Briquettes Production from Olive Mill Waste under Optimal Temperature and Pressure Conditions: Physico-Chemical and Mechanical Characterizations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Densification and Briquettes Production
2.3. Proximate Analysis
2.4. Measurement of the Compressive Strength
3. Results and Discussions
3.1. Compressive Strength Measurement
3.2. The Unit Density and the Bulk Density Measurements
3.3. High Heating Value Measurement
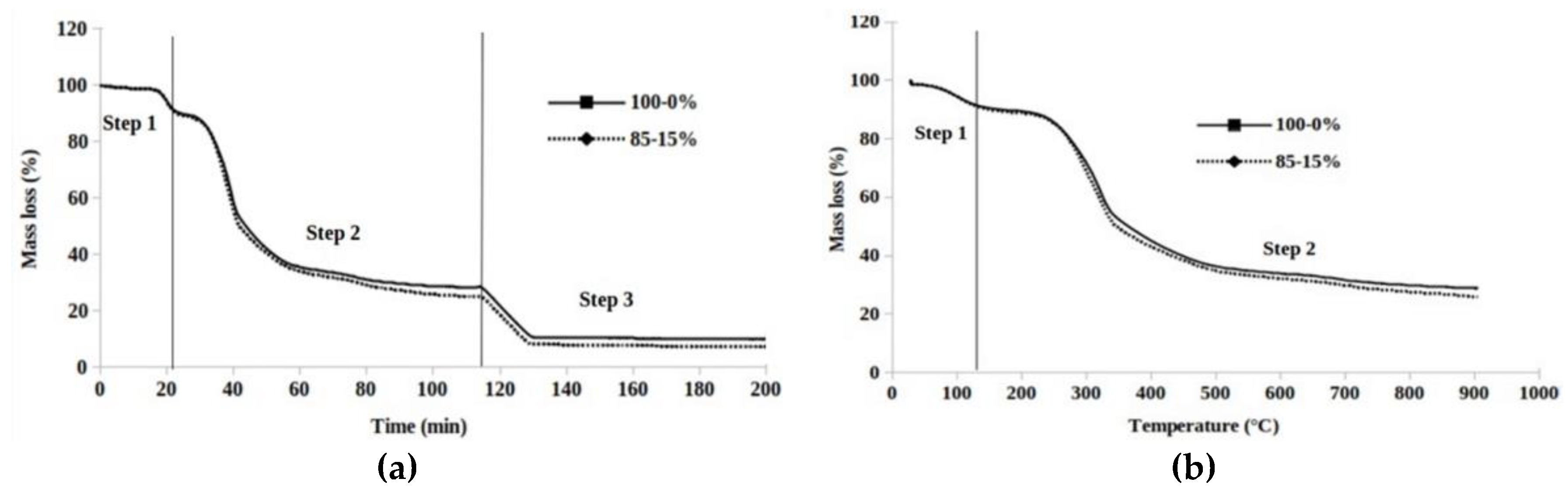
3.4. Thermogravimetry Analysis
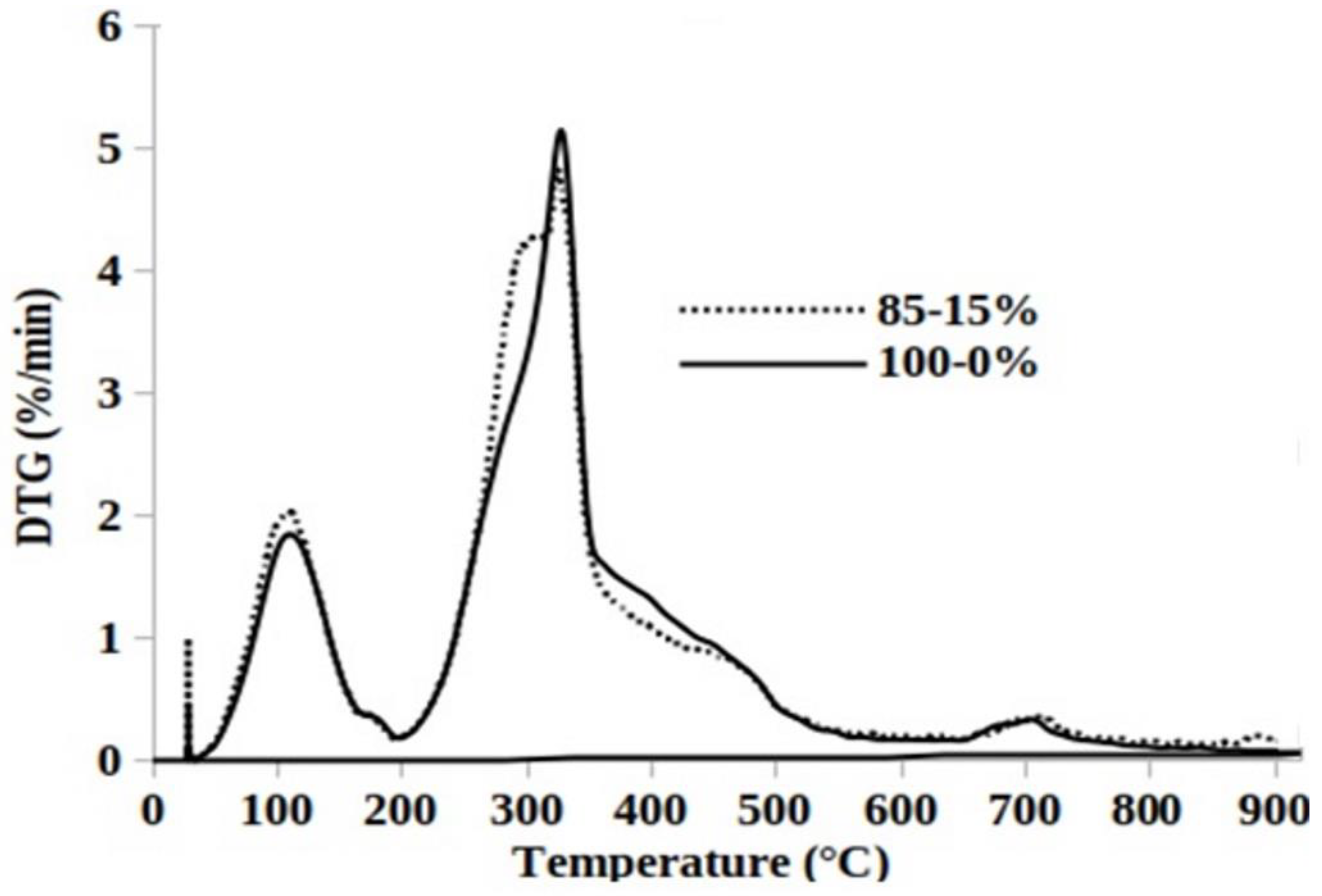
3.5. Mean Reactivity during Pyrolysis and Char Oxidation
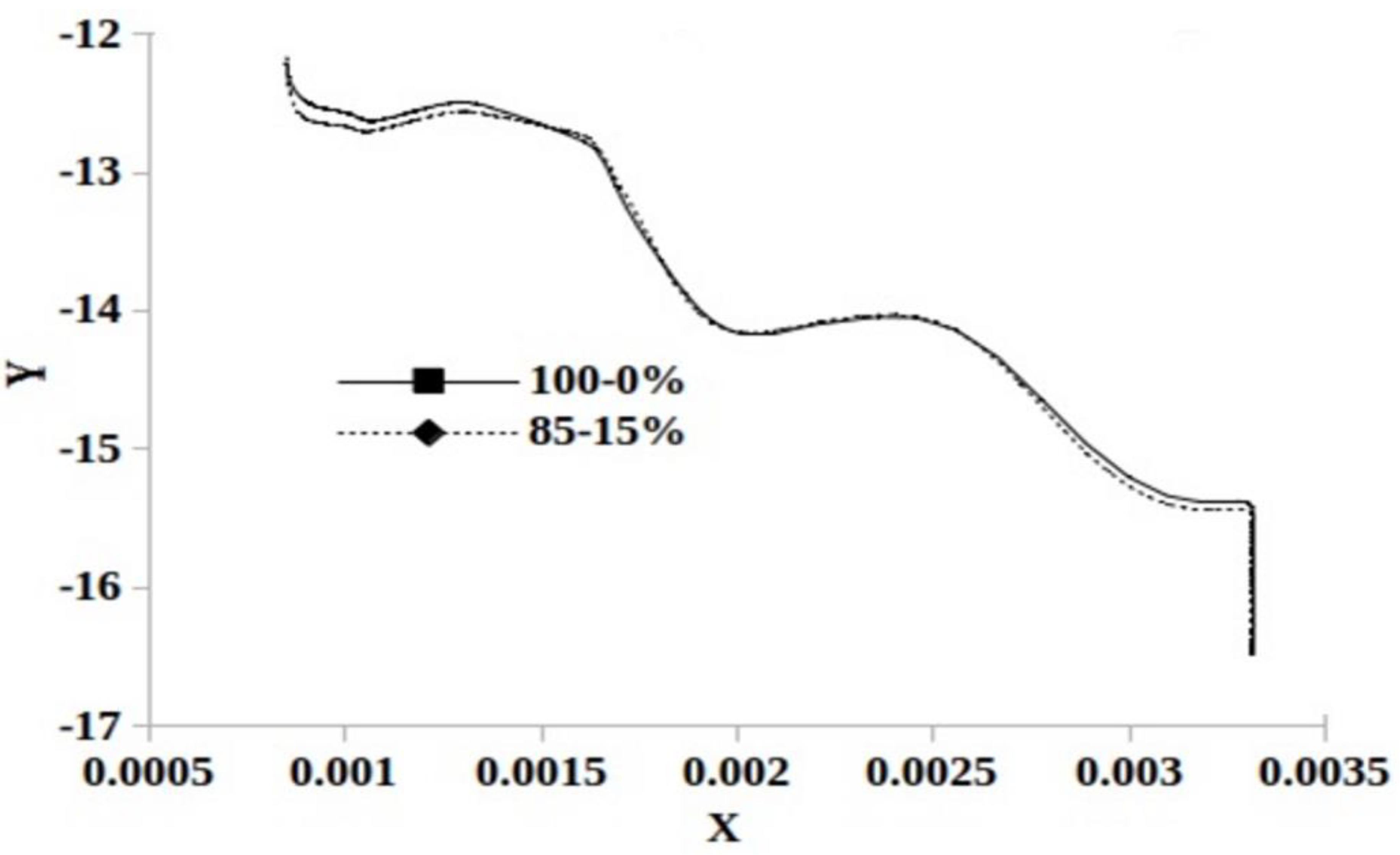
3.6. Kinetic Study and Pyrolysis Parameters Determination
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Popp, J.; Lakner, Z.; Harangi-Rákos, M.; Fári, M. The effect of bioenergy expansion: Food, energy, and environment. Renew. Sust. Energy Rev. 2014, 32, 559–578. [Google Scholar] [CrossRef] [Green Version]
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 357–1378. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (Part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Limousy, L.; Jeguirim, M.; Dutournié, P.; Kraeim, N.; Lajili, M.; Said, R. Gaseous products and particulate matter emissions of biomass residential boiler fired with spent coffee ground pellets. Fuel 2013, 107, 323–329. [Google Scholar] [CrossRef]
- Merpati Mitan, N.M.; Azmi, A.H.; Nur Fathiah, M.N.; Se, S.M. Binder application in durian peels briquette as a solid biofuel. Appl. Mech. Mater. 2015, 761, 494–498. [Google Scholar] [CrossRef]
- Limousy, L.; Jeguirim, M.; Labbe, S.; Balay, F.; Fossard, E. Performance and emissions characteristics of compressed spent coffee ground/wood chip logs in a residential stove. Energy Sustain. Dev. 2015, 28, 52–59. [Google Scholar] [CrossRef]
- Khlifi, S.; Lajili, M.; Tabet, F.; Boushaki, T.; Sarh, B. Investigation of the combustion characteristics of briquettes prepared from olive mill solid waste blended with and without a natural binder in a fixed bed reactor. Biomass Convers. Bior. 2019. [Google Scholar] [CrossRef]
