Sensitivity Analysis of an Implanted Antenna within Surrounding Biological Environment
Abstract
:1. Introduction
2. Implanted Antenna Scenario and Transmission Link
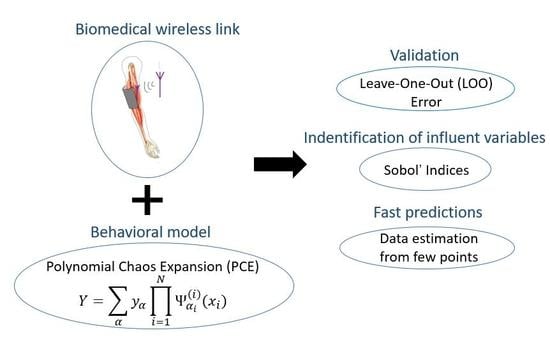
3. Polynomial Chaos Expansion (PCE)
Surrogate Model Based on PCE
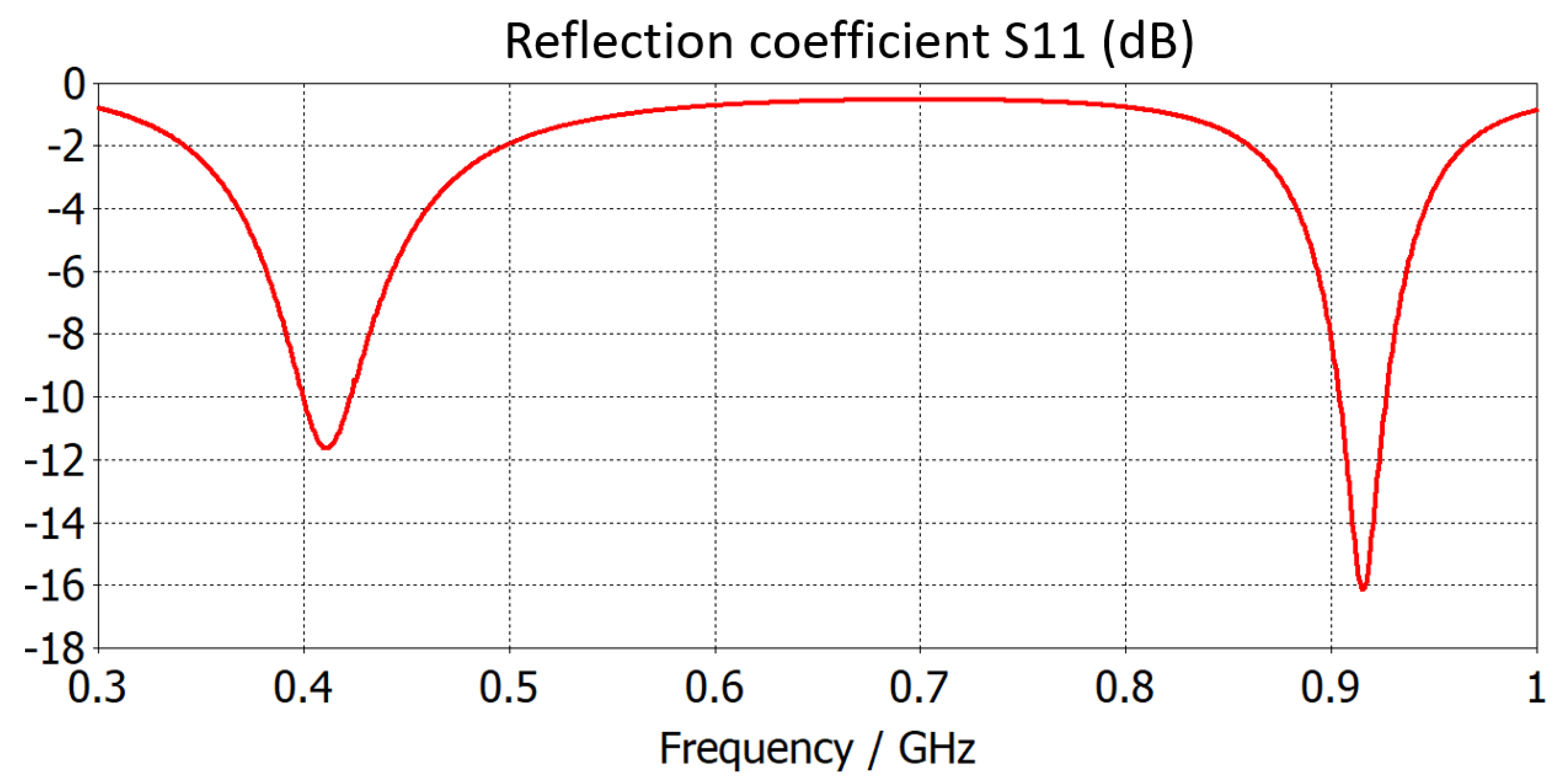
4. Numerical Results and Discussion
4.1. LOO Error
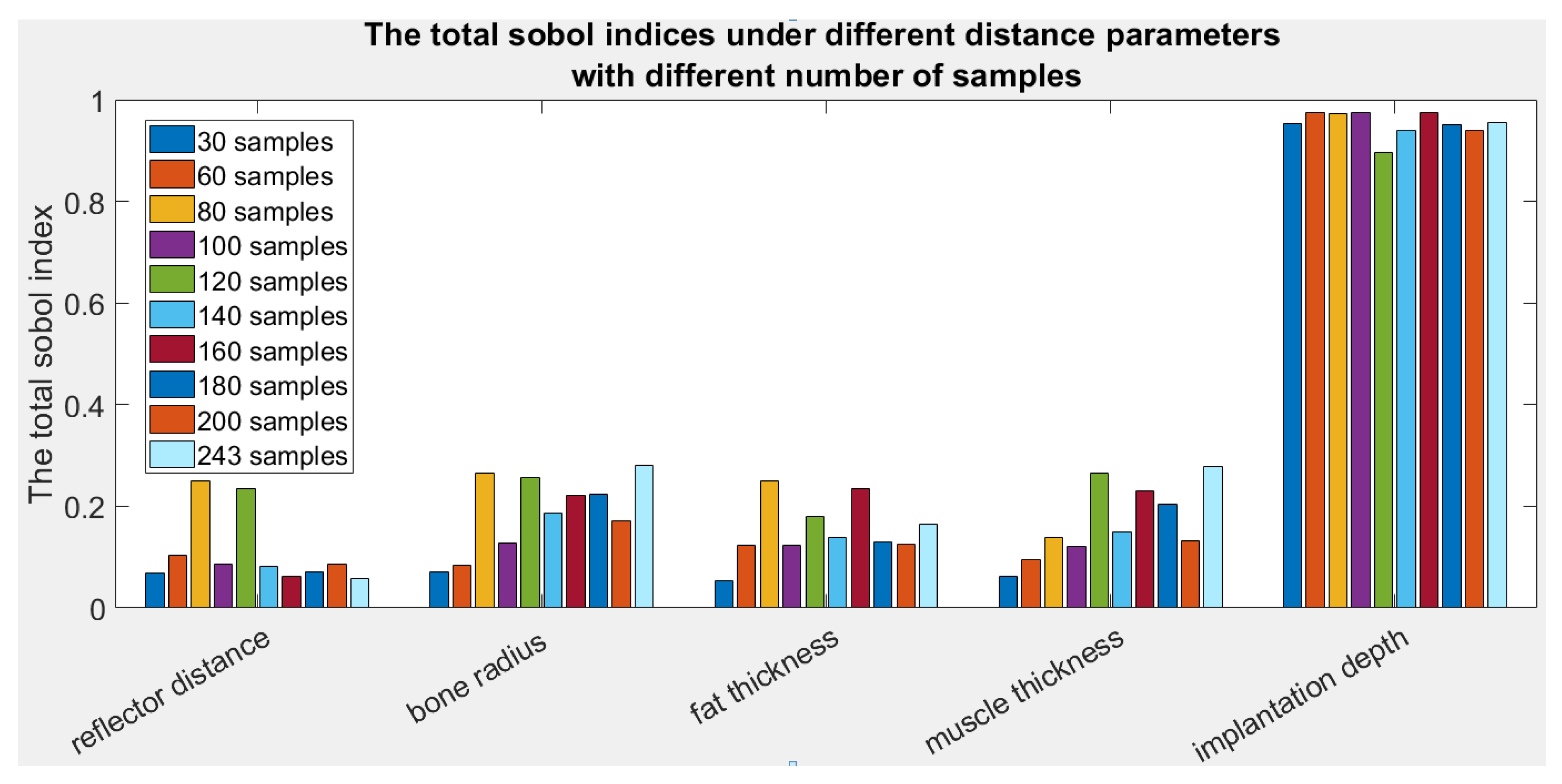
4.2. Sobol Indices
4.3. Data Prediction
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kiourti, A.; Nikita, K.S. A review of implantable patch antennas for biomedical telemetry: Challenges and solutions. IEEE Antennas Propag. Mag. 2012, 54, 210–228. [Google Scholar] [CrossRef]
- Campi, T.; Cruciani, S.; Palandrani, F.; De Santis, V.; Hirata, A.; Feliziani, M. Wireless Power Transfer Charging System for AIMDs and Pacemakers. IEEE Trans. Microw. Theory Tech. 2016, 64, 633–642. [Google Scholar] [CrossRef]
- Basar, M.R.; Ahmad, M.Y.; Cho, J.; Ibrahim, F. An Improved Wearable Resonant Wireless Power Transfer System for Biomedical Capsule Endoscope. IEEE Trans. Ind. Electron. 2018, 65, 7772–7781. [Google Scholar] [CrossRef]
- Hall, P.S.; Hao, Y. Antennas and Propagation for Body-Centric Wireless Communications; Artech House: Norwood, MA, USA, 2006. [Google Scholar]
