An Unstructured Model for Anaerobic Treatment of Raw Cheese Whey for Volatile Fatty Acids Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Mathematical Modelling
2.2.1. Temperature and pH Effects
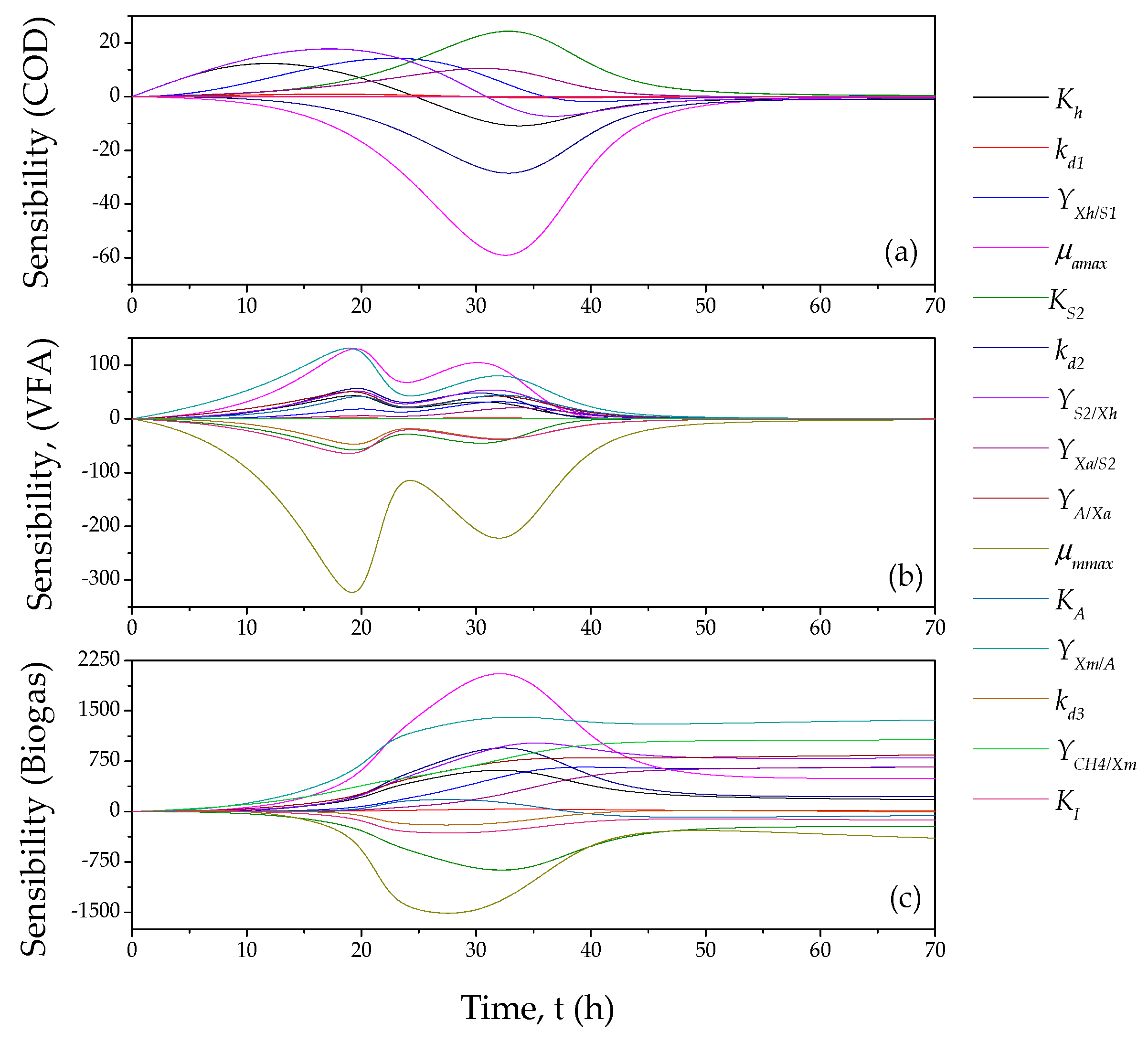
2.2.2. Sensibility Analysis
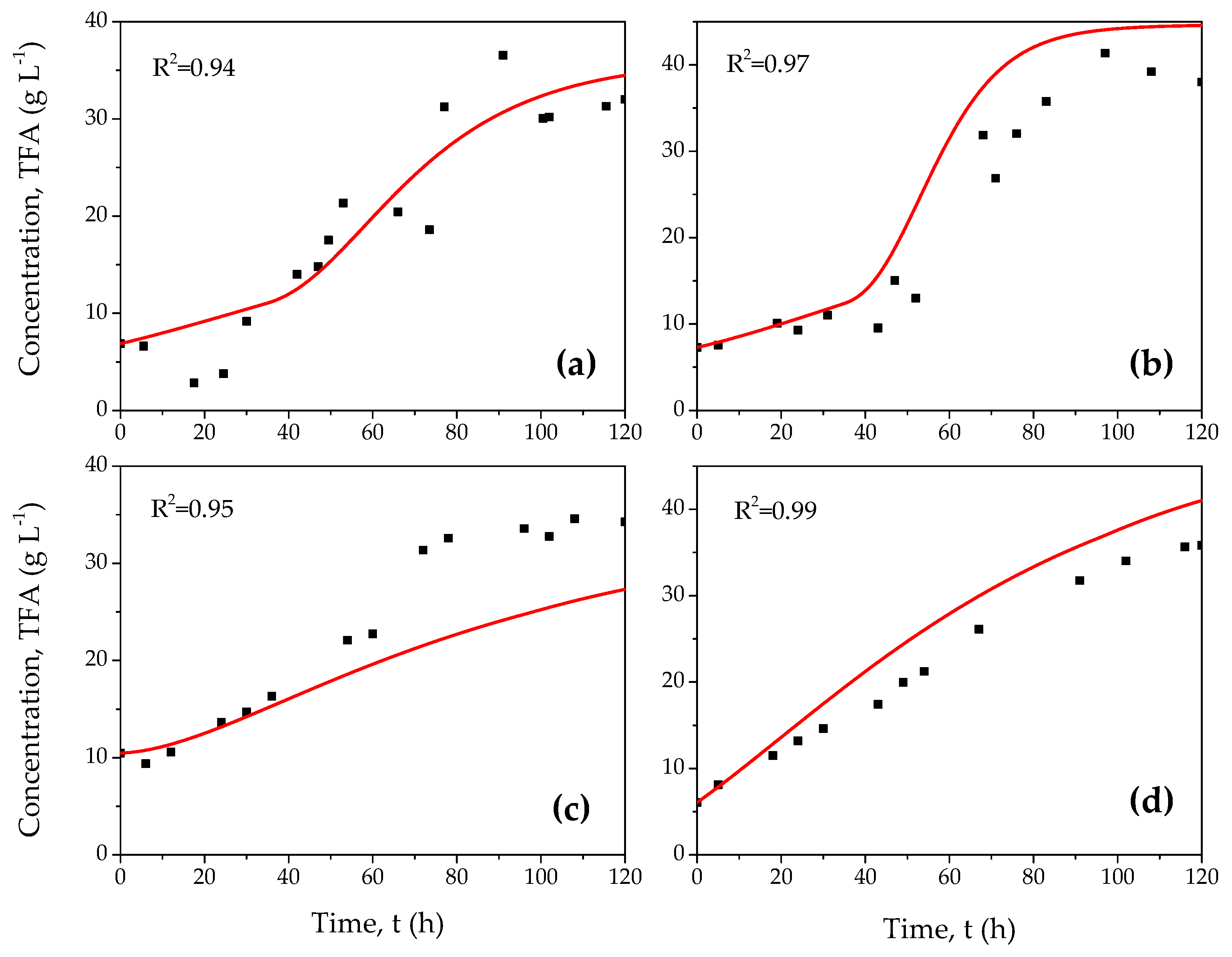
