 11b shows the second group of experiments. It can be seen from
Figure 11a that in the initial stage of the experiment, part of the methane gas was gradually dissolved into the preparation water with the progress of bubbling after methane gas was injected into the preparation kettle, because the temperature in the preparation kettle was relatively low, and the pressure in the preparation kettle was reduced accordingly, and the pressure decreased from 7 MPa to 6.51 MPa after lasting for 1.85 h. As the experiment went on, the temperature and pressure had a step change at the same time. There was a sudden drop in pressure and a sudden increase in temperature. The pressure dropped sharply from 6.51 MPa to 4.51 MPa, and the temperature rose abruptly from 2.5 °C to 4.05 °C. The rapid growth period ended after the experiment was carried out for 10.1 h. In the experimental process of preparing hydrate by bubbling method, the induction period was so short that it is difficult to observe from the diagram. Subsequently, the hydrate preparation entered a stable growth period, the experimental pressure dropped to 3.3 MPa and the experiment ended, and the cumulative preparation time was 60.39 h. It can be seen from
Figure 11b that in the initial stage of the experiment, part of the methane gas was gradually dissolved into the preparation water with the progress of bubbling after methane gas was injected into the preparation kettle, because the temperature in the preparation kettle was relatively low, and the pressure in the preparation kettle was reduced accordingly, and the pressure decreased from 7 MPa to 6.55 MPa after lasting for 1.9 h. As the experiment went on, the temperature and pressure had a step change at the same time. There was a sudden drop in pressure and a sudden increase in temperature. The pressure dropped sharply from 6.4 MPa to 4.4 MPa, and the temperature rose abruptly from 2.54 °C to 3.55 °C. The rapid growth period ended after the experiment was carried out for 9.93 h. In the experimental process of preparing hydrate by bubbling method, the induction period was so short that it is difficult to observe from the diagram. Subsequently, the hydrate preparation entered a stable growth period, the experimental pressure dropped to 3.3 MPa and the experiment ended, and the cumulative preparation time was 60.22 h. The average cumulative preparation time of the two groups was 64.63 h.
Figure 12 shows the resistivity curve in the preparation process of gas hydrate by bubbling method,
Figure 12a shows the first group of experiments, and
Figure 12b shows the second group of experiments. It can be seen that in the initial stage of the experiment, part of the methane gas was gradually dissolved into the preparation water with the progress of bubbling after methane gas was injected into the preparation kettle. The measured resistivity increased with the continuous increase of methane gas dissolution. In the hydrate preparation process by spray method, the induction period was very short, and the hydrate directly entered the fast growing period after the dissolution period; we can see from the resistivity curve that the resistivity also had the phenomenon of sudden step increase. During the fast growth period of hydrate, the resistivity of hydrate increased rapidly with the reaction time because of the fast hydrate preparation rate. After entering the stable growth period of hydrate, the rate of hydrate preparation decreased, and the resistivity increased with the increase of reaction time, but at a slower rate.
Figure 13 shows the experimental phenomenon in the preparation of gas hydrate by three-in-one method. It can be seen that as the stirring, spraying, and bubbling continue, the amount of gas hydrate generated increased rapidly and covered the whole liquid surface in a short period.
Figure 14 shows the temperature and pressure curves during the preparation of gas hydrate by three-in-one method,
Figure 14a shows the first group of experiments, and
Figure 14b shows the second group of experiments. It can be seen from
Figure 14a that in the initial stage of the experiment, part of the methane gas was gradually dissolved into the preparation water with the progress of spraying after methane gas was injected into the preparation kettle, because the temperature in the preparation kettle was relatively low, and the pressure in the preparation kettle was reduced accordingly, and the pressure decreased from 7 MPa to 6.4 MPa after lasting for 1.2 h. As the experiment went on, the temperature and pressure had a step change at the same time. There was a sudden drop in pressure and a sudden increase in temperature. The pressure dropped sharply from 6.49 MPa to 4.52 MPa, and the temperature rose abruptly from 2.5 °C to 4.15 °C. The rapid growth period ended after the experiment was carried out for 5.1 h. The induction period was so short that it is difficult to observe from the diagram. Subsequently, the hydrate preparation entered a stable growth period, the experimental pressure dropped to 3.3 MPa and the experiment ended, and the cumulative preparation time was 19.1 h. It can be seen from
Figure 14b that in the initial stage of the experiment, part of the methane gas was gradually dissolved into the preparation water with the progress of spraying after methane gas was injected into the preparation kettle, because the temperature in the preparation kettle was relatively low, and the pressure in the preparation kettle was reduced accordingly, and the pressure decreased from 7 MPa to 6.5 MPa after lasting for 1.25 h. As the experiment went on, the temperature and pressure had a step change at the same time. There was a sudden drop in pressure and a sudden increase in temperature. The pressure dropped sharply from 6.33 MPa to 4.47 MPa, and the temperature rose abruptly from 2.55 °C to 4.2 °C. The rapid growth period ended after the experiment was carried out for 5.16 h. The induction period was so short that it is difficult to observe from the diagram. Subsequently, the hydrate preparation entered a stable growth period, the experimental pressure dropped to 3.3 MPa and the experiment ended, and the cumulative preparation time was 19.29 h. The average cumulative preparation time of the two groups was 19.20 h.
Figure 15 shows the resistivity curve in the preparation process of gas hydrate by three-in-one method,
Figure 15a shows the first group of experiments, and
Figure 15b shows the second group of experiments. It can be seen that in the initial stage of the experiment, part of the methane gas was gradually dissolved into the preparation water with the progress of stirring, spraying, and bubbling after methane gas was injected into the preparation kettle. The measured resistivity increased with the continuous increase of methane gas dissolution. In the hydrate preparation process by three-in-one method, the induction period was very short, and the hydrate directly entered the fast-growing period after the dissolution period. It can be seen from the resistivity curve that the resistivity saw a step sudden increase phenomenon. During the fast growth period of hydrate, the resistivity of hydrate increased rapidly with the reaction time because of the fast hydrate preparation rate. After entering the stable growth period of hydrate, the rate of hydrate preparation decreased, and the resistivity increased with the increase of reaction time, but at a slower rate.
Through the analysis of the experimental data of the different preparation methods of gas hydrate, it can be seen that the single methods of stirring, spraying, and bubbling need a longer experimental time, but the preparation period of natural gas hydrate by the three-in-one method is obviously shortened. It can be seen from
Figure 16 that the preparation times of the single preparation methods of stirring method, spraying method, and bubbling method are about 5.13, 3.59, and 3.37 times of that of the three-in-one method, respectively. Therefore, the preparation efficiency of gas hydrate by three-in-one method is much higher than that by a single method.