- Fachinger, F.; Drewnick, F.; Gieré, R.; Borrmann, S. How the user can influence particulate emissions from residential wood and pellet stoves: Emission factors for different fuels and burning conditions. Atmos. Environ. 2017, 158, 216–226. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Brito, P.; Vilarinho, C. A Holistic Review on Biomass Gasification Modified Equilibrium Models. Energies 2019, 12, 160. [Google Scholar] [CrossRef] [Green Version]
- Zribi, M.; Lajili, M.; Escudero Sanz, R.F. Hydrogen enriched syngas production via gasification of biofuels pellets/powders blended from olive mill solid wastes and pine sawdust under different water steam/nitrogen atmospheres. Int. J. Hyd. Energy 2019, 44, 11280–11288. [Google Scholar] [CrossRef]
- Zhang, F.; Fang, Z.; Wang, Y.T. Biodiesel production directly from oils with high acid value by magnetic Na2SiO3@Fe3O4/C catalyst and ultrasound. Fuel 2015, 150, 370–377. [Google Scholar] [CrossRef]
- Hahn-Hägerdal, B.; Galbe, M.; Gorwa-Grauslund, M.F.; Lidén, G.; Zacchi, G. Bio-ethanol—The fuel of tomorrow from the residues of today. Trends Biothechnol. 2006, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Hamelinck, C.N.; Faaij, A.P.C. Outlook for advanced biofuels. Energy Policy 2006, 34, 3268–3283. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.F.; Monforti-Ferrario, F.; Nita, V. The role of biomass and bioenergy in a future bioeconomy: Policies and facts. Environ. Dev. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Sahu, S.G.; Sarkar, P.; Mukherjee, A.; Adak, A.K.; Chakraborty, N. Studies on the co-combustion behavior of coal/biomass blends using thermogravimetric analysis. IJETAE 2013, 3, 131–138. [Google Scholar]
- Azbar, N.; Bayram, A.; Filibeli, A.; Muezzinoglu, A.; Sengul, F.; Ozer, A. A review of waste management options in olive oil production. Crit. Rev. Env. Sci. Technol. 2004, 34, 209–247. [Google Scholar] [CrossRef]
- Ouazzane, H.; Laajine, F.; ElYamani, M.; el Hilaly, J.; Rharrabti, Y.; Amarouch, M.Y.; Mazouzi, D. Olive Mill Solid Waste Characterization and Recycling opportunities: A review. J. Mater. Environ. Sci. 2017, 8, 2632–2650. [Google Scholar]
- Niaounakis, M.; Halvadakis, C.P. Olive Processing Waste Management; Literature Review and Patent Survey: London, UK, 1 February 2006. [Google Scholar]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Tumuluru, J.S. Effect of process variables on the density and durability of the pellets made from high moisture corn stover. Biosyst. Eng. 2014, 119, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Tumuluru, J.S. Specific energy consumption and quality of wood pellets made from high moisture lodgepole pine biomass. Chem. Eng. Res. Des. 2016, 110, 82–97. [Google Scholar] [CrossRef] [Green Version]