3. Results and Discussion
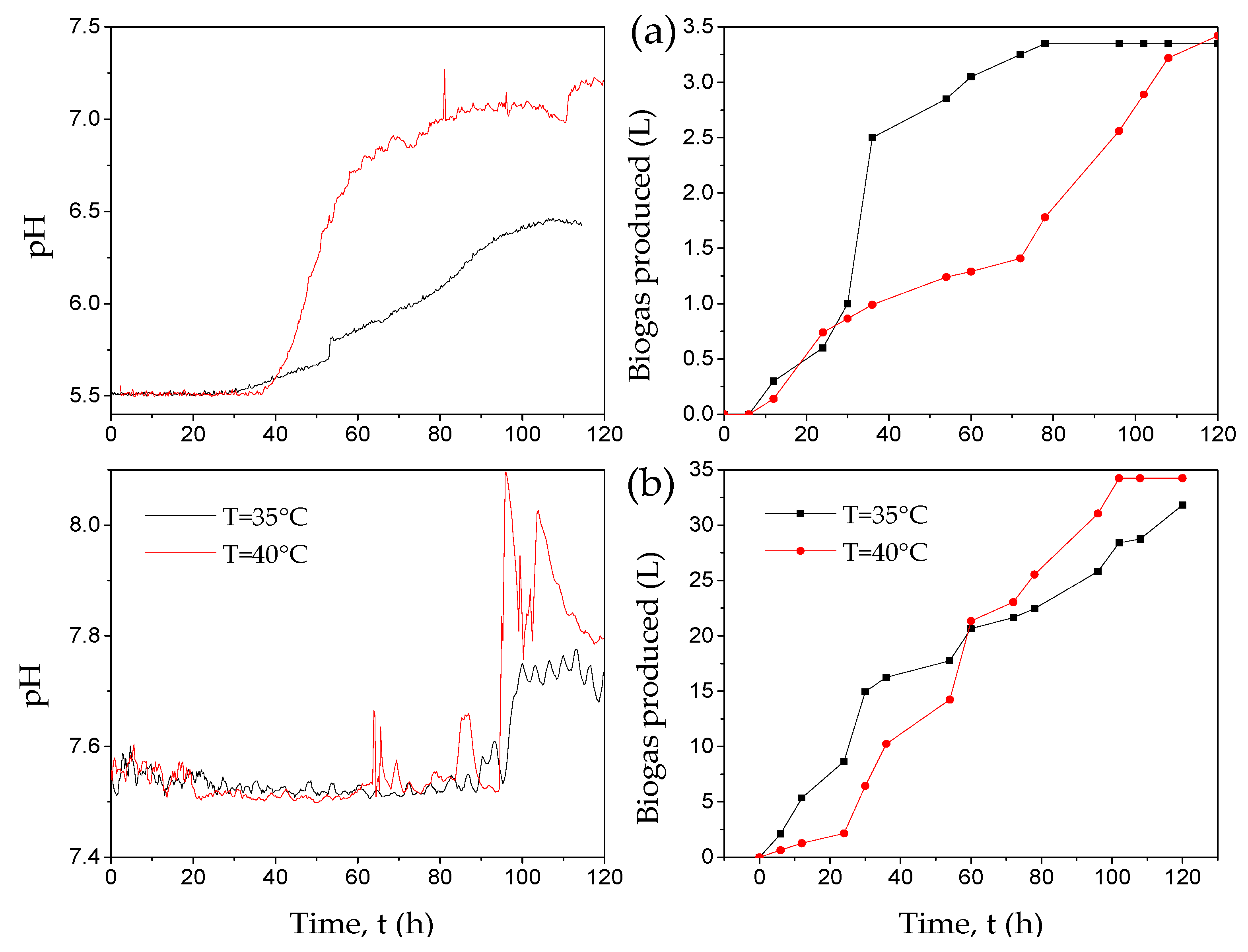
3.1. Experimental Tests
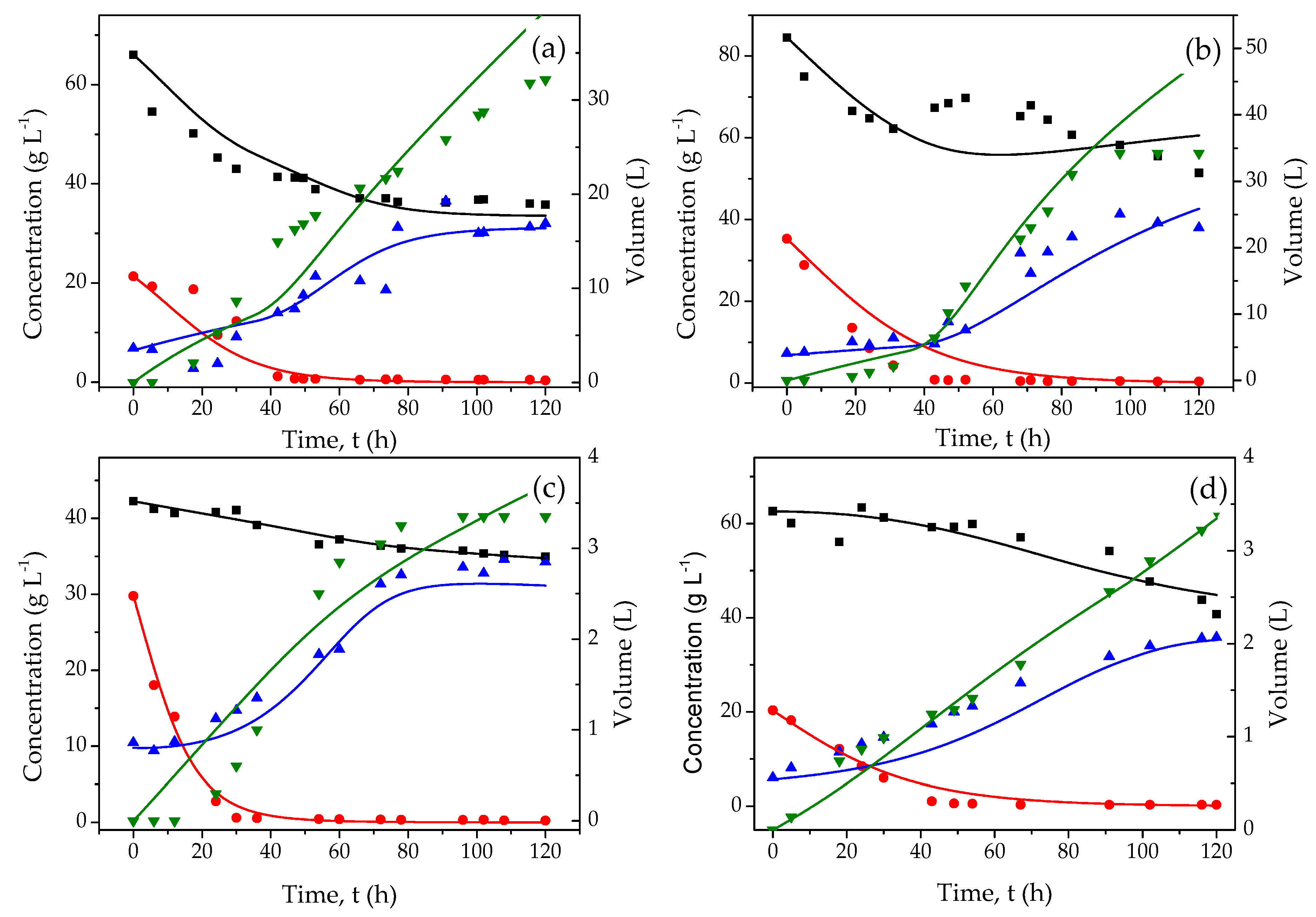
3.2. Mathematical Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations Statistics Division. Available online: http://www.fao.org/faostat/en/#data/QP/visualize (accessed on 2 December 2019).