- Karacolak, T.; Hood, A.; Topsakal, E. Design of a dual-band implantable antenna and development of skin mimicking gels for continuous glucose monitoring. IEEE Trans. Microw. Theory Tech. 2008, 56, 1001–1008. [Google Scholar] [CrossRef]
- Huang, F.-J.; Lee, C.-M.; Chang, C.-L.; Chen, L.-K.; Yo, T.-C.; Luo, C.-H. Rectenna application of miniaturized implantable antenna design for trible-band biotelemetry communication. IEEE Trans. Antennas Propag. 2011, 59, 2646–2653. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Sun, H.; Xiao, S. Design and safety considerations of an implantable rectenna for far-field wireless power transfer. IEEE Trans. Antennas Propag. 2014, 62, 5798–5806. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Liu, X. Circularly Polarized Implantable Antenna for 915 MHz ISM-Band Far-Field Wireless Power Transmission. IEEE Antennas Wirel. Propag. Lett. 2018, 17, 373–376. [Google Scholar] [CrossRef]
- Cheng, H.; Yu, T.; Luo, C. Direct current driving impedance matching method for rectenna using medical implant communication service band for wireless battery charging. IET Microw. Antennas Propag. 2013, 7, 277–282. [Google Scholar] [CrossRef]
- DeLong, B.J.; Kiourti, A.; Volakis, J.L. A Radiating Near-Field Patch Rectenna for Wireless Power Transfer to Medical Implants at 2.4 GHz. IEEE J. Electromagn. RF Microw. Med. Biol. 2018, 2, 64–69. [Google Scholar] [CrossRef]
