The Relation of Microstructure, Materials Properties and Impedance of SOFC Electrodes: A Case Study of Ni/GDC Anodes
Abstract
:1. Introduction
2. Impedance Model for Porous SOFC Electrodes
2.1. Porous Electrode Impedance Models in Literature
2.2. Electronion Conduction and Electrochemical Reactions in a Porous Electrode
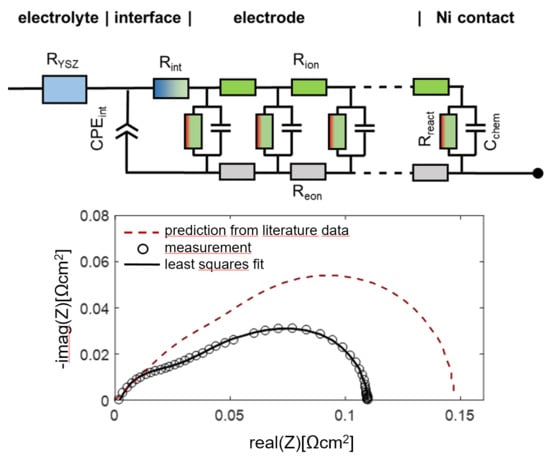
2.3. Equivalent Circuit Representation of Reactions in Porous Electrodes
2.4. The Need for Parameter Constraints in “Thick” Electrodes
2.5. Treatment of Interfacial Resistances and Wiring
2.6. Simulated Impedance Spectra
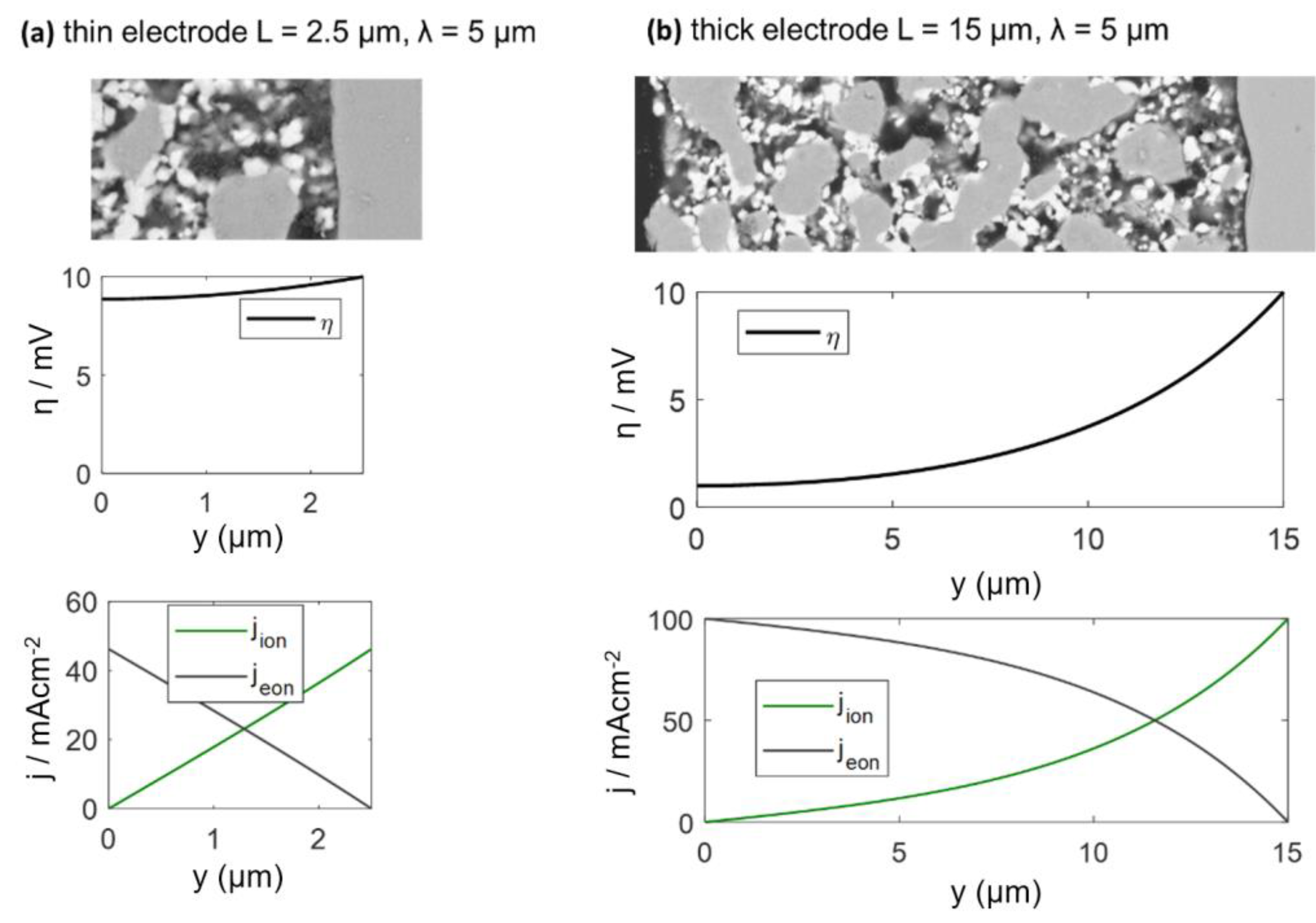
2.7. Distribution of the Local Overpotential η and Electrochemically Active Thickness λ
2.8. Prediction and Minimization of Gas Diffusion Resistances
2.9. Gas Diffusion ASR for Typical Testing Conditions
3. Materials and Methods
3.1. Symmetric Cell Fabrication
3.2. Impedance Spectroscopic Characterization and EIS Fitting
3.3. SEM Analysis of Screen-Printed Electrodes
4. Results and Discussion
4.1. Gas Diffusion Impedance
4.2. Chemical Capacitance
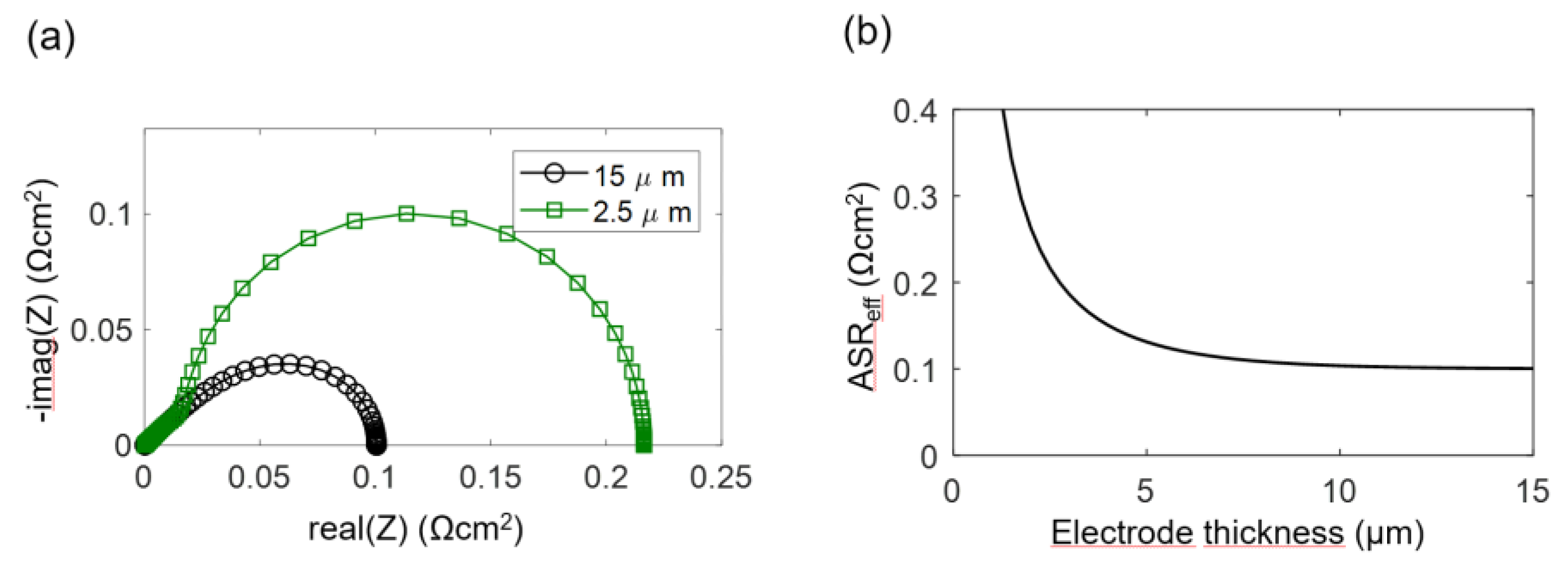
4.3. Effect of Electrode Thickness
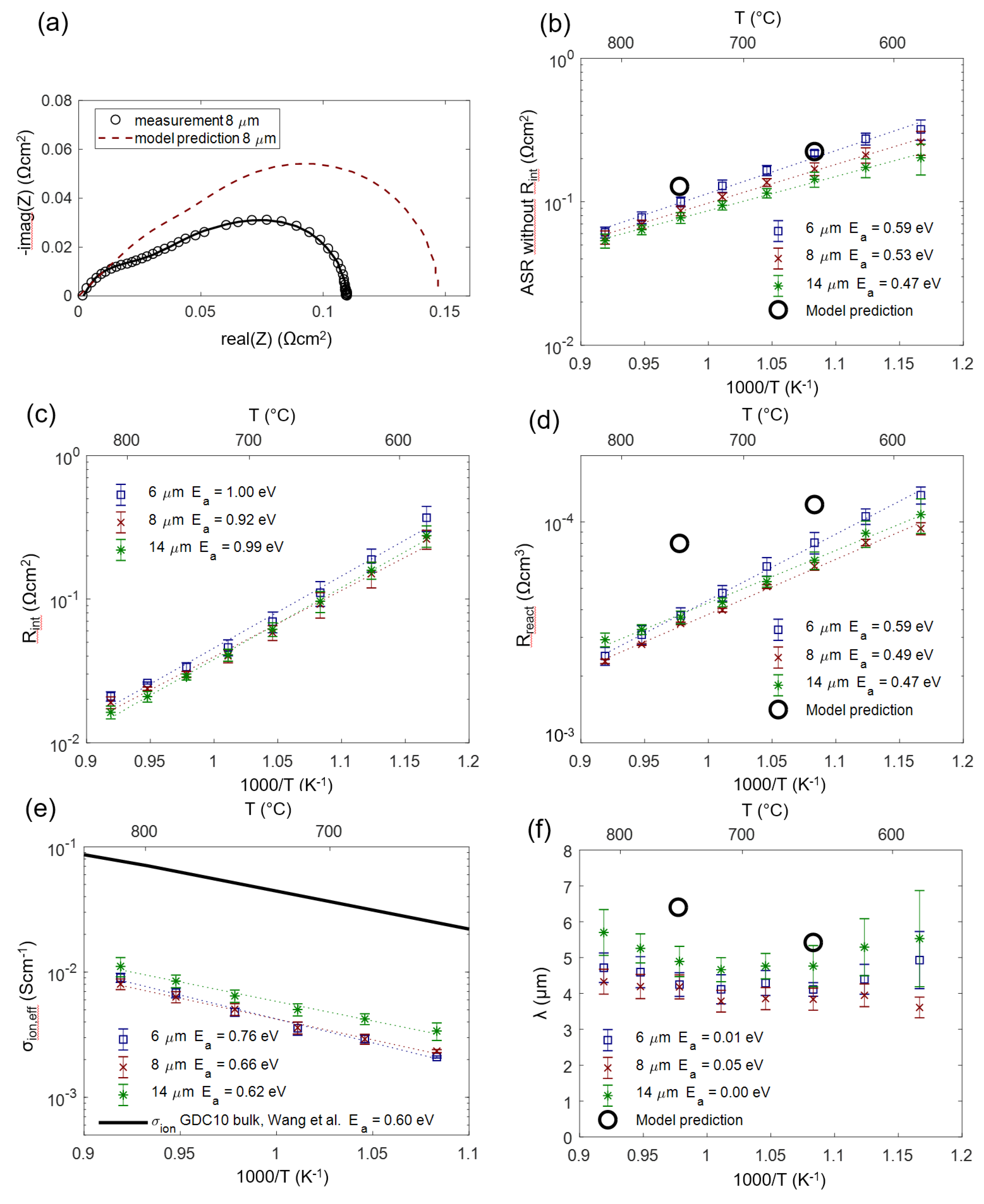
4.4. Temperature Dependence and Comparison with Model Studies
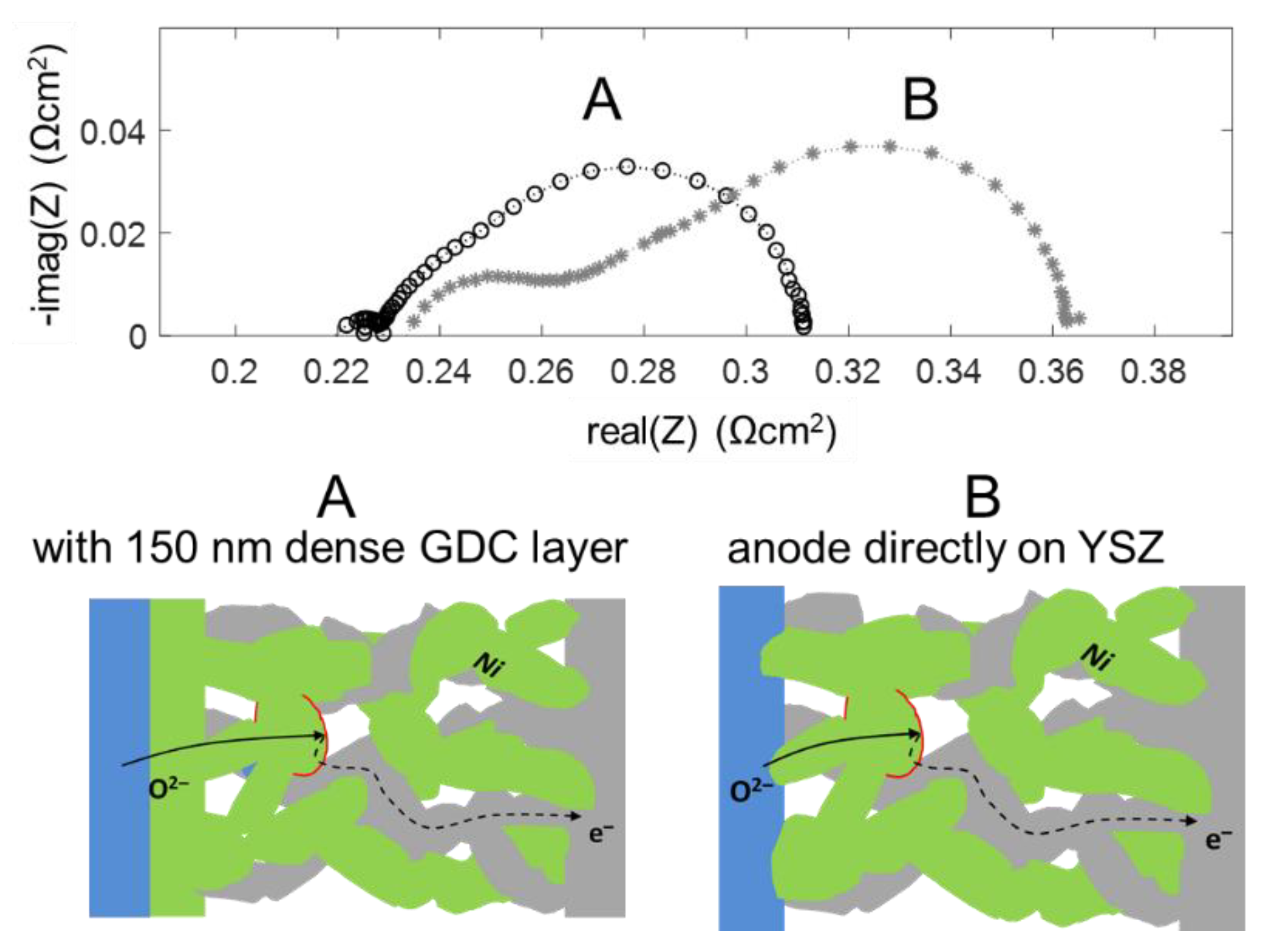
4.5. Enhancing Electrolyte-Electrode Interface Adhesion and Ionic Transfer Resistance by a Thin Film GDC Layer
4.6. Why Ceria-Based Anodes are Optimal for Metal-Supported Cells
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Equivalent Circuit Fitting in Zview

| Parameter Name in Zview | Parameter Name in Equation (A1) | Fixed/Free Parameter | Unit |
|---|---|---|---|
| DX | None | fixed (11) | None; selector for circuit model “Bisquert 2” |
| DX-R | None | fixed (0) | Ωcm |
| DX-T | None | fixed (0) | F/cm |
| DX-P | None | fixed (1) | 1 |
| DX-U | Rion | free | Ωcm |
| DX-A | None | fixed (0) | F/cm |
| DX-B | None | fixed (1) | 1 |
| DX-C | Rreact | free | Ωcm3 |
| DX-D | Cchem,eff | free * | F/cm3 |
| DX-E | None | fixed (1) | 1 |
| DX-F | L | fixed (thickness measurement) | cm |
| Qint-P | pint | may be free or fixed to a value 0.8–1 | 1 |
| Qint-T | Qint | free | Fractional capacitance |
Particle Packing Model
Appendix B. List of Used Variables
| Symbol | Meaning | Unit |
|---|---|---|
| Gibbs free energy of H2 oxidation | J/mol | |
| δ | oxygen deficiency, e.g., in Ce0.9Gd0.1O1.95-δ | 1 |
| μx | chemical potential, species x | J/mol |
| electrochemical potential, species x | J/mol | |
| τx | tortuosity, phase x | 1 |
| εx | volume fraction, phase x | 1 |
| Aspec | specific surface area (of the GDC phase) per electrode volume | cm2/cm3 |
| ASRsurf | ASR of the oxygen exchange (H2 oxidation) reaction normalized to the GDC surface area | Ωcm2 |
| ASRtot | measurable ASR of a porous electrode normalized to the geometric area | Ωcm2 |