- Kaliyan, N.; Morey, R.V. Natural binders and solid bridge type binding mechanisms in briquettes and pellets made from corn Stover and switch grass. Bioresour. Technol. 2010, 101, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Zanella, K.; Concentino, V.O.; Taranto, O.P. Influence of the type of Mixture and Concentration of Different Binders on the Mechanical Properties of “Green” Charcoal Briquettes. Chem. Eng. Trans. 2017, 57, 199–204. [Google Scholar]
- Christoforou, E.; Fokaides, P. A review of olive mill solid wastes to energy utilization techniques. Waste Manag. 2016, 49, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Ganvir, K.D.; Tulankar, P.G.; Bawankar, P.P.; Rahimkar, K.T.; Singh, S.L. Analysis and Comparison of Biomass Pellets with Various Fuels. IJSRD 2017, 5, 1272–1274. [Google Scholar]
- Davies, R.M.; Davies, O.A. Effect of Briquetting Process Variables on Hygroscopic Property of Water Hyacinth Briquettes. Hindawi Publ. Corp. J. Renew. Energy 2013, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Stelte, W.; Holm, J.K.; Sanadi, A.R.; Barsberg, S.; Ahrenfeldt, J.; Henriksen, U.B. A study of bonding and failure mechanisms in fuel pellets from different biomass resources. Biomass Bioenergy 2011, 35, 910–918. [Google Scholar] [CrossRef] [Green Version]
- Adapa, P.K.; Tabil, L.G.; Schoenau, G.J.; Sokhansanj, S. Pelleting Characteristics of Fractionated and Sun-Cured Dehydrate Alfalfa Grinds. Appl. Eng. Agric. 2004, 20, 813–820. [Google Scholar] [CrossRef]
- Swietochowski, A.; Lisowski, A.; Dabrowska-Salwin, M. Strength of briquettes and pellets from energy crops. Eng. Rural Dev. 2016, 5, 25–27. [Google Scholar]
- Sprenger, C.J.; Tabil, L.G.; Soleimani, M.; Agnew, J.; Harrison, A. Pelletization of Refuse-Derived Fuel Fluff to Produce High Quality Feedstock. J. Energy Resour. Technol. 2018, 140. [Google Scholar] [CrossRef]
- Richards, S.R. Physical testing of fuel briquettes. Fuel Process. Technol. 1990, 25, 89–100. [Google Scholar] [CrossRef]
- Lajili, M.; Limousy, L.; Jeguirim, M. Physico-chemical properties and thermal degradation characteristics of agropellets from olive mill by-products/sawdust blends. Fuel Process. Technol. 2014, 126, 215–221. [Google Scholar] [CrossRef]
