Design and Implementation of a Wireless Charging-Based Cardiac Monitoring System Focused on Temperature Reduction and Robust Power Transfer Efficiency
Abstract
:1. Introduction
2. Analysis of Wireless Power Transfer System for the IMDs
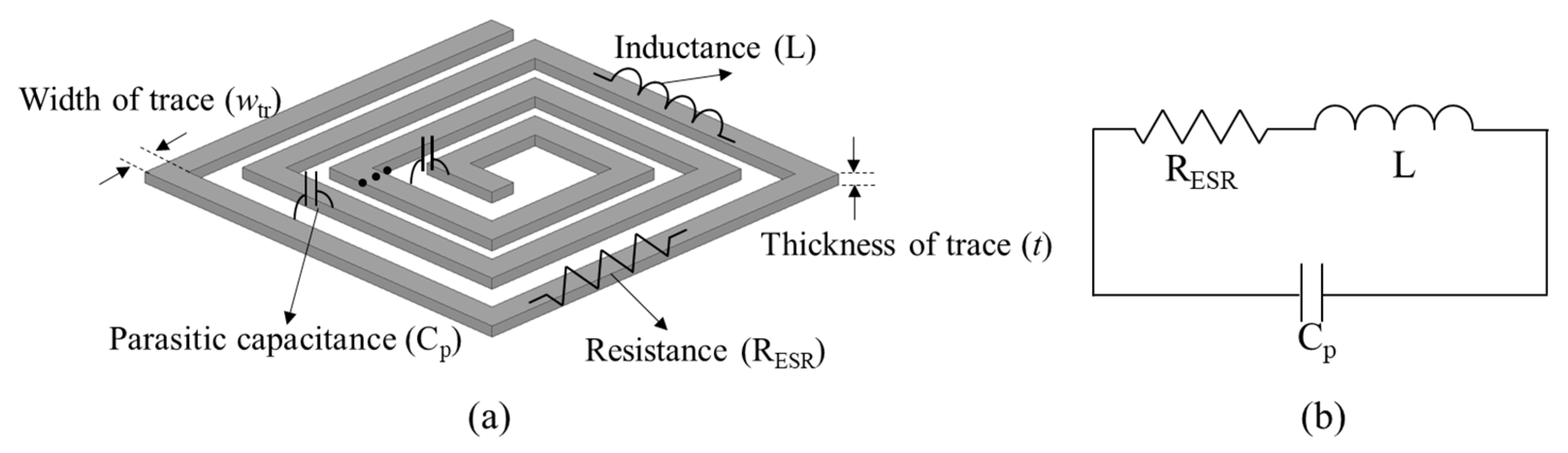
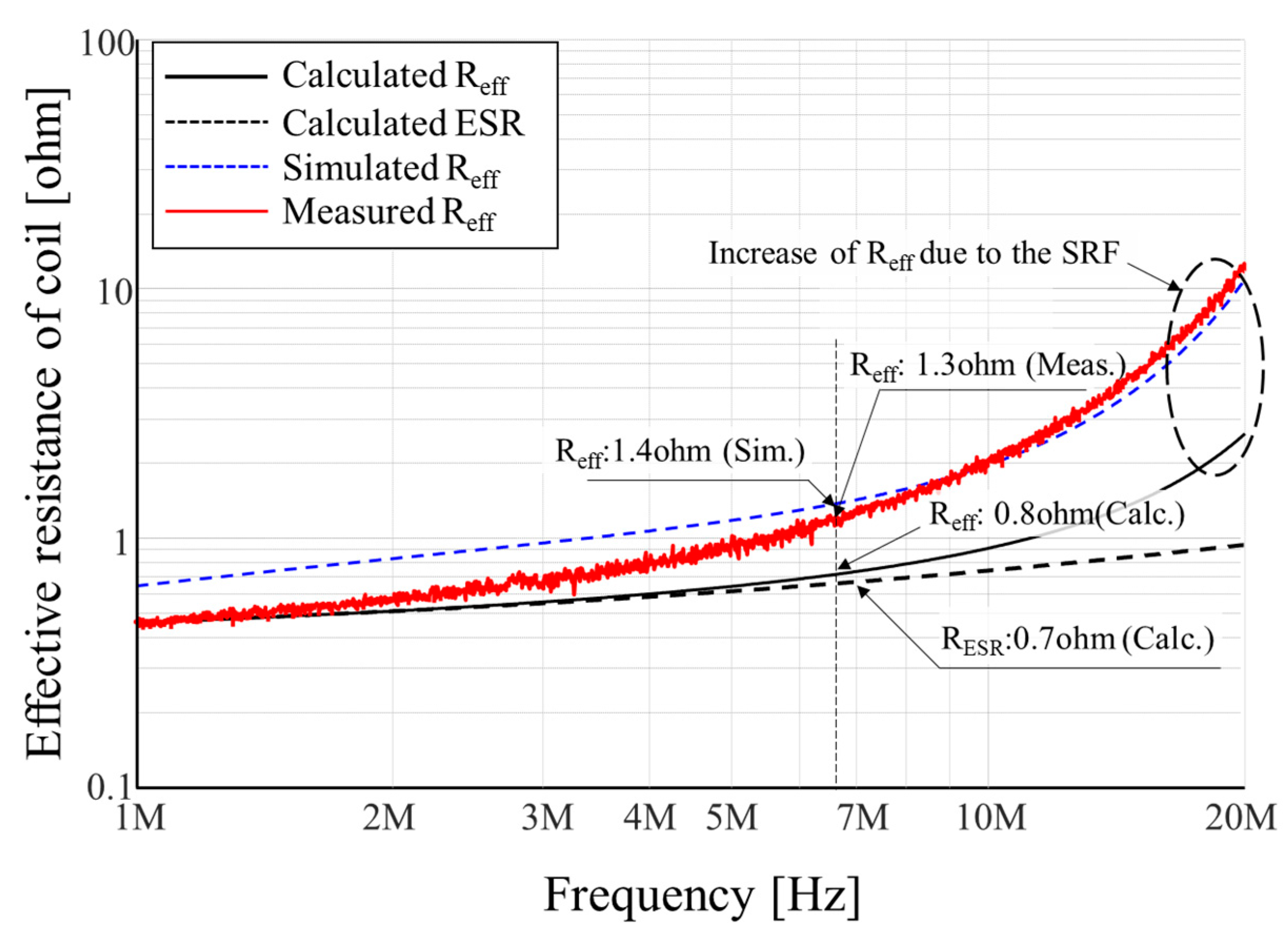
2.1. Design of the Transmitting Coil to Minimize Ohmic Loss
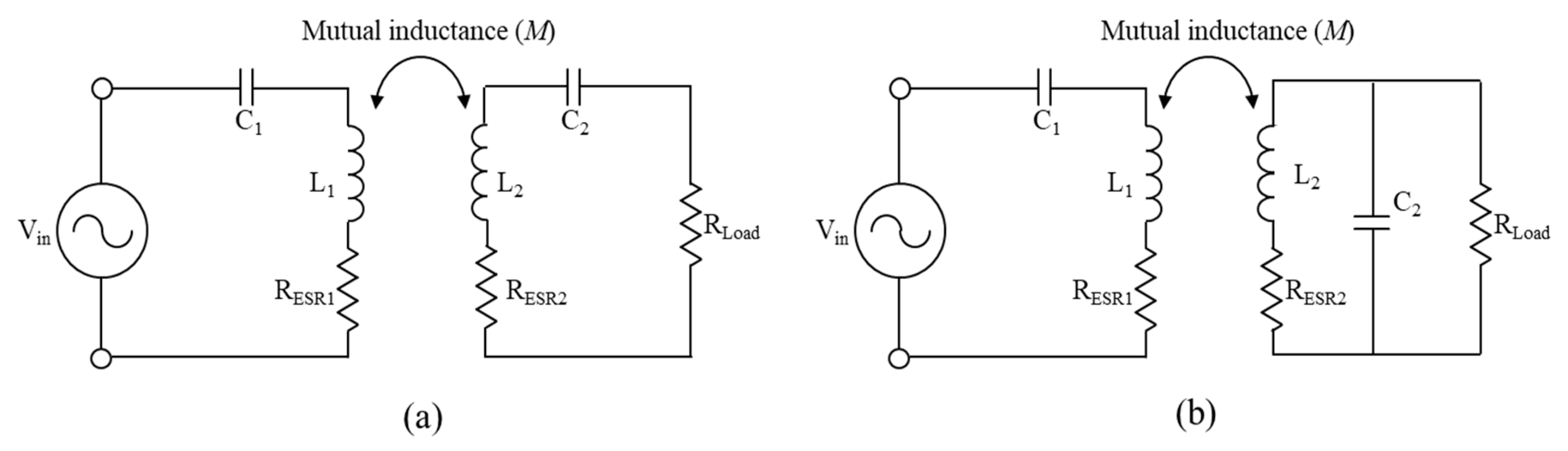
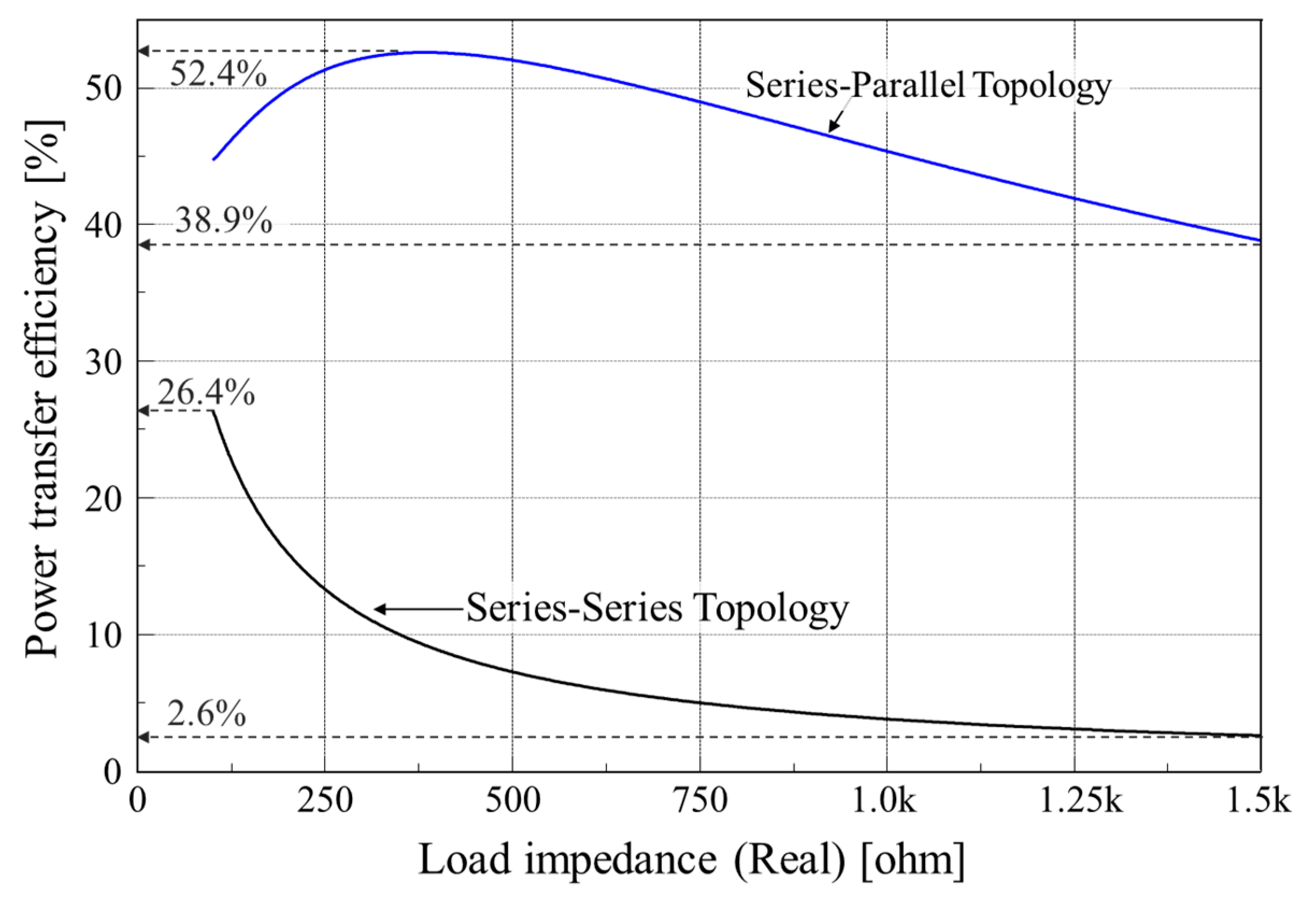
2.2. Topology Analysis for Robust Powering
3. Analysis of Wireless Power Transfer System for the IMDs
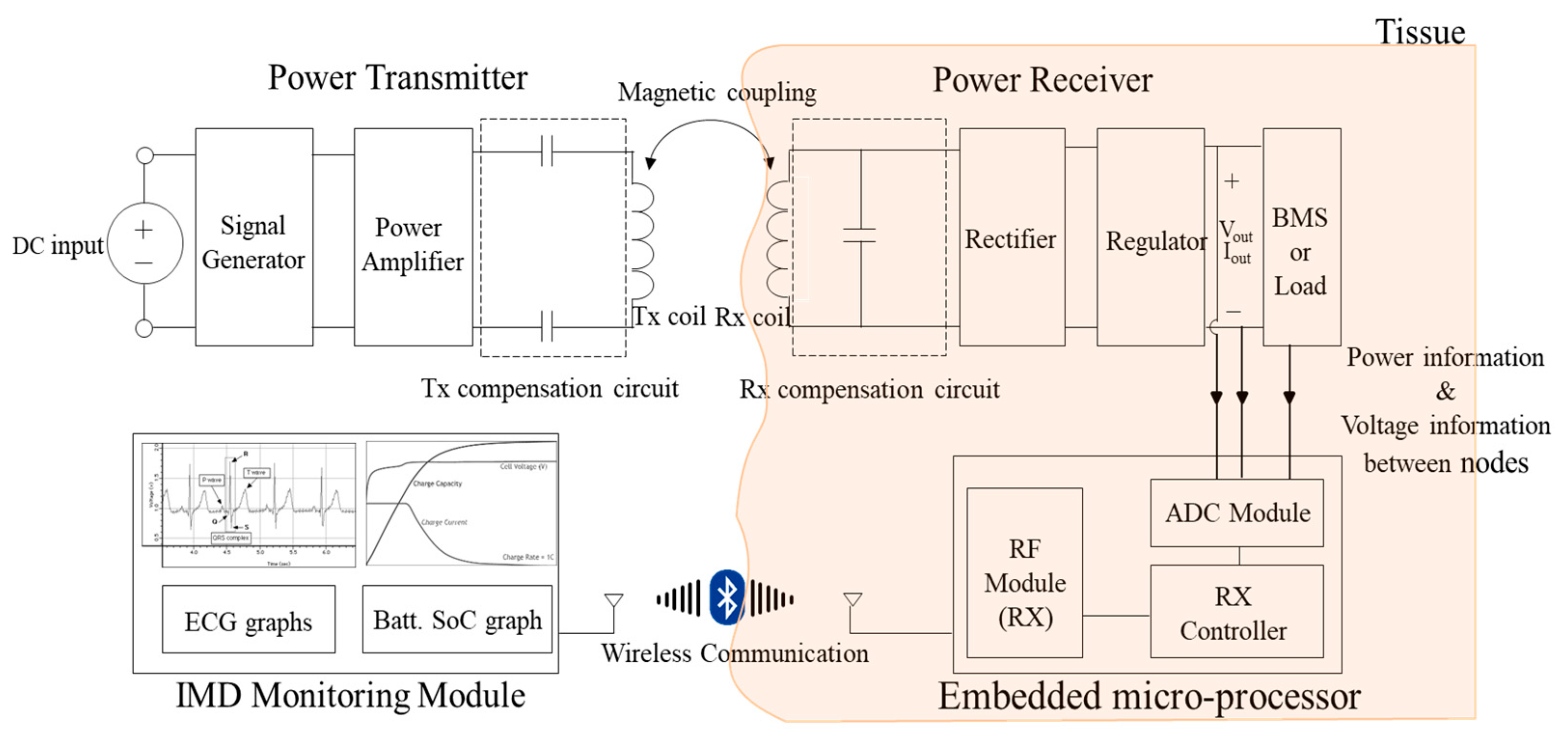
3.1. System Architecture of Cardiac Monitoring System
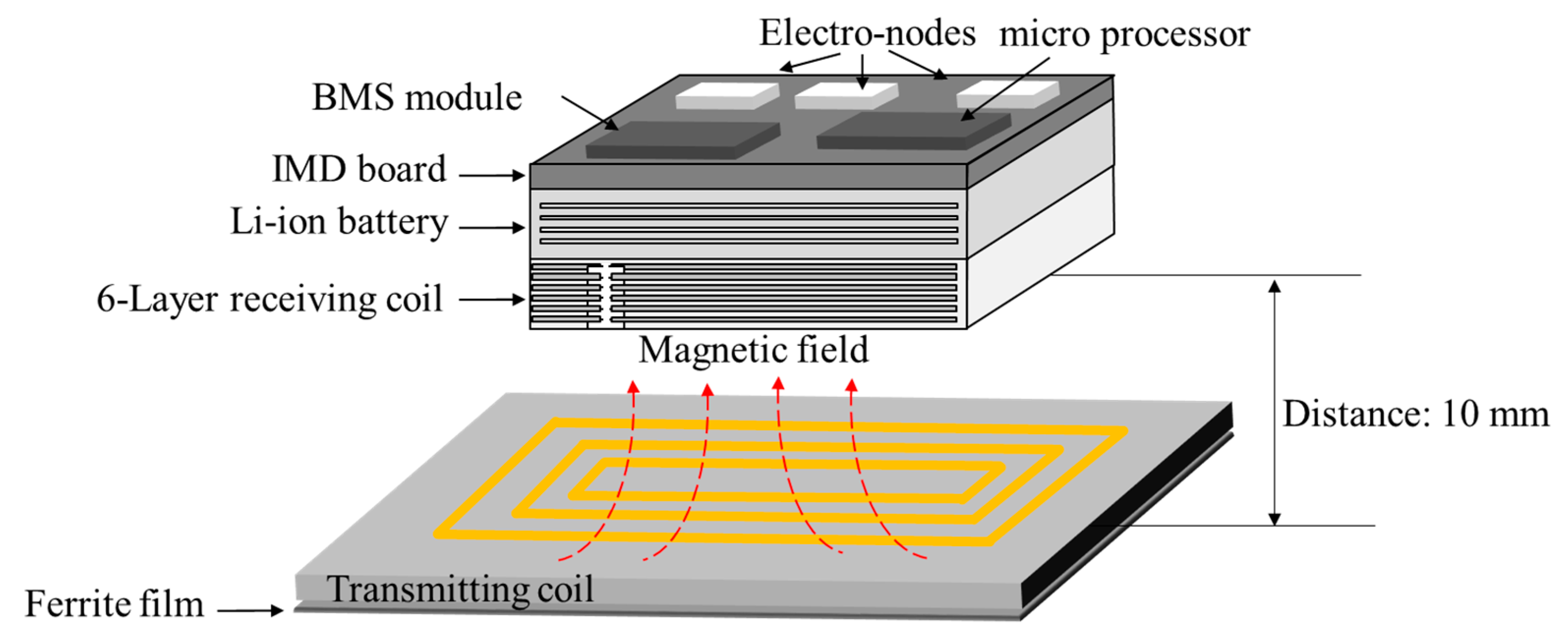
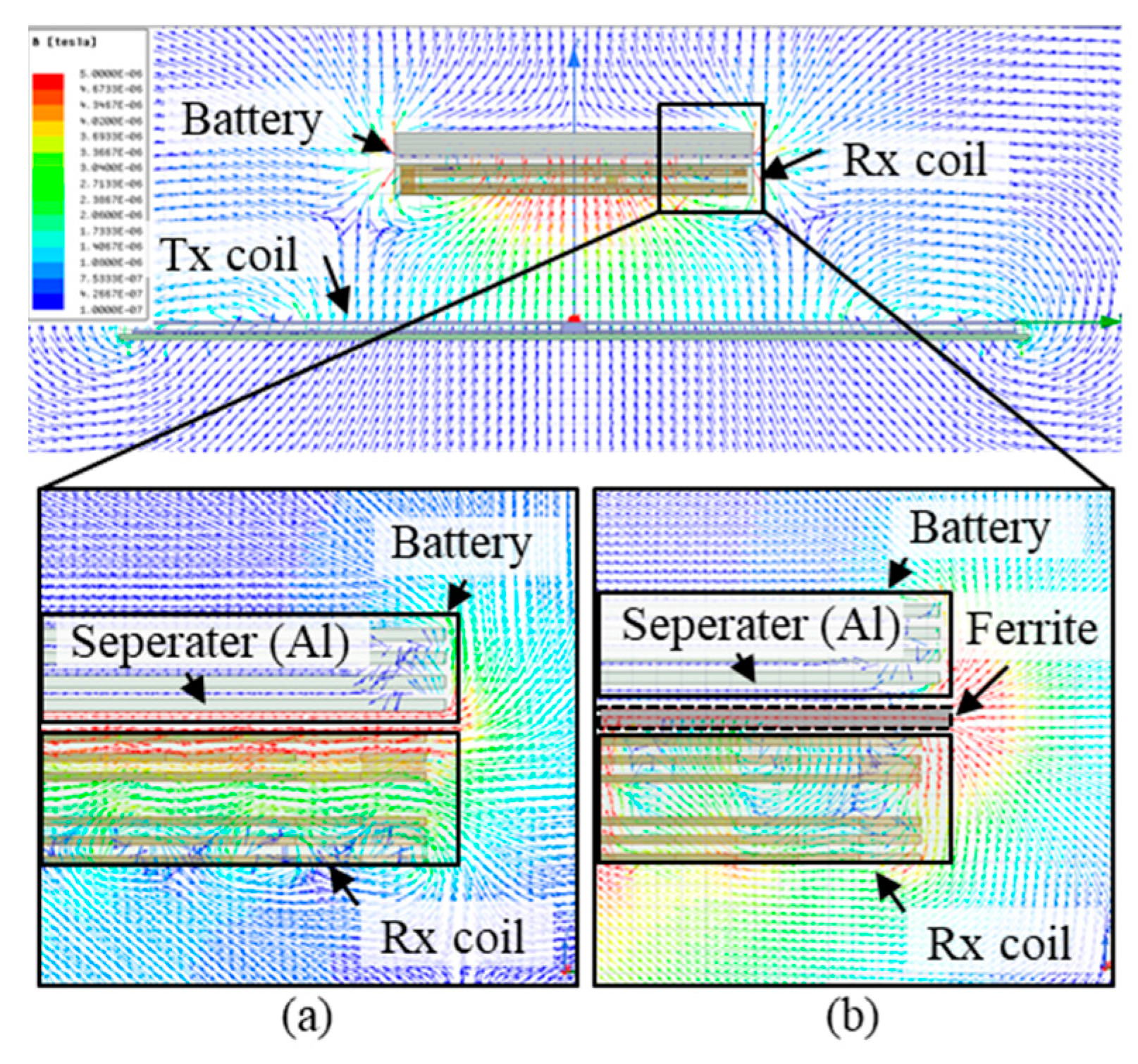
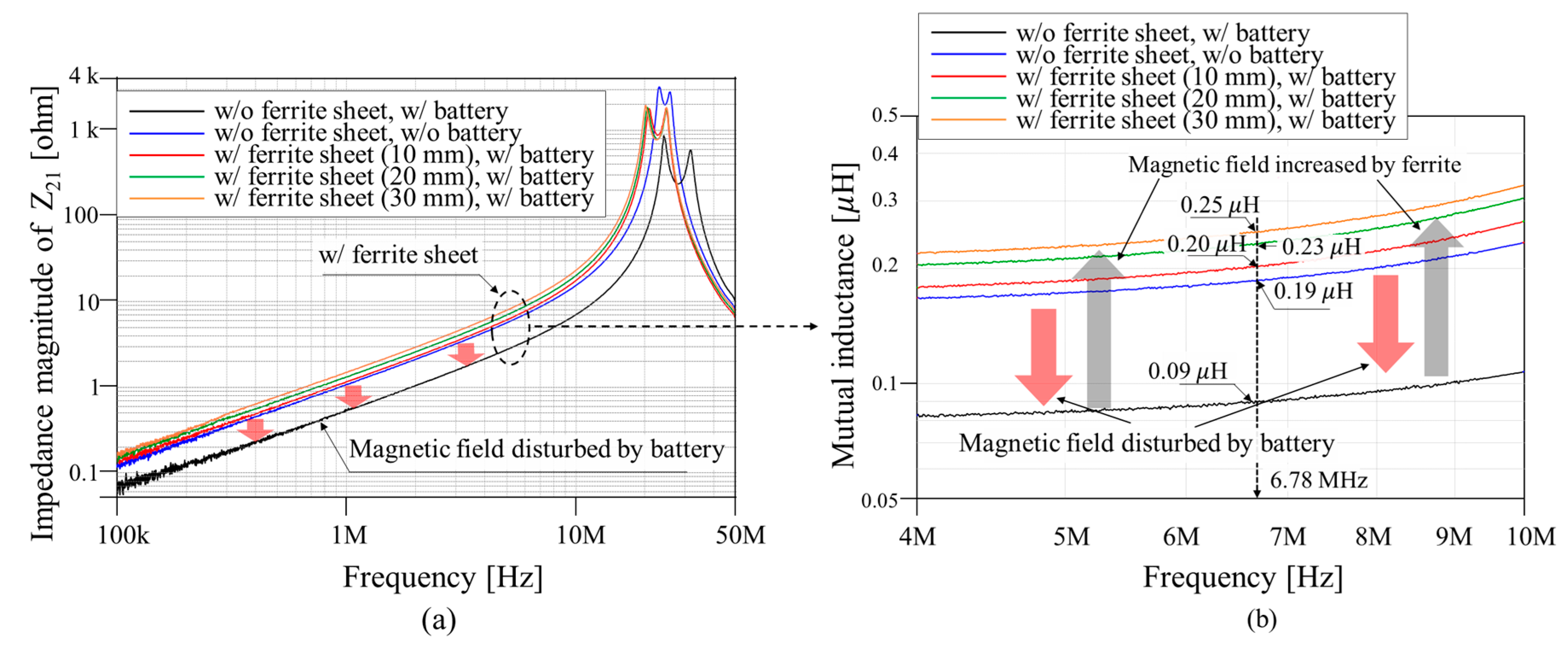
3.2. Magnetic Guidance and Determination of Thickness for Strong Magnetic Coupling
4. Experimental Verification of Proposed Design
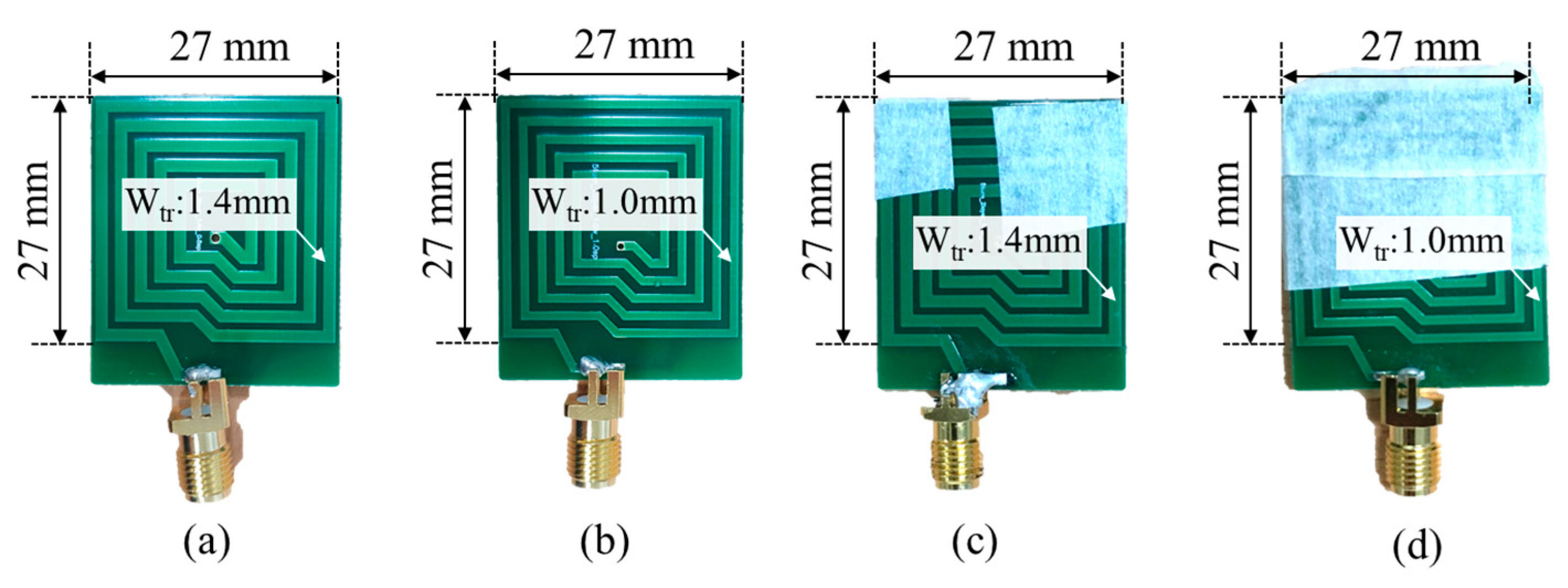
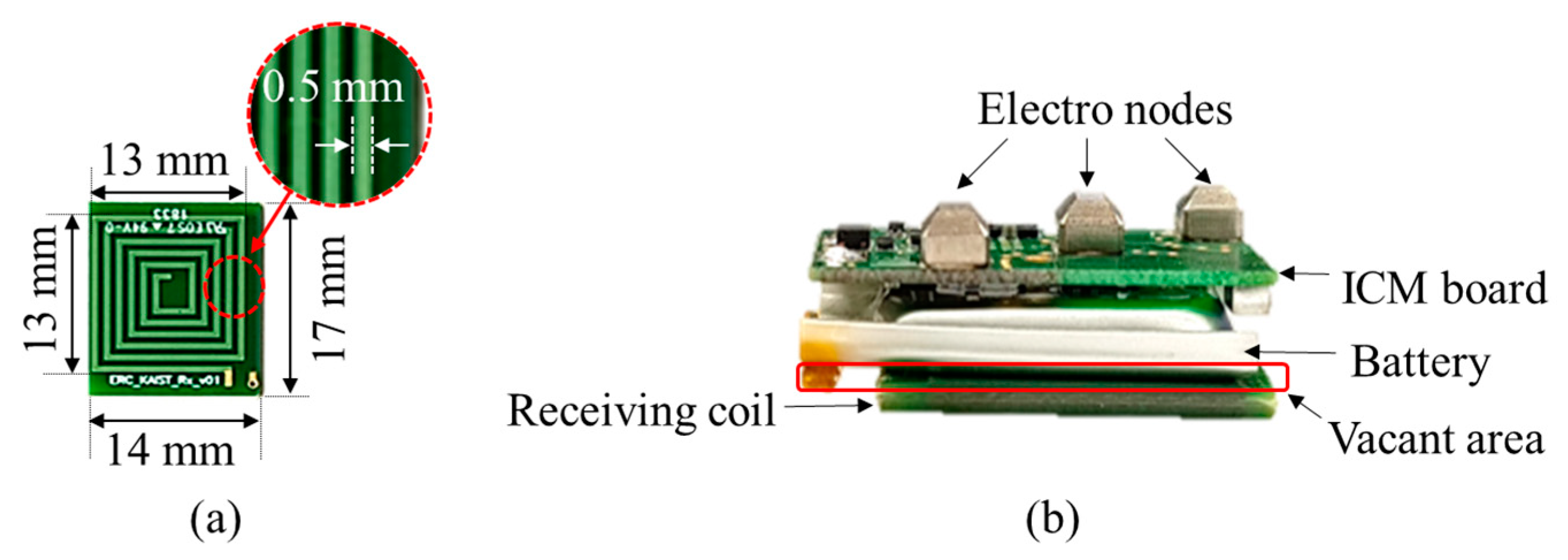
4.1. Fabricated Transmitting Coil and Receiving Coil
4.2. Measurement Results
5. Animal Experiment
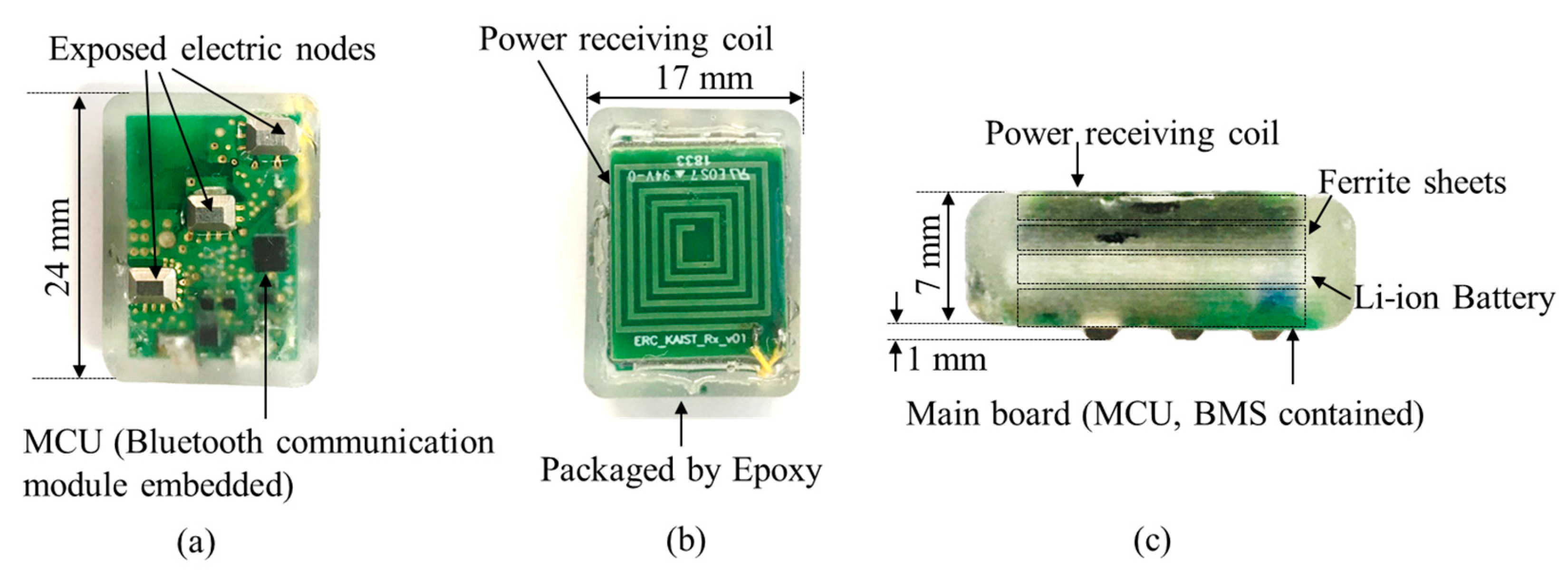
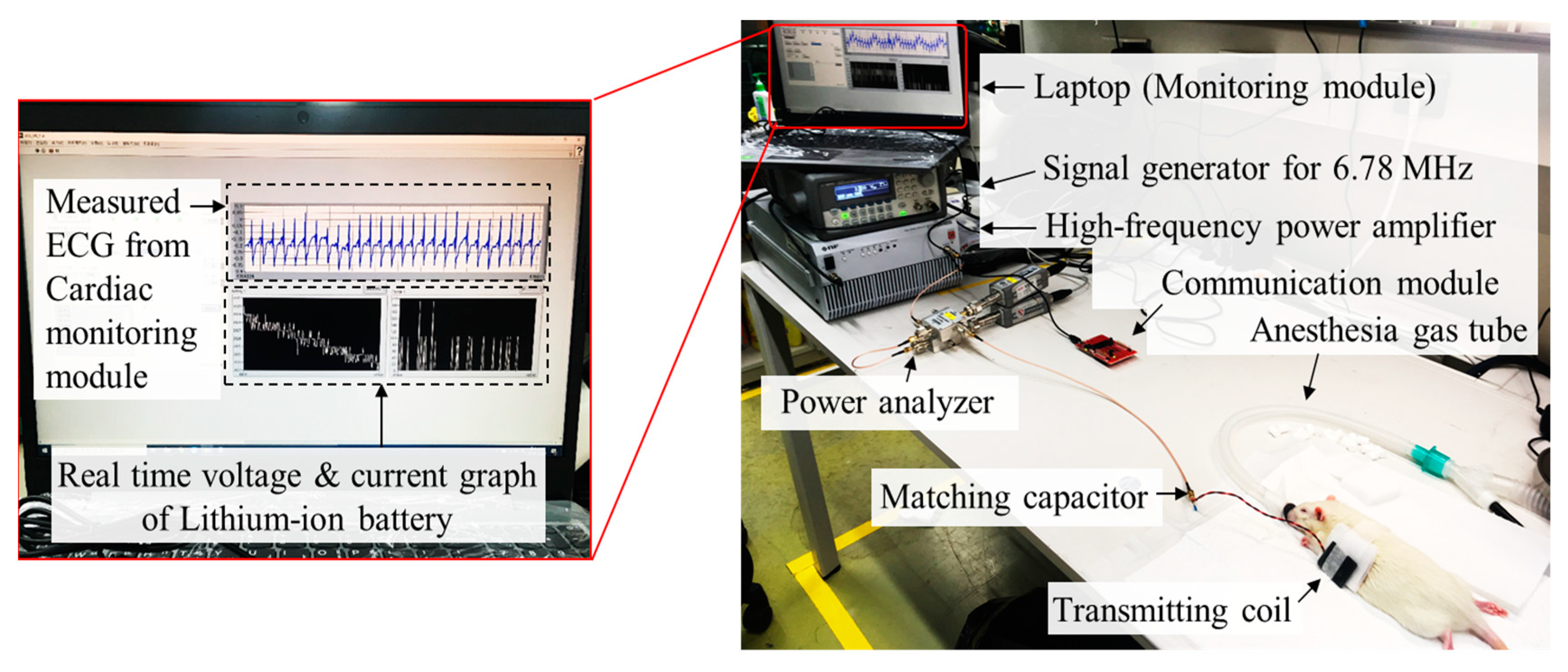
5.1. Fabricated Cardiac Monitoring System Module
5.2. Animal Experiment Setup