| jx | (effective) electrical current density per electrode area, carried by species x | A/cm2 |
| jsurf | electrical current density of released oxygen ions on the GDC surface | A/cm2 |
| jreact | (effective) density of reaction current per electrode volume | A/cm3 |
| σeff | effective ionic/electronic conductivity | S/cm |
| z | charge number | 1 |
| F | Faraday’s constant | C/mol |
| Δ(x) | perturbation of quantity x relative to the equilibrium with the gas phase and grounded electrical potential | Unit of x |
| pseudoelectrical potential, species x | V | |
| η | local overpotential in the porous electrode | V |
| ηgas | concentration overpotential of the gas phase | V |
| Cchem | chemical capacitance of the GDC phase | F/cm3 |
| Cchem,eff | effective chemical capacitance of the porous electrode | F/cm3 |
| Rion,Reon | effective ion/electron conduction resistance inverse of effective conductivity | Ωcm |
| Rint | ionic electrode/electrolyte interface resistance | Ωcm2 |
| D | diffusion coefficient | cm2/s |
Appendix C. Mathematical Isomorphisms and Differences to the Adler-Lane-Steele Model
References
- Leah, R.T.; Bone, A.; Lankin, M.; Selcuk, A.; Rahman, M.; Clare, A.; Rees, L.; Phillip, S.; Mukerjee, S.; Selby, M. Ceres power steel cell technology: Rapid progress towards a truly commercially viable SOFC. ECS Trans. 2015, 68, 95–107. [Google Scholar] [CrossRef]
- Leah, R.T.; Bone, A.; Hammer, E.; Selcuk, A.; Rahman, M.; Clare, A.; Mukerjee, S.; Selby, M. Development progress on the ceres power steel cell technology platform: Further progress towards commercialization. ECS Trans. 2017, 78, 87–95. [Google Scholar] [CrossRef]
- Thaler, F.; Udomsilp, D.; Schafbauer, W.; Bischof, C.; Fukuyama, Y.; Miura, Y.; Kawabuchi, M.; Taniguchi, S.; Takemiya, S.; Nenning, A.; et al. Redox stability of metal-supported fuel cells with nickel/gadolinium-doped ceria anode. J. Power Sources 2019, 434, 226751. [Google Scholar] [CrossRef]
- Thaler, F.; Nenning, A.; Bischof, C.; Udomsilp, D.; de Haart, L.G.J.; Opitz, A.K.; Bram, M. Optimized cell processing as the key of high electrochemical performance of metal-supported solid oxide fuel cells. ECS Trans. 2019, 91, 887–900. [Google Scholar] [CrossRef]
- Nielsen, J.; Persson, A.H.; Muhl, T.T.; Brodersen, K. Towards high power density metal supported solid oxide fuel cell for mobile applications. ECS Trans. 2017, 78, 2029–2037. [Google Scholar] [CrossRef] [Green Version]
- Rojek, V.; Roehrens, D.; Brandner, M.; Menzler, N.H.; Guillon, O.; Opitz, A.K.; Bram, M. Development of high performance anodes for metal-supported fuel cells. Ion. Mix. Conduct. Ceram. 2015, 68, 1297–1307. [Google Scholar] [CrossRef]