- Okba, A.; Takacs, A.; Aubert, H. Compact Rectennas for Ultra-Low-Power Wireless Transmission Applications. IEEE Trans. Microw. Theory Tech. 2019, 67, 1697–1707. [Google Scholar] [CrossRef]
- Bakogianni, S.; Palaiologos, M.; Koulouridis, S. Performance evaluation and sensitivity analysis of a novel rectenna system for deep implanted devices. In Proceedings of the 2017 11th European Conference on Antennas and Propagation (EUCAP), Paris, France, 19–24 March 2017. [Google Scholar]
- Zhang, H.; Gao, S.; Ngo, T.; Wu, W.; Guo, Y. Wireless Power Transfer Antenna Alignment Using Intermodulation for Two-Tone Powered Implantable Medical Devices. IEEE Trans. Microw. Theory Tech. 2019, 67, 1708–1716. [Google Scholar] [CrossRef]
- Kiourti, A.; Nikita, K.S. Miniature scalp-implantable antennas for telemetry in the MICS and ISM bands: Design, safety considerations and link budget analysis. IEEE T. Antenn. Propag. 2012, 60, 3568–3575. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Sharawi, M.S. Miniaturized dual-wideband circular patch antenna for biomedical telemetry. In Proceedings of the 2017 11th European Conference on Antennas and Propagation (EUCAP), Paris, France, 19–24 March 2017; pp. 1027–1030. [Google Scholar]
- Ding, S.; Koulouridis, S.; Pichon, L. A Dual-Band Miniaturized Circular Antenna for Deep in Body Biomedical Wireless Applications. In Proceedings of the 13th European Conference on Antennas and Propagation (EuCAP), Krakow, Poland, 31 March–5 April 2019. [Google Scholar]