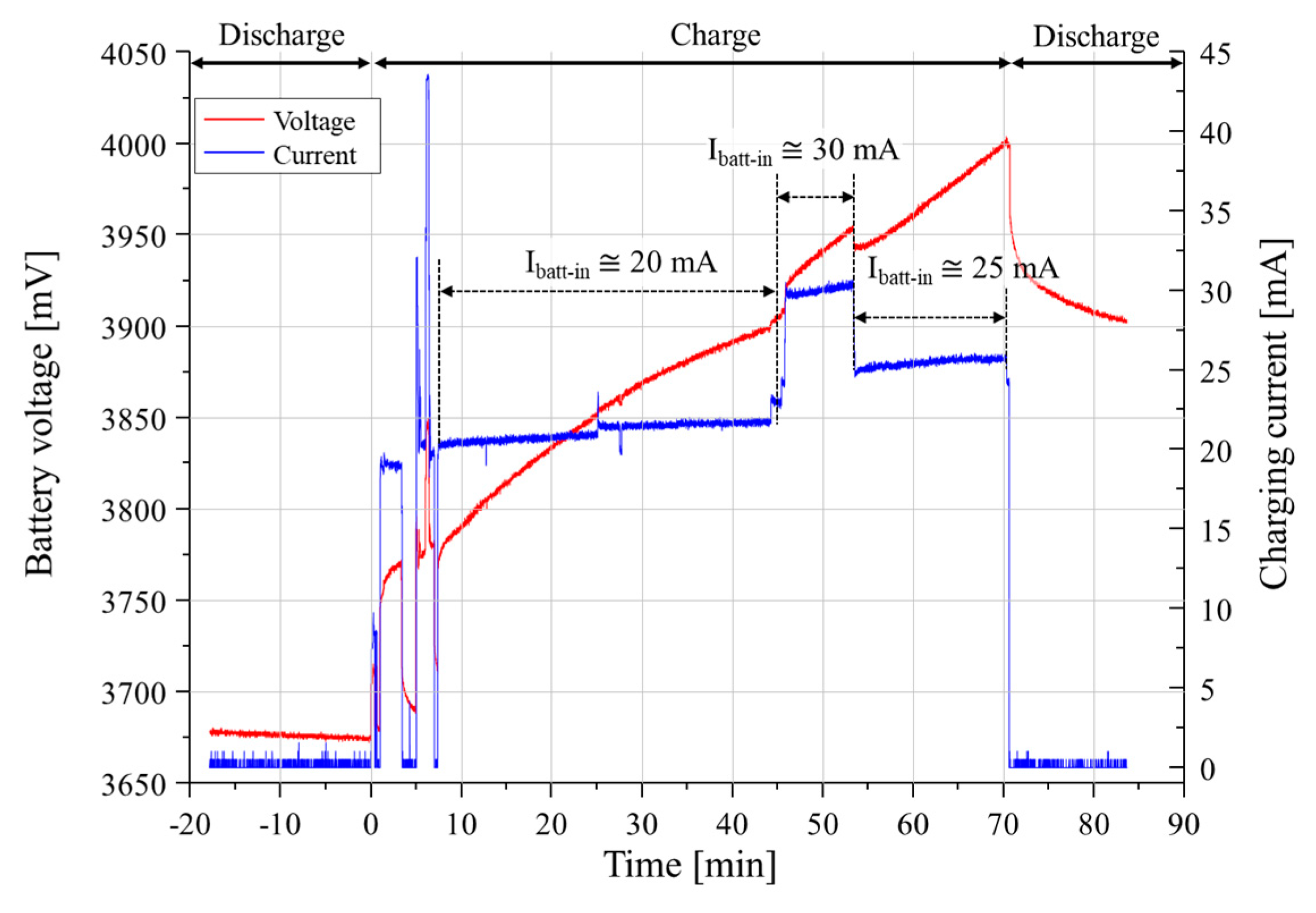
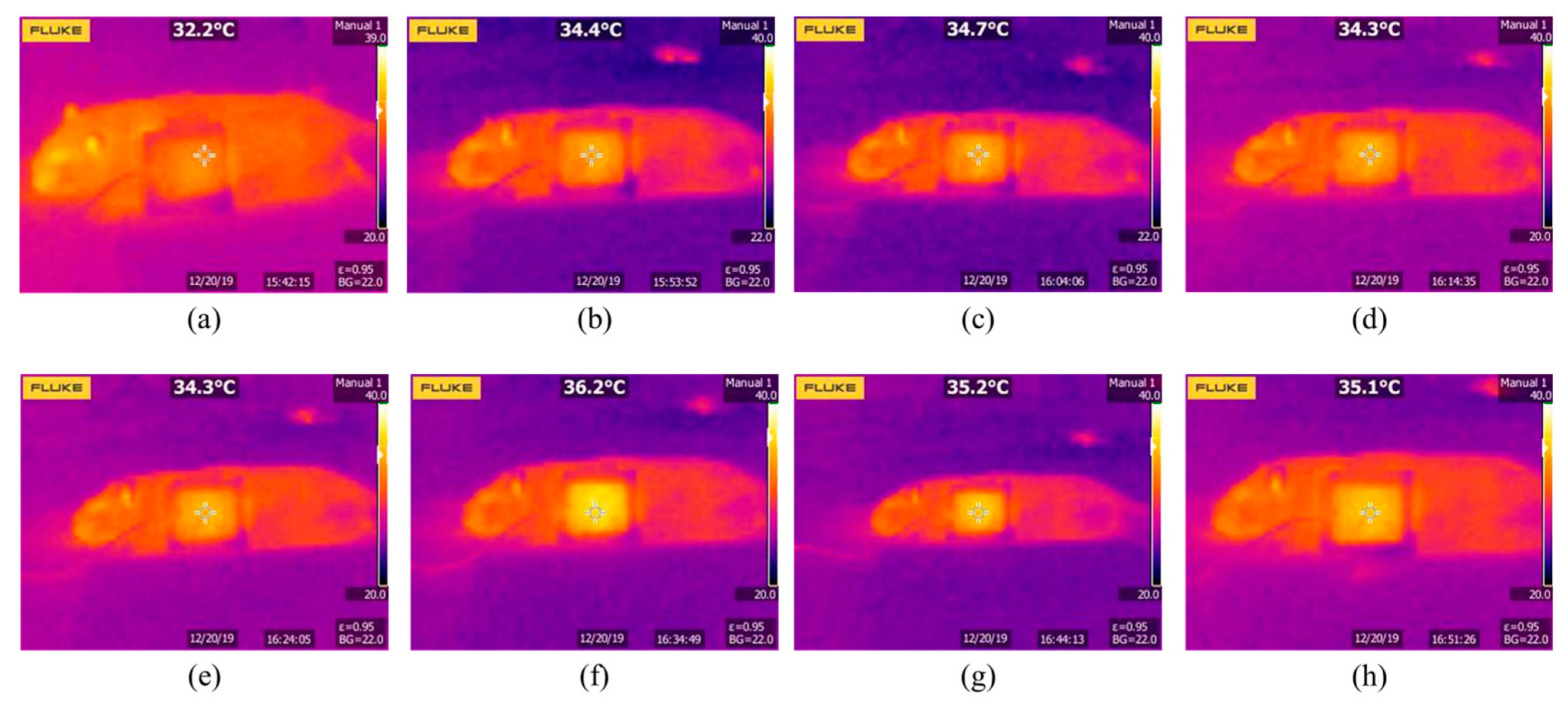
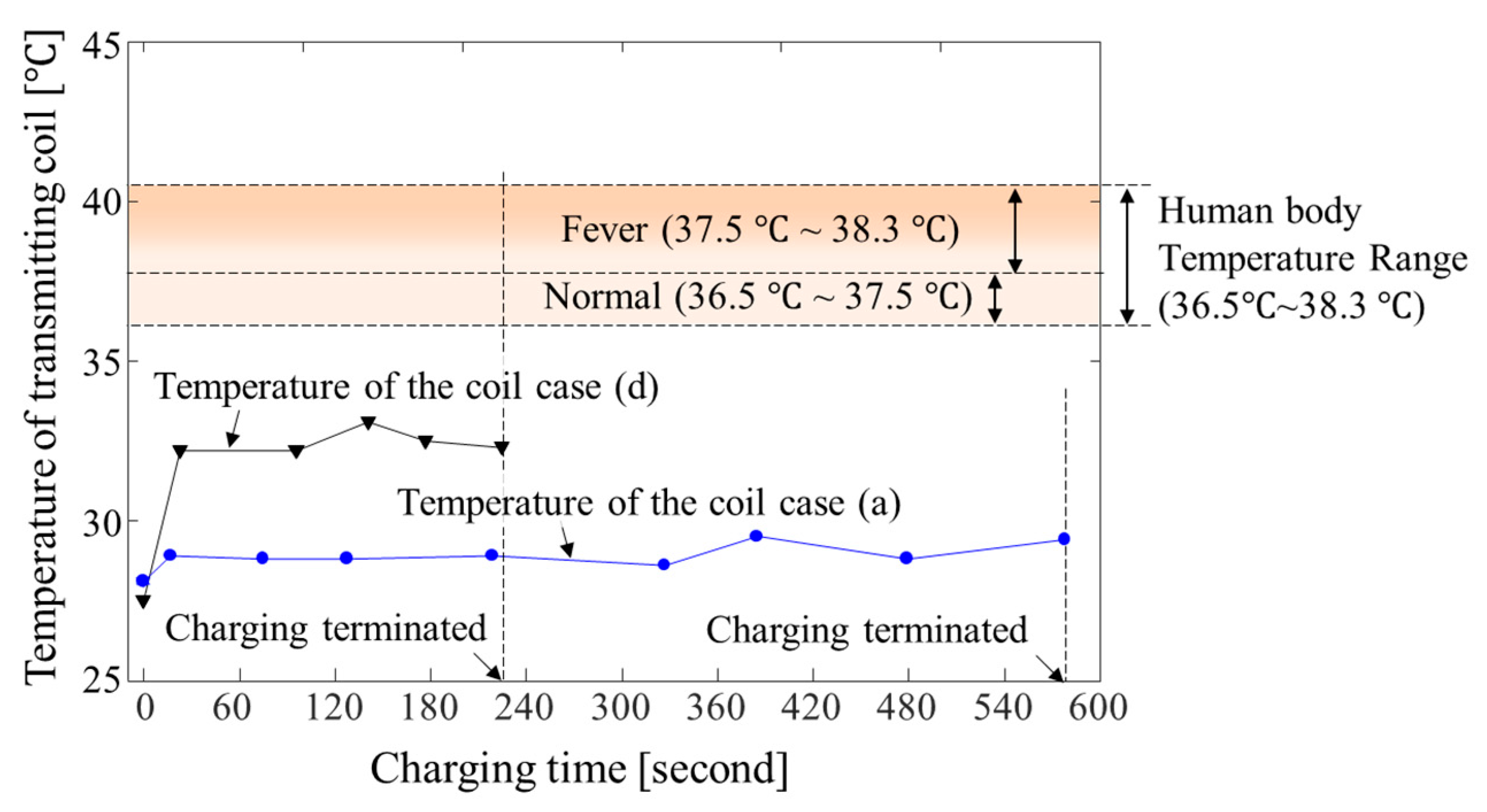
5.3. Experiment Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shiba, K.; Morimasa, A.; Hirano, H. Design and development of low-loss transformer for powering small implantable medical devices. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.; Kim, N.; Kim, S.; Lee, C.; Park, S.M. Deep ECG-respiration network (DeepER net) for recognizing mental stress. Sensors (Switzerland) 2019, 19, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Kim, M.; Yoo, J.; Park, H.H.; Ahn, S. Magnetic resonant wireless power transfer for propulsion of implantable micro-robot. J. Appl. Phys. 2015, 117, 17E712. [Google Scholar] [CrossRef]
- Li, J.; De Ávila, B.E.; Gao, W.; Zhang, L.; Wang, J. Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification. Science Robotics 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, J.; Park, H.H.; Ahn, S. Generation of Magnetic Propulsion Force and Torque for Microrobot Using Wireless Power Transfer Coil. IEEE Trans. Magn. 2015, 51, 2–5. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Jia, L.; Mathewson, K.E.; Jang, K.I.; Kim, J.; Fu, H.; Huang, X.; Chava, P.; Wang, R.; et al. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 2014, 344, 70–74. [Google Scholar] [CrossRef]