- Mehdipour, I.; Khayat, K.H. Effect of supplementary Cementitious Material Content and Binder Dispersion on Packing Density and Compressive Strength of Sustainable Cement Paste. ACI Mater. J. 2016, 113, 361–372. [Google Scholar]
- Eliche-Quesada, D.; Leite-Costa, J. Use of bottom ash from olive pomace combustion in the production of eco-friendly fired clay bricks. Waste Manag. 2016, 48, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Eija Alakangas, V.T.T. New European Pellets Standards; EUBIONET 3: Swedish, Finland, March 2011. [Google Scholar]
- Long, Y.; Meng, A.; Chen, S.; Zhou, H.; Zhang, Y.; Li, Q. Pyrolysis and Combustion of Typical Wastes in a Newly Designed Macro Thermogravimetric Analyzer: Characteristics and Simulation by Model Components. Energy Fuels 2017, 31, 7582–7590. [Google Scholar] [CrossRef]
- Munir, S.; Daood, S.S.; Nimmo, W.; Cunliffe, A.M.; Gibbs, B.M. Thermal analysis and devolatilization kinetics of cotton stalk, sugar cane bagasse and shea meal under nitrogen and air atmospheres. Bioresour. Technol. 2009, 100, 1413–1418. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S. Effect of co-combustion on the burnout of lignite/biomass blends: A Turkish case study. Waste Manag. 2008, 28, 2077–2084. [Google Scholar] [CrossRef]
- Chouchene, A.; Jeguirim, M.; Khiari, B.; Zagrouba, F.; Trouvé, G. Thermal degradation of olive solid waste: Influence of particle size and oxygen concentration. Resour. Conserv. Recy. 2010, 54, 271–277. [Google Scholar] [CrossRef]
- Vamvuka, D.; Kakaras, E.; Kastanaki, E.; Grammelis, P. Pyrolysis characteristics and kinetics of biomass residuals mixtures with lignite. Fuel 2003, 82, 1949–1960. [Google Scholar] [CrossRef]
- Miranda, T.; Esteban, A.; Rojas, S.; Montero, I.; Ruiz, A. Combustion Analysis of Different Olive Residues. Int. J. Mol. Sci. 2008, 9, 512–525. [Google Scholar] [CrossRef]
- Borello, D.; De Caprariis, B.; De Filippis, P.; Di Carlo, A.; Marchegiani, A.; Marco Pantaleo, A.; Shah, N.; Venturini, P. Thermo-Economic Assessment of a olive pomace Gasifier for Cogeneration Applications. Energy Procedia 2015, 75, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Alrawashdeh, K.A.b.; Slopiecka, K.; Alshorman, A.A.; Bartocci, P.; Fantozzi, F. Pyrolytic Degradation of Olive Waste Residue (OWR) by TGA: Thermal Decomposition Behavior and Kinetic Study. J. Energy Power Eng. 2017, 11, 497–510. [Google Scholar]