- Udomsilp, D.; Roehrens, D.; Menzler, N.H.; Bischof, C.; de Haart, L.G.J.; Opitz, A.K.; Guillon, O.; Bram, M. High-performance metal-supported solid oxide fuel cells by advanced cathode processing. J. Electrochem. Soc. 2017, 164, F1375–F1384. [Google Scholar] [CrossRef] [Green Version]
- Tucker, M.C. Progress in metal-supported solid oxide fuel cells: A review. J. Power Sources 2010, 195, 4570–4582. [Google Scholar] [CrossRef]
- Krishnan, V.V. Recent developments in metal-supported solid oxide fuel cells. Wiley Interdiscip. Rev. Energy Environ. 2017, 6, e246. [Google Scholar] [CrossRef]
- Yang, Z.; Weil, K.S.; Paxton, D.M.; Stevenson, J.W. Selection and evaluation of heat-resistant alloys for SOFC interconnect applications. J. Electrochem. Soc. 2003, 150, A1188–A1201. [Google Scholar] [CrossRef]
- Udomsilp, D.; Rechberger, J.; Neubauer, R.; Bischof, C.; Thaler, F.; Schafbauer, W.; Menzler, N.H.; de Haart, L.G.J.; Nenning, A.; Opitz, A.K.; et al. High performing metal-supported solid oxide fuel cells for novel range extender systems in battery electric vehicles. Rev. 2020. [Google Scholar]
- Bischof, C.; Nenning, A.; Malleier, A.; Martetschläger, L.; Gladbach, A.; Schafbauer, W.; Opitz, A.K.; Bram, M. Microstructure optimization of nickel/gadolinium-doped ceria anodes as key to significantly increasing power density of metal-supported solid oxide fuel cells. Int. J. Hydrog. Energy 2019, 44, 31475–31487. [Google Scholar] [CrossRef]
- Dogdibegovic, E.; Wang, R.; Lau, G.Y.; Tucker, M.C. High performance metal-supported solid oxide fuel cells with infiltrated electrodes. J. Power Sources 2019, 410–411, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Coduri, M.; Checchia, S.; Longhi, M.; Ceresoli, D.; Scavini, M. Rare earth doped ceria: The complex connection between structure and properties. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, R.; Nenning, A.; Kraynis, O.; Korobko, R.; Frenkel, A.I.; Lubomirsky, I.; Haile, S.M.; Rupp, J.L.M. A review of defect structure and chemistry in ceria and its solid solutions. Chem. Soc. Rev. 2020, 49, 554–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chueh, W.C.; Lai, W.; Haile, S.M. Electrochemical behavior of ceria with selected metal electrodes. Solid State Ion. 2008, 179, 1036–1041. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic properties of ceria and CeO2-containing materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Chueh, W.C.; Hao, Y.; Jung, W.; Haile, S.M. High electrochemical activity of the oxide phase in model ceria-Pt and ceria-Ni composite anodes. Nat. Mater. 2012, 11, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Jamnik, J.; Maier, J. Generalised equivalent circuits for mass and charge transport: Chemical capacitance and its implications. Phys. Chem. Chem. Phys. 2001, 3, 1668–1678. [Google Scholar] [CrossRef]