- Zheng, G.; Shankaran, R.; Orgun, M.A.; Qiao, L.; Saleem, K. Ideas and Challenges for Securing Wireless Implantable Medical Devices: A Review. IEEE Sens. J. 2017, 17, 562–576. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V. Implantable devices: Issues and challenges. Electronics 2013, 2, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.Y.; Qian, X.H.; Cheng, M.S.; Liang, Y.A.; Chen, W.M. A 13.56 MHz 40 mW CMOS high-efficiency inductive link power supply utilizing on-chip delay-compensated voltage doubler rectifier and multiple ldos for implantable medical devices. IEEE J. Solid-State Circuits 2014, 49, 2397–2407. [Google Scholar] [CrossRef]
- Hwang, H.; Jo, B.; Kim, S.W.; Moon, J.; Kwon, C. 6.78 MHz resonance coupling for implantable medical devices. BMEiCON 2015-8th Biomed. Eng. Int. Conf. 2016, 2, 1–3. [Google Scholar]
- Campi, T.; Cruciani, S.; De Santis, V.; Maradei, F.; Feliziani, M. Near field wireless powering of deep medical implants. Energies 2019, 12, 2720. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Park, B.; Shin, Y.; Park, H.H.; Ahn, S. Propulsion and Rotation of Microrobot Based on a Force on a Magnetic Material in a Time-Varying Magnetic Field Using a Wireless Power Transfer System. IEEE Trans. Magn. 2020, 56, 1–5. [Google Scholar] [CrossRef]