- Silly-Carette, J.; Lautru, D.; Wong, M.-F.; Gati, A.; Wiart, J.; Fouad Hanna, V. Variability on the Propagation of a Plane Wave Using Stochastic Collocation Methods in a Bio Electromagnetic Application. IEEE Microw. Wirel. Compon. Lett. 2009, 19, 185–187. [Google Scholar] [CrossRef]
- Liorni, I.; Parazzini, M.; Fiocchi, S.; Ravazzani, P. Study of the Influence of the Orientation of a 50-Hz Magnetic Field on Fetal Exposure Using Polynomial Chaos Decomposition. Int. J. Environ. Res. Public Health 2015, 12, 5934–5953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voyer, D.; Musy, F.; Nicolas, L.; Perrussel, R. Probabilistic methods applied to 2D electromagnetic numerical dosimetry. Compel Int. J. Comput. Math. Electr. Electron. Eng. 2008, 27, 651–667. [Google Scholar] [CrossRef] [Green Version]
- Larbi, M.; Stievano, I.S.; Canavero, F.G.; Besnier, P. Variability Impact of Many Design Parameters: The Case of a Realistic Electronic Link. IEEE Trans. Electromagn. Compat. 2018, 60, 34–41. [Google Scholar] [CrossRef]
- International Telecommunications Union. Recommendation ITU-R RS.1346; ITU: Geneva, Switzerland, 1998. [Google Scholar]
- FCC. Standard Specification for Radiated Emission Limits, General Requirements; FCC 15. 209; FCC: Washington, DC, USA, 1990.