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Escalante, H.; Castro, L.; Amaya, M.P.; Jaimes, L.; Jaimes-Estévez, J. Anaerobic digestion of cheese whey: Energetic and nutritional potential for the dairy sector in developing countries. Waste Manag. 2018, 71, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Bastidas-Oyanedel, J.R.; Bonk, F.; Thomsen, M.H.; Schmidt, J.E. Dark fermentation biorefinery in the present and future (bio)chemical industry. Rev. Environ. Sci. Biotechnol. 2015, 14, 473–498. [Google Scholar] [CrossRef]
- Imeni, S.M.; Pelaz, L.; Corchado-Lopo, C.; Busquets, A.M.; Ponsá, S.; Colón, J. Techno-economic assessment of anaerobic co-digestion of livestock manure and cheese whey (Cow, Goat & Sheep) at small to medium dairy farms. Bioresour. Technol. 2019, 291, 121872. [Google Scholar]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Calero, R.R.; Lagoa-Costa, B.; Fernandez-Feal, M.M.D.C.; Kennes, C.; Veiga, M.C. Volatile fatty acids production from cheese whey: Influence of pH, solid retention time and organic loading rate. J. Chem. Technol. Biotechnol. 2018, 93, 1742–1747. [Google Scholar] [CrossRef]
- Jankowska, E.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Volatile fatty acids production during mixed culture fermentation–The impact of substrate complexity and pH. Chem. Eng. J. 2017, 326, 901–910. [Google Scholar] [CrossRef]
- Yang, K.; Yu, Y.; Hwang, S. Selective optimization in thermophilic acidogenesis of cheese-whey wastewater to acetic and butyric acids: Partial acidification and methanation. Water Res. 2003, 37, 2467–2477. [Google Scholar] [CrossRef]
- De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Biohydrogen production from dark fermentation of cheese whey: Influence of pH. Int. J. Hydrogen Energy 2014, 39, 20930–20941. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Mailier, J.; Martin, C.; Rodríguez, J.; Aceves-Lara, C.A.; Wouwer, A.V. Model selection, identification and validation in anaerobic digestion: A review. Water Res. 2011, 45, 5347–5364. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Retamal, C.; Carballa, M.; Ruiz-Filippi, G.; Chamy, R. Influence of temperature on the hydrolysis, acidogenesis and methanogenesis in mesophilic anaerobic digestion: Parameter identification and modeling application. Water Sci. Technol. 2009, 60, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, J.; Appels, L.; Thompson, I.P.; Degrève, J.; Van Impe, J.F.; Dewil, R. Mathematical modelling of anaerobic digestion of biomass and waste: Power and limitations. Prog. Energy Combust. Sci. 2013, 39, 383–402. [Google Scholar] [CrossRef] [Green Version]
- Brulé, M.; Oechsner, H.; Jungbluth, T. Exponential model describing methane production kinetics in batch anaerobic digestion: A tool for evaluation of biochemical methane potential assays. Bioprocess Biosyst. Eng. 2014, 37, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- García-Ochoa, F.; Santos, V.E.; Naval, L.; Guardiola, E.; Lopez, B. Kinetic model for anaerobic digestion of livestock manure. Enzyme Microb. Technol. 1999, 25, 55–60. [Google Scholar] [CrossRef]