- Campi, T.; Cruciani, S.; Palandrani, F.; De Santis, V.; Hirata, A.; Feliziani, M. Wireless power transfer charging system for AIMDs and pacemakers. IEEE Trans. Microw. Theory Tech. 2016, 64, 633–642. [Google Scholar] [CrossRef]
- Pavelec, V.; Rotenberg, B.W.; Maurer, J.T.; Gillis, E.; Verse, T. A novel implantable device for the treatment of obstructive sleep apnea: Clinical safety and feasibility. Nat. Sci. Sleep 2016, 8, 137–144. [Google Scholar] [PubMed] [Green Version]
- Tokudaiji, Y.; Miura, D.; Hattori, Y.; Murasato, K.; Bu, Y.; Mizuno, T. AC Resistance Reduction of a Flexible Wireless Power Transmission Coil Using Magnetic Path Control Technology at 13.56 MHz. IEEE Trans. Magn. 2019, 55, 1–7. [Google Scholar] [CrossRef]
- Implants for Surgery–Active Implantable Medical Devices–Part 3: Implantable Neurostimulators, 2nd ed.; International Standard, rev.: Geneva, Switzerland, 2017.
- Lee, J.H. Human Implantable Arrhythmia Monitoring Sensor with Wireless Power and Data Transmission Technique. Austin J. Biosens. Bioelectron. 2015. [Google Scholar]