- Sudret, B. Global sensitivity analysis using polynomial chaos expansions. Reliab. Eng. Syst. Saf. 2008, 93, 964–979. [Google Scholar] [CrossRef]
- Blatman, G.; Sudret, B. Adaptive sparse polynomial chaos expansion based on least angle regression. J. Comput. Phys. 2011, 230, 2345–2367. [Google Scholar] [CrossRef]
- Sobol, I.M. Sensitivity estimates for nonlinear mathematical models. Math. Model. Comput. Exp. 1993, 1, 407–414. [Google Scholar]
- Marelli, S.; Sudret, B. UQLab: A framework for uncertainty quantification in Matlab. In Proceedings of the 2nd International Conference on Vulnerability, Risk Analysis and Management (ICVRAM2014), Liverpool, UK, 24 April 2014; pp. 2554–2563. [Google Scholar]
- Computer Simulation Technology (CST). STUDIO SUITE; Ver 2017, CST AG; CST: Danvers, MA, USA, 2017. [Google Scholar]
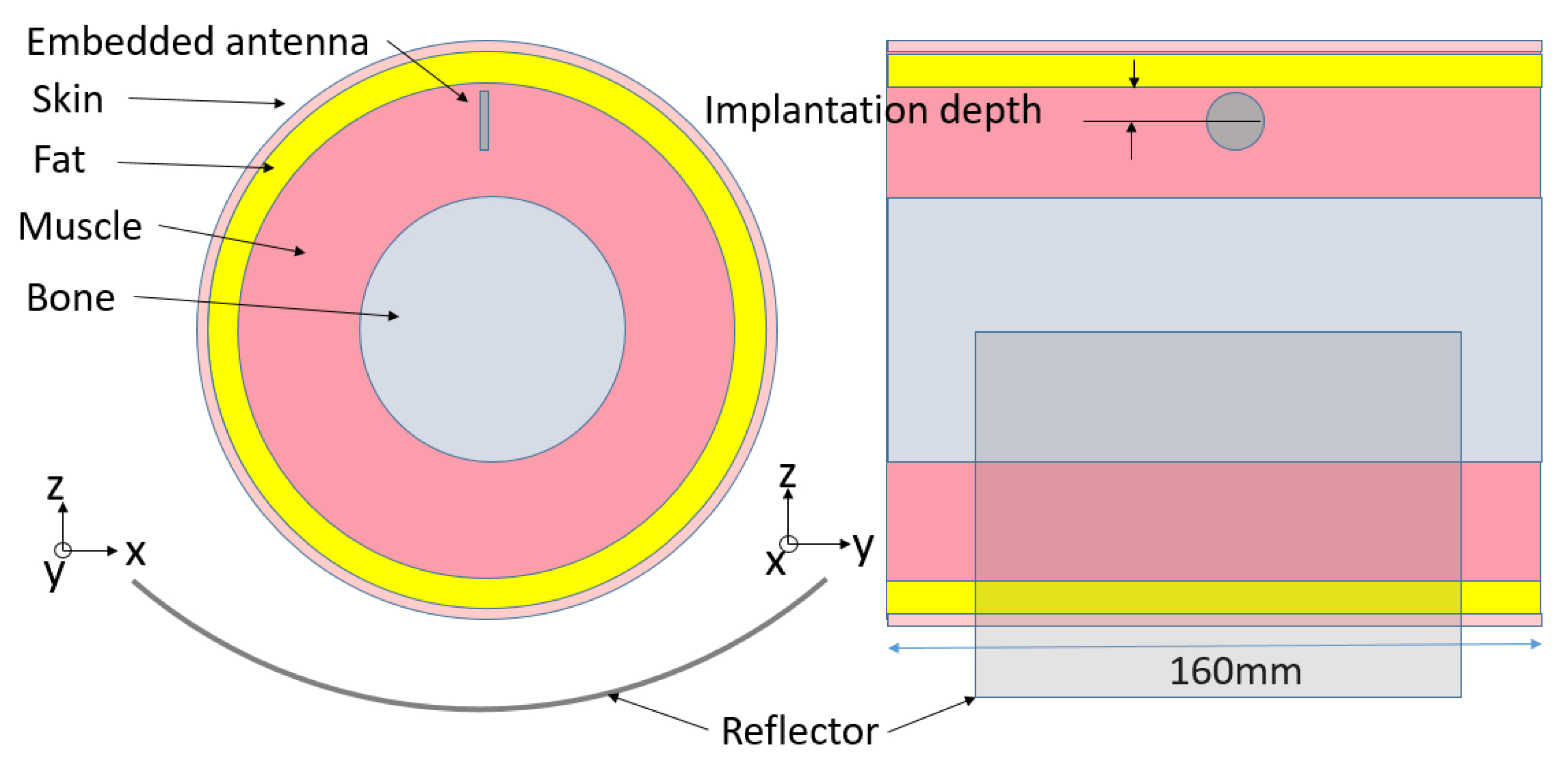
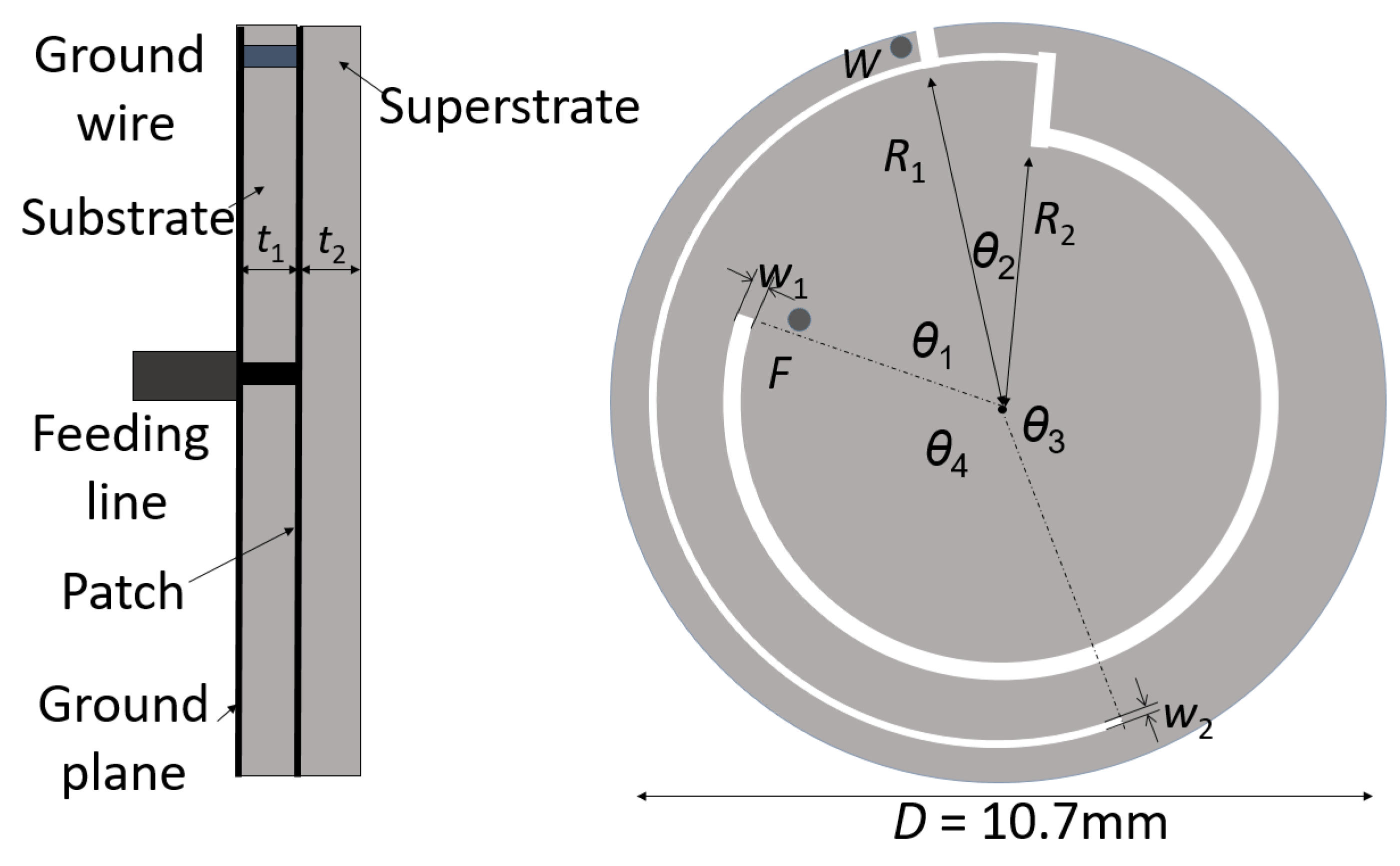

| Frequency | Bone | Muscle | Skin | Fat | |
|---|---|---|---|---|---|
| 915 MHz | εr | 12.45 | 54.98 | 41.35 | 5.46 |
| σ (S/m) | 0.15 | 0.93 | 0.85 | 0.10 | |
| Parameter Name | Value (mm) | Parameter Name | Value (mm) | Parameter Name | Value (deg) |
|---|---|---|---|---|---|
| R1 | 4.9 | t1 | 0.64 | θ1 | 70 |
| R2 | 3.76 | t2 | 0.64 | θ2 | 18 |
| w1 | 0.15 | D | 10.7 | θ3 | 163 |