- Lai, W.; Haile, S.M. Impedance spectroscopy as a tool for chemical and electrochemical analysis of mixed conductors: A case study of ceria. J. Am. Ceram. Soc. 2005, 88, 2979–2997. [Google Scholar] [CrossRef] [Green Version]
- Adler, S.B.; Lane, J.A.; Steele, B.C.H. Electrode kinetics of porous mixed-conducting oxygen electrodes. J. Electrochem. Soc. 1996, 143, 3554–3564. [Google Scholar] [CrossRef]
- Flura, A.; Nicollet, C.; Vibhu, V.; Zeimetz, B.; Rougier, A.; Bassat, J.-M.; Grenier, J.-C. Application of the adler-lane-steele model to porous La2NiO4+δ SOFC cathode: Influence of interfaces with gadolinia doped ceria. J. Electrochem. Soc. 2016, 163, F523–F532. [Google Scholar] [CrossRef]
- Adler, S.B. Mechanism and kinetics of oxygen reduction on porous La1–xSrxCoO3–δ electrodes. Solid State Ion. 1998, 111, 125–134. [Google Scholar] [CrossRef]
- Boukamp, B.A.; Verbraeken, M.; Blank, D.H.A.; Holtappels, P. SOFC-anodes, proof for a finite-length type Gerischer impedance? Solid State Ion. 2006, 177, 2539–2541. [Google Scholar] [CrossRef]
- Boukamp, B.A.; Rolle, A. Analysis and application of distribution of relaxation times in solid state ionics. Solid State Ion. 2017, 302, 12–18. [Google Scholar] [CrossRef]
- Ivers-Tiffée, E.; Weber, A. Evaluation of electrochemical impedance spectra by the distribution of relaxation times. J. Ceram. Soc. Jpn. 2017, 125, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, Y.; Yan, M.; Chen, F. Reconstruction of relaxation time distribution from linear electrochemical impedance spectroscopy. J. Power Sources 2015, 283, 464–477. [Google Scholar] [CrossRef]
- Bisquert, J.; Garcia-Belmonte, G.; Fabregat-Santiago, F.; Compte, A. Anomalous transport effects in the impedance of porous film electrodes. Electrochem. Commun. 1999, 1, 429–435. [Google Scholar] [CrossRef]
- Bisquert, J. Theory of the impedance of electron diffusion and recombination in a thin layer. J. Phys. Chem. B 2002, 106, 325–333. [Google Scholar] [CrossRef]
- Dierickx, S.; Mundloch, T.; Weber, A.; Ivers-Tiffée, E. Advanced impedance model for double-layered solid oxide fuel cell cermet anodes. J. Power Sources 2019, 415, 69–82. [Google Scholar] [CrossRef]
- Adler, S.B. Limitations of charge-transfer models for mixed-conducting oxygen electrodes. Solid State Ion. 2000, 135, 603–612. [Google Scholar] [CrossRef]
- Nielsen, J.; Jacobsen, T.; Wandel, M. Impedance of porous IT-SOFC LSCF:CGO composite cathodes. Electrochim. Acta 2011, 56, 7963–7974. [Google Scholar] [CrossRef]
- Nielsen, J.; Hjelm, J. Impedance of SOFC electrodes: A review and a comprehensive case study on the impedance of LSM:YSZ cathodes. Electrochim. Acta 2014, 115, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Nenning, A.; Gerstl, M.; Bram, M.; Opitz, A.K. Mechanistic insight into porous electrode impedance: An example of Ni + YSZ cermet anodes. ECS Trans. 2019, 91, 479–490. [Google Scholar] [CrossRef]