- Heo, J.C.; Kim, B.; Kim, Y.N.; Kim, D.K.; Lee, J.H. Induction of inflammation in vivo by electrocardiogram sensor operation using wireless power transmission. Sensors 2017, 17, 2905. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Li, W.; Luo, H.; Sin, J.K.O.; Yue, C.P. Design and characterization of wireless power links for brain-machine interface applications. IEEE Trans. Power Electron. 2014, 29, 5462–5471. [Google Scholar] [CrossRef]
- Jow, U.M.; Ghovanloo, M. Modeling and optimization of printed spiral coils in Air, Saline, and Muscle tissue environments. IEEE Trans. Biomed. Circuits Syst. 2009, 3, 339–347. [Google Scholar]
- Khripkov, A.; Hong, W.; Pavlov, K. Integrated resonant structure for simultaneous wireless power transfer and data telemetry. IEEE Antennas Wireless Propag. Lett. 2012, 11, 1659–1662. [Google Scholar] [CrossRef]
- Xue, R.F.; Cheng, K.W.; Je, M. High-efficiency wireless power transfer for biomedical implants by optimal resonant load transformation. IEEE Trans. Circuits Syst. I Reg. Papers 2013, 60, 867–874. [Google Scholar] [CrossRef]
- Monti, G.; Arcuti, P.; Tarricone, L. Resonant inductive link for remote power of pacemakers, IEEE Trans. Microw. Theory Techn. 2015, 63, 3814–3822. [Google Scholar] [CrossRef]