- Bartocci, P.; D’Amico, M.; Moriconi, N.; Bidini, G.; Fantozzi, F. Pyrolysis of olive stone for energy purposes. Energy Procedia 2015, 82, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Tamošiūnas, A.; Chouchène, A.; Valatkevičius, P.; Gimžauskaitė, D.; Aikas, M.; Uscila, R.; Ghorbel, M.; Jeguirim, M. The Potential of Thermal Plasma Gasification of Olive Pomace Charcoal. Energies 2017, 10, 710. [Google Scholar] [CrossRef] [Green Version]
- Ghouma, I.; Jeguirim, M.; Guizani, C.; Ouederni, A.; Limousy, L. pyrolysis of Olive Pomace: Degradation kinetics. Gaseous analysis and char characterization. Waste Biomass Valorization 2017, 8, 1689–1697. [Google Scholar] [CrossRef]
- Chiti, Y.; Salvador, S.; Commandré, J.M.; Broust, B. Thermal decomposition of bio-oil: Focus on the products yields under different pyrolysis conditions. Fuel 2012, 102, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Abed, I.; Paraschiv, M.; Loubar, K.; Zagrouba, F.; Tazerout, M. Thermogravimetric investigation and thermal conversion kinetics of typical North African and Middle Eastern lignocellulosic wastes. Bioresources 2012, 7, 1200–1220. [Google Scholar]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. The thermal performance of the polysaccharides extracted from hardwood: Cellulose and hemicellulose. Carbohydr. Polym. 2010, 82, 39–45. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Physicochemical Characterization and Pyrolysis Kinetic Study of Sugarcane Bagasse Using Thermogravimetric Analysis. J. Energy Resour. Technol. 2016, 138, 11. [Google Scholar] [CrossRef]
- Ounas, A.; Aboulkas, A.; El harfi, K.; Bacaoui, A.; Yaacoubi, A. Pyrolysis of olive residue and sugar cane bagasse: Non-isothermal thermogravimetric kinetic analysis. Bioresour. Technol. 2011, 102, 11234–11238. [Google Scholar] [CrossRef]
- Cheng, K.; Winter, W.T.; Stipanovic, A.J. A modulated-TGA approach to the kinetics of lignocellulosic biomass pyrolysis/combustion. Polym. Degrad. Stabil. 2012, 97, 1606–1615. [Google Scholar] [CrossRef]
- Gani, A.; Naruse, I. Effect of cellulose and lignin content on pyrolysis and combustion characteristics for several types of biomass. Renew. Energy 2007, 32, 649–661. [Google Scholar] [CrossRef]