- Cowin, P.I.; Petit, C.T.; Lan, R.; Irvine, J.T.; Tao, S. Recent progress in the development of anode materials for solid oxide fuel cells. Adv. Energy Mater. 2011, 1, 314–332. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Su, C.; Wang, W.; Liu, M.; Tadé, M.O.; Shao, Z. Progress and prospects in symmetrical solid oxide fuel cells with two identical electrodes. Adv. Energy Mater. 2015, 5, 1500188. [Google Scholar] [CrossRef]
- Izzo, J.R.; Joshi, A.S.; Grew, K.N.; Chiu, W.K.S.; Tkachuk, A.; Wang, S.H.; Yun, W. Nondestructive reconstruction and analysis of SOFC anodes using X-ray computed tomography at sub-50 nm resolution. J. Electrochem. Soc. 2008, 155, B504–B508. [Google Scholar] [CrossRef] [Green Version]
- Kanno, D.; Shikazono, N.; Takagi, N.; Matsuzaki, K.; Kasagi, N. Evaluation of SOFC anode polarization simulation using three-dimensional microstructures reconstructed by FIB tomography. Electrochim. Acta 2011, 56, 4015–4021. [Google Scholar] [CrossRef]
- Masashi, K.; Marina, L.; Enrique, R.-T.; Nigel, B. Numerical modeling of nickel-infiltrated gadolinium-doped ceria electrodes reconstructed with focused ion beam tomography. Electrochim. Acta 2016, 190, 178–185. [Google Scholar]
- Shearing, P.R.; Golbert, J.; Chater, R.J.; Brandon, N.P. 3D reconstruction of SOFC anodes using a focused ion beam lift-out technique. Chem. Eng. Sci. 2009, 64, 3928–3933. [Google Scholar] [CrossRef]
- Tjaden, B.; Brett, D.J.L.; Shearing, P.R. Tortuosity in electrochemical devices: A review of calculation approaches. Int. Mater. Rev. 2018, 63, 47–67. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Bertei, A.; Tariq, F.; Brandon, N.; Cai, Q. Progress in 3D electrode microstructure modelling for fuel cells and batteries: Transport and electrochemical performance. Prog. Energy 2019, 1, 012003. [Google Scholar] [CrossRef]
- Gerstl, M.; Hutterer, A.; Fleig, J.; Bram, M.; Opitz, A.K. Model composite microelectrodes as a pathfinder for fully oxidic SOFC anodes. Solid State Ion. 2016, 298, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gerstl, M.; Nenning, A.; Iskandar, R.; Rojek-Woeckner, V.; Bram, M.; Hutter, H.; Opitz, A.K. The sulphur poisoning behaviour of gadolinia doped ceria model systems in reducing atmospheres. Materials 2016, 9, 649. [Google Scholar] [CrossRef] [Green Version]
- Chueh, W.C.; Haile, S.M. Electrochemical studies of capacitance in cerium oxide thin films and its relationship to anionic and electronic defect densities. Phys. Chem. Chem. Phys. 2009, 11, 8144–8148. [Google Scholar] [CrossRef]
- Schmid, A.; Rupp, G.M.; Slouka, C.; Navickas, E.; Andrejs, L.; Hutter, H.; Volgger, L.; Nenning, A.; Fleig, J. The chemical capacitance as a fingerprint of defect chemistry in mixed conducting oxides. Acta Chim. Slov. 2016, 63, 509–518. [Google Scholar]