- Sun, G.; Muneer, B.; Li, Y.; Zhu, Q. Ultracompact implantable design with integrated wireless power transfer and RF transmission capabilities, IEEE Trans. Biomed. Circuits Syst. 2018, 12, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Bauer, P. Analysis and design considerations for a contactless power transfer system. INTELEC Int. Telecommun. Energy Conf. 2011, 1–6. [Google Scholar] [CrossRef]
- Lu, Y.; Qiu, D.; Meng, X.; Zhang, B.; Tang, S.C. S-PS resonant topology of WPT system for implantable spinal cord stimulator. IET Power Electron. 2018, 11, 2499–2506. [Google Scholar] [CrossRef]
- Aditya, K.; Williamson, S.S. Comparative study of series-series and series-parallel topology for long track EV charging application. IEEE Transp. Electrif. Conf. Expo Components, Syst. Power Electron. - From Technol. to Bus. Public Policy ITEC 2014, 2014, 1–5. [Google Scholar]
- Gati, E.; Kokosis, S.; Patsourakis, N.; Manias, S. Comparison of series compensation topologies for inductive chargers of biomedical implantable devices. Electronics 2019, 9, 1–21. [Google Scholar] [CrossRef] [Green Version]




| Series–Series (SS) | Series–Parallel (SP) | |
|---|---|---|
| Tx Inductance (L1) | ||
| Rx Inductance (L2) | ||
| Mutual inductance | ||
| Tx matching cap. (C1) @6.78 MHz | 204.9 nF | 206.8 nF |