- Gil, M.V.; Oulego, P.; Casal, M.D.; Pevida, C.; Pis, J.J.; Rubiera, F. Mechanical durability and combustion characteristics of pellets from biomass blends. Bioresour. Technol. 2010, 101, 8859–8867. [Google Scholar] [CrossRef] [Green Version]
- Vamvuka, D.; Karouki, E.; Sfakiotakis, S. Gasification of waste biomass chars by carbon dioxide via thermogravimetry. Part I: Effect of mineral matter. Fuel 2011, 90, 1120–1127. [Google Scholar] [CrossRef]
- Hu, Q.; Shao, J.; Haiping, Y.; Yao, D.; Wang, X.; Chen, H. Effect of binders on the properties of bio-char pellets. Appl. Energy 2015, 157, 508–516. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.; Zhai, Y.; Li, S.; Liu, X.; Wang, B.; Li, C.; Zhu, Y. Effect of molasses binder on the pelletization of food waste hydrochar for enhanced biofuels pellets production. Sustain. Chem. Pharm. 2019, 14, 100183. [Google Scholar] [CrossRef]
- Zribi, M.; Lajili, M. Study of the Pyrolysis of Biofuels Pellets Blended from Sawdust and Oleic by-Products: A Kinetic Study. IJRER 2019, 9, 561–571. [Google Scholar]
- Cai, J.; Chen, Y.; Liu, R. Isothermal kinetic predictions from nonisothermal data by using the iterative linear integral isoconversional method. J. Energy Inst. 2014, 87, 183–187. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochimca Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Wu, K. Effects of Waste Engine Oil Additive on the Pelletizing and Pyrolysis Properties of Wheat Straw. Bioresources 2019, 14, 537–553. [Google Scholar]
- Guo, J.; Lua, A.C. Kinetic study on pyrolytic process of oil-palm solid waste using two-step consecutive reaction model. Biomass Bioenergy 2001, 20, 223–233. [Google Scholar] [CrossRef]
- Wang, X.; Hu, M.; Hu, W.; Chen, Z.; Liu, S.; Hu, Z.; Xiao, B. Thermogravimetric kinetic study of agricultural residue biomass pyrolysis based on combined kinetics. Bioresour. Technol. 2016, 219, 510–520. [Google Scholar] [CrossRef] [PubMed]



| Pressure (MPa) | 100 | 125 | 150 | ||||||||||
| Binder (%) | 0 | 10 | 15 | 30 | 0 | 10 | 15 | 30 | 0 | 10 | 15 | 30 | |
| Compressive Strength (kN) | 1581 | 1039 | 775 | 626 | 1506 | 1770 | 4015 | 130 | 1557 | 2090 | 4581 | 863 | |
| Pressure (MPa) | 100 | 125 | 150 | |||||||||
| Binder (%) | 0 | 10 | 15 | 30 | 0 | 10 | 15 | 30 | 0 | 10 | 15 | 30 |
| Unit Density (kg/dm3) | 2.04 | 2.83 | 2.81 | 2.31 | 2.41 | 2.40 | 2.47 | * | 2.77 | 2.84 | 2.95 | 3.03 |