- Schmid, A.; Rupp, G.M.; Fleig, J. Voltage and partial pressure dependent defect chemistry in (La,Sr)FeO 3−δ thin films investigated by chemical capacitance measurements. Phys. Chem. Chem. Phys. 2018, 20, 12016–12026. [Google Scholar] [CrossRef] [Green Version]
- Bishop, S.R.; Duncan, K.L.; Wachsman, E.D. Defect equilibria and chemical expansion in non-stoichiometric undoped and gadolinium-doped cerium oxide. Electrochim. Acta 2009, 54, 1436–1443. [Google Scholar] [CrossRef]
- Wang, S.; Inaba, H.; Tagawa, H.; Dokiya, M.; Hashimoto, T. Nonstoichiometry of Ce0.9Gd0.1O1.95−x. Solid State Ion. 1998, 107, 73–79. [Google Scholar] [CrossRef]
- Bishop, S.R.; Duncan, K.L.; Wachsman, E.D. Surface and bulk oxygen non-stoichiometry and bulk chemical expansion in gadolinium-doped cerium oxide. Acta Mater. 2009, 57, 3596–3605. [Google Scholar] [CrossRef]
- Kuhn, M.; Fukuda, Y.; Hashimoto, S.; Sato, K.; Yashiro, K.; Mizusaki, J. Oxygen nonstoichiometry and thermo-chemical stability of perovskite-type La0. 6Sr0. 4Co1-yFeyO3-δ (y = 0, 0.2, 0.4, 0.5, 0.6, 0.8, 1) Materials. J. Electrochem. Soc. 2013, 160, F34–F42. [Google Scholar] [CrossRef]
- Bisquert, J. Influence of the boundaries in the impedance of porous film electrodes. Phys. Chem. Chem. Phys. 2000, 2, 4185–4192. [Google Scholar] [CrossRef]
- Boukamp, B.A.; Bouwmeester, H.J.M. Interpretation of the Gerischer impedance in solid state ionics. Solid State Ion. 2003, 157, 29–33. [Google Scholar] [CrossRef]
- Haydn, M.; Bischof, C.; Udomsilp, D.; Opitz, A.K.; Bimashofer, G.; Schafbauer, W.; Brandner, M.; Bram, M. Metal supported SOFCs: Electrochemical performance under various testing conditions. ECS Trans. 2017, 78, 1993–2003. [Google Scholar] [CrossRef]
- Rojek-Wöckner, V.A.; Opitz, A.K.; Brandner, M.; Mathé, J.; Bram, M. A novel Ni/ceria-based anode for metal-supported solid oxide fuel cells. J. Power Sources 2016, 328, 65–74. [Google Scholar] [CrossRef]
- Opitz, A.; Gerstl, M.; Bram, M. Model-system supported impedance simulation of composite electrodes. Fuel Cells 2019, 19, 417–428. [Google Scholar] [CrossRef] [Green Version]
- Nenning, A.; Opitz, A.K.; Huber, T.M.; Fleig, J. A novel approach for analyzing electrochemical properties of mixed conducting solid oxide fuel cell anode materials by impedance spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 22321–22336. [Google Scholar] [CrossRef]
- Primdahl, S.; Mogensen, M. Gas diffusion impedance in characterization of solid oxide fuel cell anodes. J. Electrochem. Soc. 1999, 146, 2827–2833. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 978-0-470-11539-8. [Google Scholar]
- Suwanwarangkul, R.; Croiset, E.; Fowler, M.W.; Douglas, P.L.; Entchev, E.; Douglas, M.A. Performance comparison of Fick’s, dusty-gas and Stefan–Maxwell models to predict the concentration overpotential of a SOFC anode. J. Power Sources 2003, 122, 9–18. [Google Scholar] [CrossRef]