- Arudchelvam, Y.; Perinpanayagam, M.; Nirmalakhandan, N. Predicting VFA formation by dark fermentation of particulate substrates. Bioresour. Technol. 2010, 101, 7492–7499. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Ngo, H.H.; Zhang, C.; Yang, Q.; Peng, L.; Ni, B.J. Approach of describing dynamic production of volatile fatty acids from sludge alkaline fermentation. Bioresour. Technol. 2017, 238, 343–351. [Google Scholar] [CrossRef]
- Blanco, V.M.C.; Oliveira, G.H.D.; Zaiat, M. Dark fermentative biohydrogen production from synthetic cheese whey in an anaerobic structured-bed reactor: Performance evaluation and kinetic modeling. Renew. Energy 2019, 139, 1310–1319. [Google Scholar] [CrossRef]
- Hublin, A.; Zelić, B. Modelling of the whey and cow manure co-digestion process. Waste Manag. Res. 2013, 31, 353–360. [Google Scholar] [CrossRef]
- Lovato, G.; Ratusznei, S.M.; Rodrigues, J.A.D.; Zaiat, M. Co-digestion of whey with glycerin in an AnSBBR for biomethane production. Appl. Biochem. Biotechnol. 2016, 178, 126–143. [Google Scholar] [CrossRef]
- Lovato, G.; Lazaro, C.Z.; Zaiat, M.; Ratusznei, S.M.; Rodrigues, J.A.D. Biohydrogen production by co-digesting whey and glycerin in an AnSBBR: Performance optimization, metabolic pathway kinetic modeling and phylogenetic characterization. Biochem. Eng. J. 2017, 128, 93–105. [Google Scholar] [CrossRef]
- Mainardis, M.; Flaibani, S.; Trigatti, M.; Goi, D. Techno-economic feasibility of anaerobic digestion of cheese whey in small Italian dairies and effect of ultrasound pre-treatment on methane yield. J. Environ. Manag. 2019, 246, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, P.; Shin, J.; Shin, S.G.; Vyrides, I. Anaerobic digestion of industrial dairy wastewater and cheese whey: Performance of internal circulation bioreactor and laboratory batch test at pH 5–6. Renew. Energy 2020, 147, 1–10. [Google Scholar] [CrossRef]
- Flores-Mendoza, A.P.; Hernández-García, H.; Cocotle-Ronzón, Y.; Hernandez-Martinez, E. Methanogenesis of raw cheese whey: pH and substrate-inoculum ratio evaluation at mesophyll temperature range. J. Chem. Technol. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, J.; Shin, S.G.; O’Flaherty, V.; Hwang, S. Quantitative and qualitative transitions of methanogen community structure during the batch anaerobic digestion of cheese-processing wastewater. Appl. Microbiol. Biotechnol. 2010, 87, 1963–1973. [Google Scholar] [CrossRef]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic fermentation of industrial wastewaters: Effects of chemostat retention time and pH on volatile fatty acids production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar] [CrossRef]
- HACH. Chemical Oxygen Demand, Reactor Digestion Method; Method 8000; HACH: Loveland, CO, USA, 2000. [Google Scholar]