| Rx matching cap. (C2) @6.78 MHz | 307.9 nF | 307.8 nF |
| Tx resistance (RESR1) @6.78 MHz | 3.5 ohm | 4.4 ohm |
| Tx resistance (RESR2) @6.78 MHz | 3.5 ohm | 4.4 ohm |
| Power transfer efficiency | , where | |
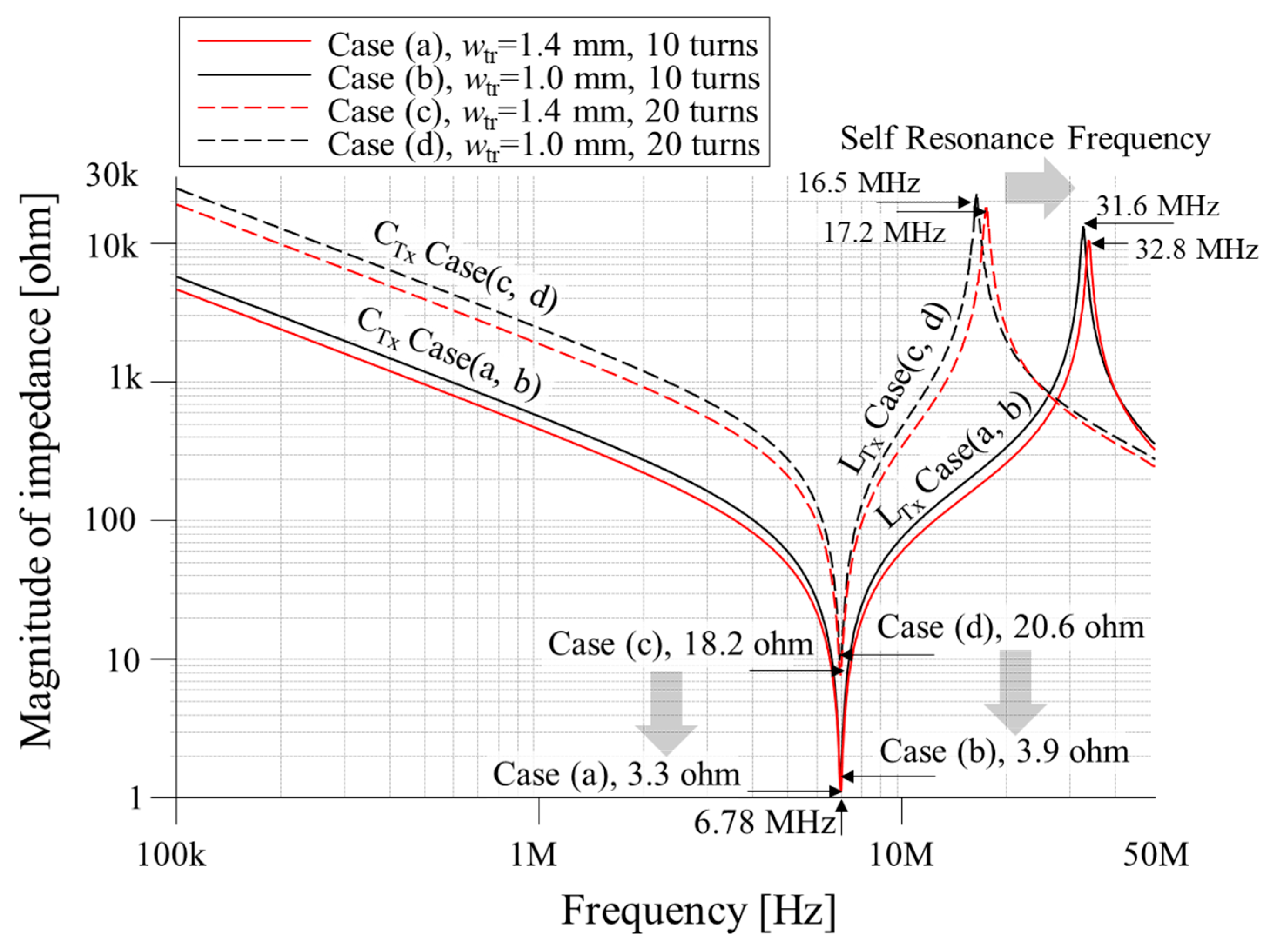
| Parameter | Case (a) | Case (b) | Case (c) | Case (d) |
|---|---|---|---|---|
| Number of turns | 10 | 10 | 20 | 20 |
| Resistance [ohm] (@ 6.78 MHz) | 3.3 | 3.9 | 18.2 | 20.6 |
| Self-inductance [H] @ 6.78 MHz | 1.6 | 2.0 | 6.6 | 8.6 |
| Matching capacitance [pF] @ 6.78 MHz | 344 | 276 | 83 | 64 |
| Self-resonance frequency (SRF) [MHz] | 32.8 | 31.6 | 16.5 | 17.2 |
| Parameter | Transmitting Coil | Receiving Coil |
|---|---|---|
| Number of turns | 10 | 36 |
| Coil size | 27 27 0.5 mm3 | 17 × 19 × 1 mm3 (coil area: 13 × 13 mm2) |
| Thickness of coil trace | 0.035 mm (1 oz) | 0.07 mm (2 oz) |
| Width of coil | 1.4 mm | 0.5 mm |
| Air gap | 10 mm | |
| Parameter | Transmitting Coil | Receiving Coil |
|---|---|---|
| Coil resistance (ReffTx, ReffRx) | 3.52 ohm | 4.37 ohm |
| Self-inductance (L1, L2) | ||
| Mutual inductance (M) | 234.19 nH | |
| Resonance matching capacitor (C1, C2) @ 6.78 MHz | 205 pF | 310 pF |
| Ref. | [19] | [20] | [21] | [22] | [23] | [24] | This Work |
|---|---|---|---|---|---|---|---|