- Iwai, H.; Shikazono, N.; Matsui, T.; Teshima, H.; Kishimoto, M.; Kishida, R.; Hayashi, D.; Matsuzaki, K.; Kanno, D.; Saito, M.; et al. Quantification of SOFC anode microstructure based on dual beam FIB-SEM technique. J. Power Sources 2010, 195, 955–961. [Google Scholar] [CrossRef]
- Kim, S.; Merkle, R.; Maier, J. Oxygen nonstoichiometry of nanosized ceria powder. Surf. Sci. 2004, 549, 196–202. [Google Scholar] [CrossRef]
- Nenning, A.; Volgger, L.; Miller, E.; Mogni, L.V.; Barnett, S.; Fleig, J. The electrochemical properties of Sr (Ti, Fe) O3-δ for anodes in solid oxide fuel cells. J. Electrochem. Soc. 2017, 164, F364–F371. [Google Scholar] [CrossRef] [Green Version]
- Gopal, C.B.; Gabaly, F.E.; McDaniel, A.H.; Chueh, W.C. Origin and tunability of unusually large surface capacitance in doped cerium oxide studied by ambient-pressure X-Ray photoelectron spectroscopy. Adv. Mater. 2016, 28, 4692–4697. [Google Scholar] [CrossRef]
- Tsoga, A.; Gupta, A.; Naoumidis, A.; Nikolopoulos, P. Gadolinia-doped ceria and yttria stabilized zirconia interfaces: Regarding their application for SOFC technology. Acta Mater. 2000, 48, 4709–4714. [Google Scholar] [CrossRef]
- Wang, S.R.; Kobayashi, T.; Dokiya, M.; Hashimoto, T. Electrical and ionic conductivity of Gd-doped ceria. J. Electrochem. Soc. 2000, 147, 3606–3609. [Google Scholar] [CrossRef]
- Joos, J.; Carraro, T.; Weber, A.; Ivers-Tiffée, E. Reconstruction of porous electrodes by FIB/SEM for detailed microstructure modeling. J. Power Sources 2011, 196, 7302–7307. [Google Scholar] [CrossRef]
- Carniglia, S.C. Construction of the tortuosity factor from porosimetry. J. Catal. 1986, 102, 401–418. [Google Scholar] [CrossRef]
- Avila-Paredes, H.J.; Choi, K.; Chen, C.-T.; Kim, S. Dopant-concentration dependence of grain-boundary conductivity in ceria: A space-charge analysis. J. Mater. Chem. 2009, 19, 4837–4842. [Google Scholar] [CrossRef]
- Zhang, T.S.; Ma, J.; Cheng, H.; Chan, S.H. Ionic conductivity of high-purity Gd-doped ceria solid solutions. Mater. Res. Bull. 2006, 41, 563–568. [Google Scholar] [CrossRef]
- Guo, X.; Waser, R. Electrical properties of the grain boundaries of oxygen ion conductors: Acceptor-doped zirconia and ceria. Prog. Mater. Sci. 2006, 51, 151–210. [Google Scholar] [CrossRef]
- Nenning, A.; Opitz, A. Low oxygen partial pressure increases grain boundary ion conductivity in Gd-doped ceria thin films. J. Phys. Energy 2019, 2, 014002. [Google Scholar] [CrossRef]