- American Public Health Association; American Water Works Association; Water Pollution Control Federation. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Parawira, W.; Murto, M.; Read, J.S.; Mattiasson, B. Volatile fatty acid production during anaerobic mesophilic digestion of solid potato waste. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2004, 79, 673–677. [Google Scholar] [CrossRef]
- Miron, Y.; Zeeman, G.; van Lier, J.; Lettinga, G. The role of sludge residence time in the hydrolysis of lipids, carbohydrates and proteins during the anaerobic treatment of domestic sewage. Water Res. 2000, 34, 1705–1713. [Google Scholar] [CrossRef]
- Drosg, B.; Braun, R.; Bochmann, G.; Al Saedi, T. Analysis and characterisation of biogas feedstocks. In The Biogas Handbook; Wellinger, A., Murphy, J., David, B., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 52–84. [Google Scholar]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Vavilin, V.A. The IWA anaerobic digestion model no 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Bernard, O.; Hadj-Sadok, Z.; Dochain, D.; Genovesi, A.; Steyer, J.P. Dynamical model development and parameter identification for an anaerobic wastewater treatment process. Biotechnol. Bioeng. 2001, 75, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Hassam, S.; Ficara, E.; Leva, A.; Harmand, J. A generic and systematic procedure to derive a simplified model from the anaerobic digestion model No. 1 (ADM1). Biochem. Eng. J. 2015, 99, 193–203. [Google Scholar] [CrossRef]
- Ficara, E.; Hassam, S.; Allegrini, A.; Leva, A.; Malpei, F.; Ferretti, G. Anaerobic digestion models: A comparative study. IFAC Proc. Vol. 2012, 45, 1052–1057. [Google Scholar] [CrossRef]
- Kiely, G.; Tayfur, G.; Dolan, C.; Tanji, K. Physical and mathematical modelling of anaerobic digestion of organic wastes. Water Res. 1997, 31, 534–540. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Wijtzes, T.; De Wit, J.C.; Riet, K.V.T. A decision support system for prediction of the microbial spoilage in foods. J. Food Prot. 1992, 55, 973–979. [Google Scholar] [CrossRef]
- Luo, K.; Yang, Q.; Li, X.M.; Yang, G.J.; Liu, Y.; Wang, D.B.; Zeng, G.M. Hydrolysis kinetics in anaerobic digestion of waste activated sludge enhanced by α-amylase. Biochem. Eng. J. 2012, 62, 17–21. [Google Scholar] [CrossRef]
- Moguel-Castañeda, J.G.; Puebla, H.; Méndez-Acosta, H.O.; Hernandez-Martinez, E. Modeling pH and temperature effects on the anaerobic treatment of tequila vinasses. J. Chem. Technol. Biotechnol. 2020. [Google Scholar] [CrossRef]
- McLean, K.A.; McAuley, K.B. Mathematical modelling of chemical processes—Obtaining the best model predictions and parameter estimates using identifiability and estimability procedures. Can. J. Chem. Eng. 2012, 90, 351–366. [Google Scholar] [CrossRef]
- Gelegenis, J.; Georgakakis, D.; Angelidaki, I.; Mavris, V. Optimization of biogas production by co-digesting whey with diluted poultry manure. Renew. Energy 2007, 32, 2147–2160. [Google Scholar] [CrossRef]