| w2 | 0.32 | - | - | θ4 | 109 |
| Parameter Name | Bone Radius B | Reflector to Skin Distance R | Fat Thickness F | Muscle Thickness M | Implantation Depth I |
|---|---|---|---|---|---|
| Range (mm) | 20–40 | 0–20 | 1–20 | 25–40 | 10–20 |
| Test Samples (B, R, F, M, I) (mm) | Simulated Gain (dB) | Estimated Gain (dB) | Absolute Error (dB) | Relative Error (%) |
|---|---|---|---|---|
| (10; 30; 10.5; 37.5; 15) | −29.7364 | −29.4675 | 0.268919 | 0.9043 |
| (10; 36.67; 10.5; 37.5; 15) | −29.0338 | −28.6122 | 0.421595 | 1.4521 |
| (3.3; 30; 10.5; 27.5; 11.67) | −27.8142 | −27.5534 | 0.260767 | 0.9375 |
| (10; 23.33; 10.5; 37.5; 15) | −28.7928 | −29.253 | 0.460239 | 1.5985 |
| (16.67; 0; 10.5; 32.5; 15) | −28.2000 | −28.6401 | 0.440114 | 1.5607 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, S.; Pichon, L. Sensitivity Analysis of an Implanted Antenna within Surrounding Biological Environment. Energies 2020, 13, 996. https://doi.org/10.3390/en13040996
Ding S, Pichon L. Sensitivity Analysis of an Implanted Antenna within Surrounding Biological Environment. Energies. 2020; 13(4):996. https://doi.org/10.3390/en13040996
Chicago/Turabian StyleDing, Shuoliang, and Lionel Pichon. 2020. "Sensitivity Analysis of an Implanted Antenna within Surrounding Biological Environment" Energies 13, no. 4: 996. https://doi.org/10.3390/en13040996