- Jung, W.; Gu, K.L.; Choi, Y.; Haile, S.M. Robust nanostructures with exceptionally high electrochemical reaction activity for high temperature fuel cell electrodes. Energy Environ. Sci. 2014, 7, 1685–1692. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Seo, J.; Jung, W. Sintering-resistant Pt@CeO2 nanoparticles for high-Temperature oxidation catalysis. Nanoscale 2016, 8, 10219–10228. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.; Dereux, J.O.; Chueh, W.C.; Hao, Y.; Haile, S.M. High electrode activity of nanostructured, columnar ceria films for solid oxide fuel cells. Energy Environ. Sci. 2012, 5, 8682–8689. [Google Scholar] [CrossRef] [Green Version]









| Gas Phase Pressures (mbar) | Dbinary (cm2/s) [60] | ASRdiff (Ωcm2) | Jlimit (Acm−2) |
|---|---|---|---|
| 25 H2 + 25 H2O + 950 Ar | 6.3 (H2-Ar); 2 (H2O-Ar) | 0.27 | 0.75 |
| 500 H2 + 500 H2O | 7.2 (H2-H2O) | 0.005 | 16 |
| 990 H2 + 10 H2O | 7.2 (H2-H2O) | 0.14 | 32 |
| 45 H2 + 25 H2O | 110 (H2-H2O) | 0.006 | 21 |
| 150 O2 + 850 N2 | 1.7 (O2-N2) | 0.01 | 2.3 |
| 10 O2 + 990 N2 | 0.15 (O2-N2) | 0.15 | 0.16 |
| Parameter | Value (Prediction) | Value (Fit, Mean) | Reference |
|---|---|---|---|
| GDC phase ion conductivity | 25 mS/cm (650 °C) 60 mS/cm (750 °C) | - | GDC10 polycrystal [67] |
| GDC phase volume fraction | 0.3 | - | Porosity estimate from SEM cross-sections |
| GDC phase tortuosity | 3 | 2.7 ± 0.4 | Estimate from SEM cross-sections [40,62,68,69] |
| Effective ion conductivity | 2.5 mS/cm (650 °C) 6 mS/cm (750 °C) | 2.5 ± 0.15 mS/cm (650 °C) 5.3 ± 0.5 mS/cm (750 °C) | |
| Effective chemical capacitance | 90 F/cm3 (650 °C) 260 F/cm3 (750 °C) | 112 ± 4 F/cm3 (650 °C) 321 ± 6 F/cm3 (750 °C) | Thermogravimetry [50,51], Equation (14) |
| GDC surface ASR | 6 Ωcm2 (650 °C) 4 Ωcm2 (750 °C) | - | Thin film electrodes (initial performance) 650 °C: [18]; 750 °C: [44] |
| Specific GDC surface area | 5 μm2/ μm3 | - | Particle packing model, Appendix A |
| Electrochemical reaction resistance (Rreact) | 120 µΩcm3(650 °C) 80 µΩcm3 (750 °C) | 70 ± 10µΩcm3 (650 °C) 36 ± 5 µΩcm3 (750 °C) | |
| Electrode ASR (6-μm-thickness) | 0.23 Ωcm2 (650 °C) 0.13 Ωcm2 (750 °C) | 0.17 ± 0.02 Ωcm2 (650 °C) 0.08 ± 0.01 Ωcm2 (750 °C) | Equation (29) |
| Electrochemically active thickness | 5.4 µm (650 °C) 6.4 µm (750 °C) | 4.4 ± 0.4 µm (650 °C) 4.2 ± 0.4 µm (750 °C) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nenning, A.; Bischof, C.; Fleig, J.; Bram, M.; Opitz, A.K. The Relation of Microstructure, Materials Properties and Impedance of SOFC Electrodes: A Case Study of Ni/GDC Anodes. Energies 2020, 13, 987. https://doi.org/10.3390/en13040987
Nenning A, Bischof C, Fleig J, Bram M, Opitz AK. The Relation of Microstructure, Materials Properties and Impedance of SOFC Electrodes: A Case Study of Ni/GDC Anodes. Energies. 2020; 13(4):987. https://doi.org/10.3390/en13040987
Chicago/Turabian StyleNenning, Andreas, Cornelia Bischof, Jürgen Fleig, Martin Bram, and Alexander K. Opitz. 2020. "The Relation of Microstructure, Materials Properties and Impedance of SOFC Electrodes: A Case Study of Ni/GDC Anodes" Energies 13, no. 4: 987. https://doi.org/10.3390/en13040987