- Kasali, G.B.; Senior, E.; Watson-Craik, I.A. Sodium bicarbonate effects on the anaerobic digestion of refuse. J. Chem. Technol. Biotechnol. 1989, 45, 279–289. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, J. Kinetics of batch anaerobic co-digestion of poultry litter and wheat straw including a novel strategy of estimation of endogenous decay and yield coefficients using numerical integration. Bioprocess Biosyst. Eng. 2016, 39, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
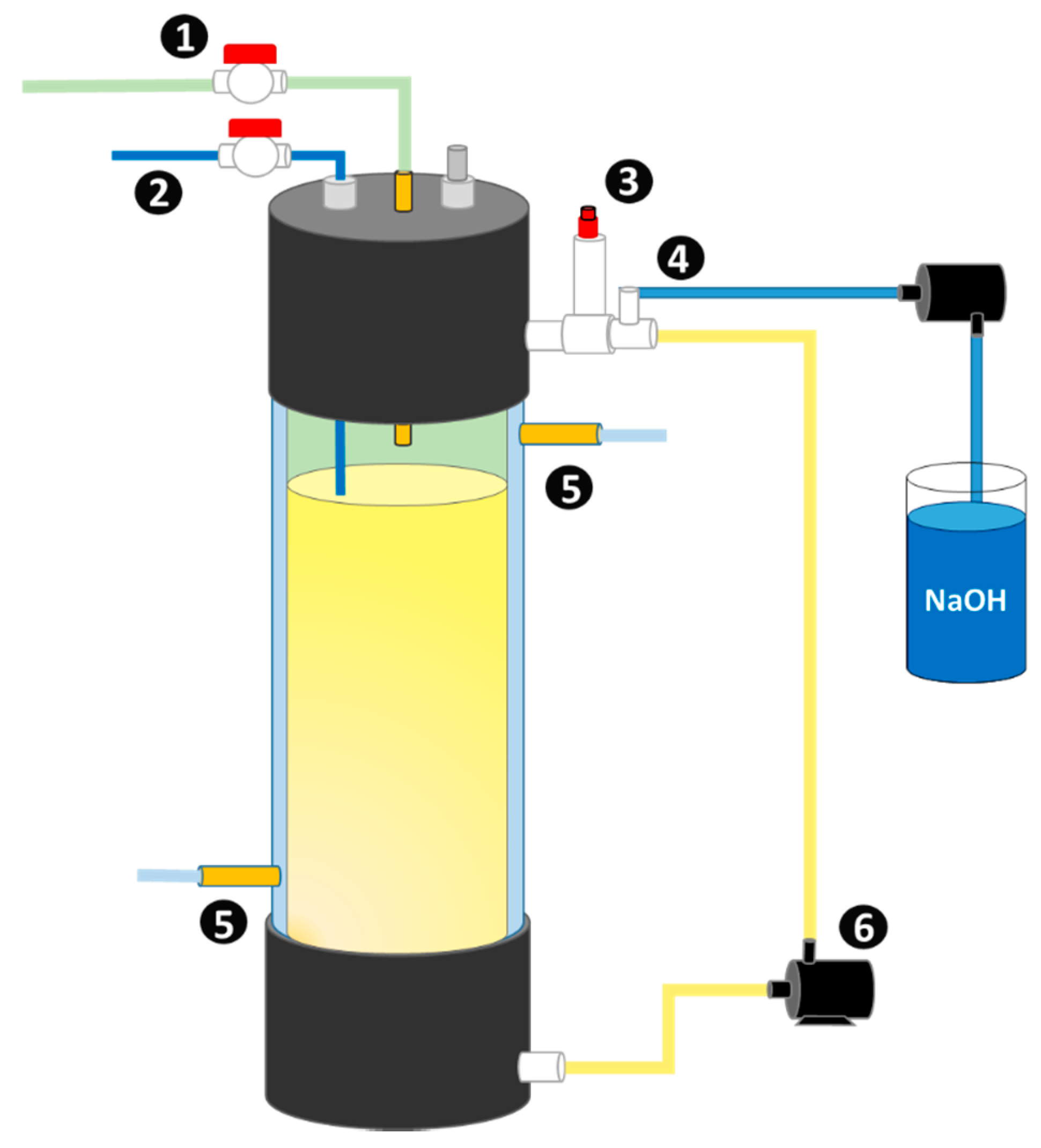

| Parameter | Cheese Whey | Inoculum |
|---|---|---|
| COD (g L−1) | 74.24 ± 1.35 | 32.52 ± 4.27 |
| Total carbohydrates (TCH) (gglucose L−1) | 35.04 ± 1.56 | 0.94 ± 0.07 |
| Total solids (g L−1) | 51.21 ± 1.36 | 49.87 ± 0.95 |
| Volatile solids (g L−1) | 36.68 ± 4.68 | 22.46 ± 4.30 |
| Volatile sedimented solids (g L−1) | 1.20 ± 0.24 | 2.15 ± 0.41 |
| Volatile fatty acids (VFA) (gCOD L−1) | 0.57 ± 0.08 | 28.56 ± 1.90 |
| Proteins (mg L−1) | 27.32 ± 2.17 | 7.22 ± 1.09 |
| pH | 4.72 ± 0.04 | 6.85 ± 0.08 |
| pH | T (°C) | TCH Degradation (%) | COD Diminution (%) | Biogas (mL) | VFA Generated (gCOD L−1) |
|---|---|---|---|---|---|
| 5.5 | 35 | 98.38 ± 0.82 | 45.81 ± 1.81 | 32,150 ± 1286 | 24.35 ± 0.61 |
| 5.5 | 40 | 98.97 ± 0.28 | 39.2 ± 1.52 | 34,241 ± 1301 | 30.71 ± 0.41 |
| 7.5 | 35 | 99.19 ± 0.16 | 21.31 ± 2.65 | 3350 ± 402 | 23.8 ± 0.44 |
| 7.5 | 40 | 98.41 ± 0.23 | 34.9 ± 3.54 | 3420 ± 335 | 29.73 ± 0.29 |
| Parameter | pH = 5.5, T = 35 °C | pH = 5.5, T = 40 °C | pH = 7.5, T = 35 °C | pH = 7.5, T = 40 °C |
|---|---|---|---|---|
| 0.0025 | 0.00086 | 0.0026 | 0.0013 | |
| 0.0019 | 0.00167 | 0.0112 | 0.003 | |
| 9.69 | 26.55 | 20.93 | 19.99 | |
| 0.551 | 0.139 | 0.303 | 0.301 | |
| 76.50 | 220.151 | 10.30 | 16.24 | |
| 0.0110 | 0.00972 | 0.099 | 0.096 | |
| 0.00039 | 0.00087 | 20.76 | 23.40 | |
| 33.65 | 45.33 | 0.9405 | 1.46 | |
| 368.465 | 994.07 | 13.85 | 17.32 | |
| 0.0497 | 0.0996 | 0.1884 | 0.125 | |
| 8.16 | 2.86 | 19.30 | 14.06 | |
| 26.89 | 84.46 | 88.74 | 147.26 | |
| 0.00195 | 0.00005 | 0.0312 | 0.00011 | |
| 489,888.98 | 250,696.16 | 152.21 | 151.146 | |
| 35.43 | 22.87 | 408.52 | 122.69 | |
| 0.908 | 1.00 | 0.7610 | 0.615 | |
| 0.1153 | 0.0854 | 0.3042 | 0.310 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
B-Arroyo, C.; Lara-Musule, A.; Alvarez-Sanchez, E.; Trejo-Aguilar, G.; Bastidas-Oyanedel, J.-R.; Hernandez-Martinez, E. An Unstructured Model for Anaerobic Treatment of Raw Cheese Whey for Volatile Fatty Acids Production. Energies 2020, 13, 1850. https://doi.org/10.3390/en13071850
B-Arroyo C, Lara-Musule A, Alvarez-Sanchez E, Trejo-Aguilar G, Bastidas-Oyanedel J-R, Hernandez-Martinez E. An Unstructured Model for Anaerobic Treatment of Raw Cheese Whey for Volatile Fatty Acids Production. Energies. 2020; 13(7):1850. https://doi.org/10.3390/en13071850
Chicago/Turabian StyleB-Arroyo, Claudio, Antonio Lara-Musule, Ervin Alvarez-Sanchez, Gloria Trejo-Aguilar, Juan-Rodrigo Bastidas-Oyanedel, and Eliseo Hernandez-Martinez. 2020. "An Unstructured Model for Anaerobic Treatment of Raw Cheese Whey for Volatile Fatty Acids Production" Energies 13, no. 7: 1850. https://doi.org/10.3390/en13071850