| Bulk Density (kg/dm3) | 0.84 | 0.86 | 0.88 | 0.84 | 0.92 | 0.93 | 0.95 | * | 0.95 | 0.98 | 1.20 | 1.10 |
| Biofuels | VM (%wt) | FC (%wt) | Moisture (%wt) | Ash (%wt) | HHV (MJ/kg) |
|---|---|---|---|---|---|
| 100%-0% (w.b.) | 61.86 | 18.75 | 9.88 | 9.49 | 16.36 |
| 85%-15% (w.b.) | 64.65 | 18.39 | 10.44 | 6.72 | 16.92 |
| Pulp [41] (d.b.) | 79.10 | 15.30 | 6.5 | 5.60 | 23.39 |
| OP [42] (d.b.) | 65 | 29.6 | 10 | 5.4 | * |
| Dry OP [43] (d.b.) | 86.71 | 7.48 | 4.52 | 5.81 | 19.88 |
| Wet OP [44] (d.b.) | 42.35 | 7.79 | 49.02 | 0.84 | 5.70 |
| Samples | Thermal Degradation | Peaks of Temperature (°C) | RDTG (mg·min−1) | RM (%.min−1·°C−1) |
|---|---|---|---|---|
| 100%-0% | Pyrolysis | 118-180-329-398-448- | 0.896-0.176-2.582-0.672 | 0.897 |
| Char Combustion | 537-544-548 | 0.636-0.635-0.590 | 0.228 | |
| 85%-15% | Pyrolysis | 102-180-295-319-458-707-882-904-902 | 1.039-0.167-2.149-2.460-0.424-0.182-0.108-0.043-0.028 | 1.199 |
| Char Combustion | 593-568-552 | 0.660-0.661-0.598 | 0.227 |
| Samples | Range of Temperature (°C) | Linear Equation | Ea (kJ·mol−1) | A (min−1) |
|---|---|---|---|---|
| 85%-15% | 54–126 | 20.94 | 11.32 | |
| 135–225 | n.d. | n.d. | ||
| 230–353 | 34.93 | 106.26 | ||
| 360–438 | 4.94 | 0.05 | ||
| 100%-0% | 60–134 | 18.55 | 4.38 | |
| 151–225 | n.d. | n.d. | ||
| 235–368 | 32.38 | 55.27 | ||
| 378–498 | 7.46 | 0.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khlifi, S.; Lajili, M.; Belghith, S.; Mezlini, S.; Tabet, F.; Jeguirim, M. Briquettes Production from Olive Mill Waste under Optimal Temperature and Pressure Conditions: Physico-Chemical and Mechanical Characterizations. Energies 2020, 13, 1214. https://doi.org/10.3390/en13051214
Khlifi S, Lajili M, Belghith S, Mezlini S, Tabet F, Jeguirim M. Briquettes Production from Olive Mill Waste under Optimal Temperature and Pressure Conditions: Physico-Chemical and Mechanical Characterizations. Energies. 2020; 13(5):1214. https://doi.org/10.3390/en13051214
Chicago/Turabian StyleKhlifi, Saaida, Marzouk Lajili, Saoussen Belghith, Salah Mezlini, Fouzi Tabet, and Mejdi Jeguirim. 2020. "Briquettes Production from Olive Mill Waste under Optimal Temperature and Pressure Conditions: Physico-Chemical and Mechanical Characterizations" Energies 13, no. 5: 1214. https://doi.org/10.3390/en13051214