| Operating frequency [MHz] | 7.4 | 13.56 | 6.78 | 13.56 | 403 | 39.86 | 6.78 |
| Dimensions of the receiver [mm2] | 4.5 4.5 | 10 | 12 | 10 | 9.5 | 10.5 | 13 |
| Coil to coil distances [mm] | 5 | 10 | 20 (air) | 10 | 10 | 10 | 10 |
| Power transfer efficiency | 33.1% | 30% | 38% | * 58.2% | 7.3% | 47.2 | 24% (* 44.5%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Jeong, D.; Kim, J.; Kim, H.; Kim, J.; Park, S.-M.; Ahn, S. Design and Implementation of a Wireless Charging-Based Cardiac Monitoring System Focused on Temperature Reduction and Robust Power Transfer Efficiency. Energies 2020, 13, 1008. https://doi.org/10.3390/en13041008
Kim D, Jeong D, Kim J, Kim H, Kim J, Park S-M, Ahn S. Design and Implementation of a Wireless Charging-Based Cardiac Monitoring System Focused on Temperature Reduction and Robust Power Transfer Efficiency. Energies. 2020; 13(4):1008. https://doi.org/10.3390/en13041008
Chicago/Turabian StyleKim, Dongwook, Dawon Jeong, Jongwook Kim, Haerim Kim, Junho Kim, Sung-Min Park, and Seungyoung Ahn. 2020. "Design and Implementation of a Wireless Charging-Based Cardiac Monitoring System Focused on Temperature Reduction and Robust Power Transfer Efficiency" Energies 13, no. 4: 1008. https://doi.org/10.3390/en13041008





